The surgical treatment of choice for complex forms of d-transposition of the great arteries associated with a ventricular septal defect and obstruction of the left ventricular outflow tract remains a controversial topic associated with essentially three surgical options.Reference Hazekamp, Gomez and Koolbergen 1
Traditionally, the Rastelli operation introduced in 1969 has been the standard surgical repair for these patients, with satisfactory short-term morbidity and mortality results. This procedure directs the flow of the left ventricle through the ventricular septal defect to the aorta, and the connection of the right ventricle to the pulmonary artery is established through an extracardiac conduit.Reference Rastelli, Wallace and Ongley 2 Long-term follow-up, however, in different series of the Rastelli operation has generally documented suboptimal results with reported survival after 20 years of follow-up around 50–60%, depending on the group.Reference Hazekamp, Gomez and Koolbergen 1 , Reference Horer, Schreiber and Dworak 3 – Reference Hörer, Schreiber and Dworak 7
The “réparation à l’étage ventriculaire” or ventricular repair, described by Lecompte in 1982, involves the extensive resection of the interventricular septum at the infundibular level, which enlarges the pathway of the left ventricle to the aorta, minimising the risk of posterior subaortic stenosis.Reference Lecompte, Neveux and Leca 8 Reconstruction of the right ventricular outflow tract does not entail the use of a prosthetic conduit, unlike in the Rastelli technique, thus avoiding conduit-related complications. The use of a unicusp valve, however, is associated with significant residual pulmonary regurgitation, with the ensuing need for medium to long-term re-intervention. This technique generally leads to better long-term results compared with the Rastelli technique, with fewer reoperations and greater long-term survival rates.Reference Hazekamp, Gomez and Koolbergen 1
The third surgical option is aortic translocation with biventricular outflow tract reconstruction described by Nikaidoh in 1984,Reference Nikaidoh 9 which is gaining increasing traction internationally in recent years.
The originally described Nikaidoh technique consists of direct posterior translocation of the aortic root, leaving the coronary arteries in situ along with resection of the infundibular septum. The right ventricular outflow tract is re-built using as much autologous tissue as possible to provide some growth potential. Otherwise, if this technique is not possible, the connection to the pulmonary branches is accomplished using a valved homograft or xenograft. Different groups including Nikaidoh’s have subsequently described modifications to the original technique. The main variations in different series have been in the form of changes to the partial or full mobilisation of the aortic root, the previous transection of the aorta and pulmonary artery, the mobilisation and re-implantation of coronary arteries, the use of non-autologous grafts to re-build the right ventricular outflow tract, or a combination with the Mustard procedure in patients with congenitally corrected transposition of the great arteries.Reference Hu, Liu and Li 10 – Reference Bautista-Hernandez, Marx, Bacha and del Nido 13
In this study, we describe the experience of our group with the modified Nikaidoh technique and report the short-term follow-up of four patients suffering from complex transposition of great arteries with ventricular septal defect and pulmonary stenosis.
Patients and methods
Patients
Between July 2007 and June 2014, four patients with d-transposition of the great arteries, ventricular septal defect and severe pulmonary stenosis were operated by our group with the modified Nikaidoh technique. The gender distribution was 50%; the average age at the time of operation was 26 months (with a range from 13 to 40), with an average weight of 10.7 kg (with a range from 8.4 to 14.5) and an average height of 82 cm (with a range from 77 to 93).
The ventricular septal defect was subpulmonary in three patients and was a non-related muscular defect in one patient, with an average size of 8 mm (with a range from 6 to 10 mm). The congenital pulmonary stenosis evaluated by echocardiography had an average peak gradient of 62 mmHg (with a range from 45 to 70), being at a valvular level in three patients – with the size of the pulmonary annulus in these three patients being 8.5 mm on average – and subvalvular in the remaining patient – whose pulmonary annulus was 10 mm in size. The average diameter of the aortic annulus was 17 mm (with a range from 14 to 18). Of the four patients, three of them also had an atrial septal defect of 13 mm on average (with a range from 8 to 17).
In all, three patients had undergone a palliative procedure previously: a Rashkind septostomy in one patient, a Blalock–Taussig shunt in one patient, and both procedures in the final patient. The fourth patient was referred from a developing country and had not undergone any palliative procedure.
Surgical technique
All patients underwent a median sternotomy and deep hypothermic cardiopulmonary bypass at 18°C, without circulatory arrest. Arterial cannulation was performed in all cases at a proximal aortic arch level, in order to enable better aortic mobilisation. Myocardial protection was by intermittent antegrade cold blood cardioplegia.
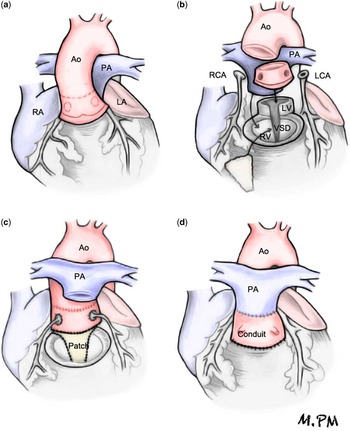
After circumferential dissection and mobilisation of the great arteries, the aortic cross-clamp was applied, and the aorta was transected at the sinotubular junction (Fig. 1). Next, the aortic root was dissected and excised from the right ventricle, creating an aortic root autograft. Both coronary buttons were excised from the aortic root and were carefully dissected along their proximal course to prevent anatomical distortion or tension when later re-implanted into the translocated aortic root.
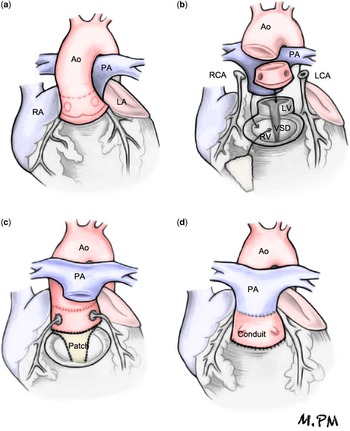
Figure 1 (a) Dissection and distal mobilisation of the great arteries. (b) Excision of the aortic root as autograft. Excision of both coronary buttons. Transection of the pulmonary artery (PA) connected to the left ventricle (LV). Release of stenosis from the left ventricular outflow tract by excision of the pulmonary valve and division of the conal septum above the ventricular septal defect (VSD). (c) Closure of the VSD with heterologous pericardium by expanding the left ventricular outflow tract. Re-implantation of the translocated aortic root on the expanded left ventricular outflow tract. Re-implantation of coronary ostia. Anastomosis of the aortic root to the ascending aorta (Ao). (d) PA translocated to the anterior position (Lecompte). Connection of the right ventricle (RV) to the PA with a valved homograft or Contegra conduit. LA=left atrium; LCA=left coronary artery; RA=right atrium; RCA=right coronary artery.
The pulmonary artery, connected to the left ventricle, was transected, and the stenosis of the left ventricular outflow tract was relieved by excision of the stenotic pulmonary valve and division of the conal septum superior to the ventricular septal defect. A bovine pericardial patch was used to close the enlarged ventricular septal defect, thus widening the left ventricular outflow tract. The aortic root was posteriorly translocated and re-implanted into this widened left ventricular outflow tract. The coronary artery ostia were re-implanted in this newly translocated aortic root, avoiding tension or distortion of their course.
The main pulmonary artery and its branches were translocated anterior to the ascending aorta (Lecompte maneuver), and the distal transected aortic root was anastomosed end to end to the proximal aorta. The previously translocated pulmonary artery was connected to the right ventricle in three patients by means of a Contegra conduit (Medtronic, Minneapolis, Minnesota, United States of America) of 18 mm in two patients and 16 mm in the third patient and by means of a 23-mm valved homograft in the fourth patient.
Results
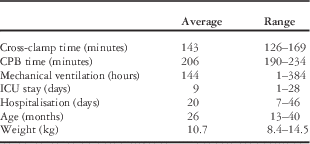
The procedure was performed in four patients (data in Table 1). The average aortic cross-clamp time was 143 minutes (with a range from 126 to 169), and the cardiopulmonary bypass time was 206 minutes (with a range from 190 to 234 minutes). The average ICU stay was 9 days.
Table 1 Perioperative data.

CPB=cardiopulmonary bypass
None of the patients required re-operation for bleeding or other complications. None of the patients required mechanical circulatory support. All patients were discharged in stable condition after an average hospital stay of 20 days (with a range from 7 to 46).
Follow-up was completed in all patients as of June 2015, with a mean follow-up of 4.5 years (with a range from 13 months to 8 years). On the most recent echocardiogram, one patient showed mild aortic insufficiency, whereas the remainder had normal aortic valve function. There were no residual ventricular or atrial septal defects in any of the patients. None of the patients had left ventricular outflow tract obstruction. The biventricular function remained normal in the four patients.
The patient with a 16-mm Contegra conduit had moderate regurgitation and stenosis, with a 25-mmHg gradient after 2 years of follow-up. The other two patients in whom an 18-mm Contegra conduit was used had no stenosis. The patient in whom a homograft was used for right ventricular outflow tract re-construction had neither pulmonary stenosis nor regurgitation.
All patients had a normal functional class with weight gain appropriate for their age. There have been no ventricular arrhythmias, atrioventricular block, or need for a pacemaker implant in any of the patients at the last follow-up.
Comment
The technique of choice to repair d-transposition of the great arteries associated with ventricular septal defect and obstruction of the left ventricular outflow tract is still a controversial topic. The most frequently applied technique globally over the last few decades has been the Rastelli procedure. Its results are satisfactory in the short term, but suboptimal survival results and a frequent need for reoperation have been found in the long-term follow-up of patients, mostly on the right ventricle, but also in up to 10% of patients because of the recurrence of obstruction of the left ventricular outflow tract.
In this study, we report our experience and results with the modified Nikaidoh procedure for the treatment of patients with d-transposition of the great arteries associated with ventricular septal defect and pulmonary stenosis, mainly obstruction of the left ventricular outflow tract. Postoperative results and short-term follow-up were all favourable, and potentially show that this procedure may be a better option than the Rastelli procedure for this subset of patients; however, as the global experience with the Nikaidoh procedure, in quantitative terms, is still limited and relatively recent, longer follow-up times are needed to establish the potential superiority of this approach.
The main theoretical advantage of Nikaidoh’s aortic translocation compared with the Rastelli or the “réparation à l’étage ventriculaire” techniques is the re-construction of a more normal intracardiac anatomy. Avoiding intracardiac tunnels minimises the risk of recurrence of left ventricular outflow tract obstruction and creates a better alignment of both outflow tracts by re-positioning the great arteries in a more natural position. This could reduce the risk of premature degeneration of the right ventricular outflow tract conduit by sternal compression. The high re-operation rates after the Rastelli procedure have been related to a non-anatomical repair, caused by implanting the right ventricular outflow tract conduit onto the infundibular portion of the right ventricle. Kreutzer reported a re-intervention rate of 79% at 15 years, with a 15-year actuarial survival of 68%.Reference Kreutzer, De Vive and Oppido 4 DearaniReference Dearani, Danielson, Puga, Mair and Schleck 5 reported an actuarial 20-year survival of 60%. HazekampReference Hazekamp, Gomez and Koolbergen 1 reported an 84% re-operation rate after 15 years, with a global survival of 68%.
Nikaidoh’s aortic translocation and biventricular outflow tract reconstruction procedure results in better anatomical repair, moving the aortic root posteriorly and creating greater space for the right ventricular outflow tract in the orthotopic position, regardless of the conduit used. These key details of the technique potentially reduce the risk of recurrent obstruction in the left ventricular outflow tract compared with the Rastelli procedure, which could foreseeably lead to a lower global re-operation rate and better long-term survival results.
The reintervention rate for the right ventricular outflow tract in different series reporting the results of the Nikaidoh procedure varies between 15% (del Nido),Reference Bautista-Hernandez, Marx, Bacha and del Nido 13 with an average follow-up of 6 years, and 46% (Nikaidoh)Reference Yeh, Ramaciotti, Leonard, Roy and Nikaidoh 11 but with a much longer follow-up time of 15 years. In a more recent series, although with a shorter follow-up time, no need of reoperation was reported at the medium-term follow-up, and these results were consistent with those of our series.Reference Hazekamp, Gomez and Koolbergen 1 , Reference Hu, Liu and Li 10 Therefore, even though the use of valved conduits in the Nikaidoh procedure is associated with the need for reoperation on the right ventricular outflow tract, this incidence along with the morbidity and mortality has been reasonable.Reference Hazekamp, Gomez and Koolbergen 1
Consistent with all the other reported series, none of the patients in our series has required a reoperation because of a left ventricular outflow tract obstruction. This also compares favourably with the Rastelli procedure.
Premature mortality after the Nikaidoh procedure in the largest seriesReference Hu, Liu and Li 10 – Reference Bautista-Hernandez, Marx, Bacha and del Nido 13 , Reference Kramer, Ovroutski, Hetzer, Hübler and Berger 14 was <5%, and medium- to long-term mortality was significantly lower compared with the Rastelli procedure, with a survival of around 95–100% versus 50–60% for the Rastelli procedure after 20 years.Reference Hazekamp, Gomez and Koolbergen 1 , Reference Horer, Schreiber and Dworak 3 – Reference Hörer, Schreiber and Dworak 7
Although in Nikaidoh’s original description the coronary arteries were not detached and re-implanted, but were moved in-block with the aortic root, our approach consists of detachment, mobilisation, and re-attachment to the anterior surface of the posteriorly translocated aortic root. This is similar to the technique used by many contemporary groups who perform this procedure. The advantage of this technique of detachment, mobilisation, and re-attachment is to minimise the risk of coronary artery distortion, a serious risk that more frequently affects the right coronary artery in patients with the usual coronary artery pattern. In a recent article,14 two deaths were reported – one secondary to coronary artery ischaemia in a patient with an anomalous (1L; 2RCx) coronary artery pattern. During the 7-year interval encompassed by our study, we have not experienced any ischaemic complications; however, we did perform a Rastelli procedure on three patients with an anomalous coronary artery pattern who were otherwise candidates for the Nikaidoh procedure. Anomalous coronary artery patterns that can increase the morbidity and mortality of the Nikaidoh procedure includeReference Kramer, Ovroutski, Hetzer, Hübler and Berger 14 origin of the circumflex and/or anterior descending artery from sinus 2 with a looping course posterior to the pulmonary artery and right coronary artery origin from sinus 1 with a long anterior course.
Another possible complication of posterior aortic translocation is the development of aortic insufficiency. Some larger series have documented an incidence up to 27% of moderate aortic regurgittion during the follow-up period.Reference Hu, Liu and Li 10 – Reference Bautista-Hernandez, Marx, Bacha and del Nido 13 Although aortic regurgittion has usually been reported as mild to moderate and without progression, one patient did require late reoperation on the aortic valve for severe regurgitation.Reference Hu, Liu and Li 10 The mechanism for the development of aortic regurgittion in these patients is probably related to distortion of the sinotubular junction and alignment errors during the implantation of the aortic autograft. In our series, one patient developed mild aortic regurgittion, and none have developed aortic root dilation. Although the incidence of aortic regurgittion appears to be low, this remains an important long-term outcome event to re-assess as more patients are followed-up over time.
The main limitation of this study is its retrospective design, the lack of a comparison group, and the small number of cases with a relatively short follow-up time.
Conclusions
Although Nikaidoh’s procedure was first described in 1984, because of its technical complexity, there are still relatively few series encompassing a relatively small number of total patients. Nevertheless, the Nikaidoh procedure is gaining increased international acceptance because it produces a more anatomically correct alignment between the ventricles and their great arteries, and because it can be consistently performed with acceptable morbidity and mortality with the potential for improved long-term survival compared with the Rastelli or the “réparation à l’étage ventriculaire” procedures.
Larger series with comparison groups and longer-term follow-up are needed to clearly establish the potential superiority of this procedure.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees (Hospital San Joan de Deu, Barcelona, Spain).