Introduction
Methiozolin [MRC-101, 5(2,6-difluorobenzyl)oxymethyl-5-methyl-3,3(3-methylthiophen-2-yl)-1,2-isoxazoline] is a herbicide in the isoxaline chemical family originally considered for weed management in rice (Oryza sativa L.) (Hwang et al. Reference Hwang, Kim, Jeon, Hong, Song and Cho2005; Ryu et al. Reference Ryu, Kim, Jeon, Song, Kim, Lee, Kim and Hong2002). It was originally registered in Korea and Japan for use in turfgrass (Koo et al. Reference Koo, Hwang, Jeon, Kim, Lim, Lee and Cho2014) and was registered for that use in 2019 in the United States (Anonymous 2019b). A primary weed target for methiozolin is P. annua in established creeping bentgrass (Agrostis stolonifera L.) and other cool- and warm-season turfgrasses (Askew and McNulty Reference Askew and McNulty2014; Brosnan et al. Reference Brosnan, Calvache, Breeden and Sorochan2013; Flessner et al. Reference Flessner, Wehtje and McElroy2013; Koo et al. Reference Koo, Hwang, Jeon, Kim, Lim, Lee and Cho2014; Venner Reference Venner2015; Yu and McCullough Reference Yu and McCullough2014). However, certain Agrostis spp. and cultivars are sensitive to methiozolin (Hoisington et al. Reference Hoisington, Flessner, Schiavon, McElroy and Baird2014).
Methiozolin is a lipophilic herbicide (logKow 3.9) that is readily absorbed by roots and shoots but is not phloem mobile and has little to moderate translocation in the xylem (Flessner et al. Reference Flessner, Wehtje and McElroy2013; McCullough et al. Reference McCullough, Barreda and Yu2013; Yu and McCullough Reference Yu and McCullough2014). The variation in methiozolin sensitivity found within and among established turfgrasses and P. annua was partially attributed to differences in absorption and translocation, but not metabolism (Flessner et al. Reference Flessner, Wehtje and McElroy2013; McCullough et al. Reference McCullough, Barreda and Yu2013; Yu and McCullough Reference Yu and McCullough2014). Target-site sensitivity or growth phenology differences were also proposed as alternative tolerance mechanisms.
Two possible mechanisms of action (MOAs) have been proposed for methiozolin. Lee et al. (Reference Lee, Koo, Hwang, Hwang, Jeon and Kim2007) proposed methiozolin either directly or indirectly inhibits cell wall biosynthesis. The MOA of cellulose biosynthesis inhibitors (CBIs), while not completely understood, causes a decrease in the incorporation of glucose into expanding cellulose microfibrils (Brabham and Debolt Reference Brabham and Debolt2012). For a herbicide to be classified as a CBI, it must meet three criteria: (1) treated seedlings are phenotypically stunted and rapidly expanding tissue appears swollen, (2) cellulose synthesis is rapidly (within 2 h after treatment [HAT]) inhibited, and (3) cellulose content is reduced in a dose-dependent manner (Brabham and Debolt Reference Brabham and Debolt2012; Sabba and Vaughn Reference Sabba and Vaughn1999). Lee et al. (Reference Lee, Koo, Hwang, Hwang, Jeon and Kim2007) found that 1 µM methiozolin caused corn (Zea mays L.) root growth to be nearly stopped 6 HAT. The incorporation of [14C]glucose into the cellulose and hemicellulose fractions of the corn roots was severely inhibited at 12 and 24 HAT, but inhibition was variable at earlier time points. Although not a rapid inhibition, the inhibition of glucose incorporation into cellulose is consistent with a CBI MOA. In addition, while both the shoot and root growth of barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] seedlings were inhibited by methiozolin, the observed symptomologies were dissimilar to those of the known CBI dichlobenil. Taken together, the results of Lee et al. (Reference Lee, Koo, Hwang, Hwang, Jeon and Kim2007) offer little support for methiozolin acting as a CBI.
Alternatively, Grossmann et al. (Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012), based on data from metabolomic profiling and metabolite supplementation of methiozolin-treated lesser duckweed (Lemna aequinoctialis Welw.; syn. Lemna paucicostata Hegelm.), suggested that methiozolin prevents formation of plastoquinone and tocopherol through inhibition of tyrosine aminotransferases (TATs; EC 2.6.1.5). TATs are responsible for the conversion of tyrosine to 4-hydroxyphenyl-pyruvate and ultimately homogentisate. Homogentisate is an essential branch-point metabolite for both the prenylquinone and tocopherol pathways (DellaPenna and Pogson Reference DellaPenna and Pogson2006). Functionally, TATs are directly upstream of the enzymatic target of 4-hydroxyphenyl-pyruvate-diooxygenase (HPPD) inhibitors. Thus, the inhibitory mechanism of methiozolin would be essentially similar to that of HPPD-inhibiting herbicides. Susceptible plants treated with HPPD inhibitors lack pigmentation or become “bleached” in new tissue, which is followed by necrosis (Shaner 2014). Consistent with TAT inhibition, Grossmann et al. (Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012) observed photobleaching in newly formed fronds of L. aequinoctialis within 72 h of methiozolin treatment. However, photobleached tissue later became “greenish with a touch of brown,” and the meristematic tissue was necrotic. Methiozolin was also shown to inhibit Arabidopsis (Arabidopsis thaliana L.) TAT7 activity in vitro. However, the inhibition only occurred at extremely high methiozolin concentrations (≥62 µM). Grossmann et al., (Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012) hypothesized that another TAT(s) or that TATs in grass species would be inhibited at lower methiozolin concentrations.
The studies described in this paper were undertaken to investigate the MOA of methiozolin in more detail. Experiments were conducted at the University of Kentucky and at BASF. In the paper, we exclude several physiological processes as the target site of methiozolin and present evidence that methiozolin is a fatty acid thioesterase (FAT) inhibitor.
Materials and Methods
Plant Growth Conditions and Measurements
Arabidopsis thaliana (ecotype Columbia) and P. annua grass seeds were surface sterilized for 15 min in Eppendorf tubes with 30% household bleach and subsequently washed three times with sterilized distilled water and kept at 4 C for 2 d. Seeds were placed on agar plates placed vertically in growth chambers (22 C and a 16-h photoperiod). Agar media was made by autoclaving a 1-L solution containing 2.25 g of Murashige and Skoog (MS) Basal Salt Mixture (Phyto Technology Laboratories, Shawnee Mission, KS), 0.6 g of 2-(N-Morpholino)-ethanesulfuric acid (MES), and 11 g of agar. The pH of the solution was 5.7 to 5.8. Agar plates were made with or without methiozolin by pouring 40 ml of autoclaved MS-agar media into a 50-ml Falcon tube, which was then capped and shaken, and the solution was poured directly into plates and allowed to solidify. Compounds were added to the tubes before capping. The plates used in all experiments were a sterile square petri dish with grids (100 mm by 100 mm by 15 mm; Simport). Methiozolin (purity 99.71%) was supplied by the Moghu Research Center. A 100 mM stock solution was made by dissolving methiozolin in dimethyl sulfoxide (DMSO), and this was subsequently diluted with DMSO to obtain appropriate concentrations. Concentrations of DMSO above 0.1% in agar plates are injurious to A. thaliana, and thus no more than 40 µl of the methiozolin solution was added to obtain the desired methiozolin rates. DMSO (0.05% v/v) alone was included in the untreated control in all experiments. Arabidopsis thaliana seeds germinate very quickly, and the first day after plating was considered 1 d after treatment (DAT). Poa annua seed was pre-germinated on water saturated filter paper in petri dishes before being transferred to the treatment plates. Poa annua that had a protruding radicle <1 mm were selected and transferred. For A. thaliana, each plate had two rows of seeds, and each row had roughly 10 seeds. For P. annua, only one row of 12 pre-germinated seeds was used.
Root and hypocotyl lengths were measured at 7 DAT. Plates were photographed, and ImageJ (Schneider et al. Reference Schneider, Rasband and Eliceiri2012) was used to measure root length. The grid pattern on square plates used in these experiments had an area of 1 cm2. This allowed us to convert pixel number into centimeters. Lengths are expressed as a percentage of the untreated control. At 14 DAT, shoot tissue from four randomly sampled plants was harvested, and fresh weight was measured. The four-plant sample was considered a replication, and three replications were taken from each treatment plate. To determine the effect of methiozolin on hypocotyl growth, plates were exposed to light for 2 h, wrapped in aluminum foil, and then placed vertically into the growth chamber.
Effect of Methiozolin on Plant Growth
Dose Response Experiments
Arabidopsis thaliana and P. annua seedlings were grown on agar plates with different concentrations (0, 10, 25, 37, 50, 75, 100, 500 nM, or 1 µM) of methiozolin as described earlier. In the case of the dark-grown A. thaliana (hypocotyl expansion determination) experiment, the methiozolin concentrations used were: 0, 1, 5, 10, 25, 50, or 100 µM. A minimum of 10 plants were measured per treatment plate, and treatment plates were replicated three times in time. Dose–response curves were generated in R using the drc package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhand2015).
Chlorophyll Measurement
To determine whether methiozolin causes a loss in chlorophyll content, A. thaliana seedlings were treated with methiozolin (0, 5 nM, 50 nM, 500 nM, 5 µM, and 50 µM) as described earlier. At 7 and 14 DAT, shoot tissue was harvested, weighed, placed into Eppendorf tubes, frozen in liquid nitrogen, and stored at −80 C until being used for chlorophyll determinations. Chlorophyll was extracted as follows (Ritchie Reference Ritchie2006): shoot tissue was homogenized with a micropestle in Eppendorf tubes with 300 µl of extraction buffer (1:1:1 ratio of 90% v/v acetone:methanol:ethanol). This step was done under dim light. Tubes were centrifuged for 5 min at 2,300 × g. Afterward, 50 µl of the supernatant was combined with 950 ml of ethanol. Chlorophyll a and b were measured spectrophotometrically at 665 nm and 649 nm, respectively. Absorbance values were used to calculate chlorophyll content per milligram using Equations 1–3 (Ritchie Reference Ritchie2006):
Root Cell Length Measurement
Arabidopsis thaliana seedlings expressing Plasma membrane Intrinsic Protein 2:Red Fluorescent Protein (PIP2:RFP) were grown on agar plates supplemented with DMSO (0.05% v/v), methiozolin (100 nM), or indaziflam (2 nM) as described earlier. Fluorescence from PIP2:RFP colocalizes with the plasma membrane and can be used to outline cells (Cutler et al. Reference Cutler, Ehrhardt, Griffitts and Somerville2000). Images of roots from 3-d-old PIP2:RFP plants were taken on an Olympus IX83 confocal microscope using a 40× water objective. Images of roots from 10 plants per treatment plate were taken, and the experiment was repeated. Six cells from the zone of elongation and maturation were measured per plant using ImageJ (National Institutes of Health, Bethesda, MD 20892). Scale bars acquired during imaging were used to convert pixels to millimeter lengths.
Metabolite Supplementation Studies
To see whether methiozolin phytotoxicity could be reversed by metabolite supplementation, A. thaliana seedlings were grown on agar plates with methiozolin (5 or 50 nM) with or without supplements. To test whether methiozolin is a TAT-inhibiting herbicide, agar plates were supplemented with 10 µM chorismate, tyrosine + phenylalanine, 4-hydroxyphenyl-pyruvate, or homogentisate. In addition, mesotrione (20 nM) was included as a positive control for supplements’ efficacy. For the mevalonate supplementation assay, the positive control, lovastatin (1 µM; ([1S-[1α(R*),3α,7β,8β(2 S*,4 S*),8aβ]]-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy- 6-oxo-2 H-pyran-2-yl)ethyl]-1-naphthalenyl 2-methylbutanoate)), methiozolin, or DMSO (0.05% v/v) were supplemented with 20 µM of trans, trans-farnesol, or squalene. Lovastatin, farnesol, and squalene were kindly provided by the laboratory of Joseph Chappell at the University of Kentucky Department College of Pharmacy and were diluted in DMSO, methanol, and acetone, respectively. Otherwise, all supplements were from Sigma and were dissolved in water. Root lengths were measured at 7 DAT and shoot fresh weights at 14 DAT as described earlier. The study was repeated three times in time.
Measurement of α-Tocopherol
Arabidopsis thaliana plants were grown on agar plates containing DMSO or methiozolin (5, 50, or 500 nM) as described earlier. Shoot tissue was harvested at 7 and 14 DAT, weighed, placed in Eppendorf tubes, frozen in liquid nitrogen, and stored at −80 C until used. Tocopherols were extracted and quantified using the official AOCS method (Xu Reference Xu2002; modified for Eppendorf tube volume). Briefly, tissue was ground in the Eppendorf tube using a micropestle with 500 µl of 90% (v/v) ethanol and 50 µl 800 g L−1 potassium hydroxide. Samples were placed in a 70 C water bath for 30 min followed 5 min in an ice bath. Then, 300 µl of water and 500 µl of hexane were added, and the samples were vortexed and centrifuged at 1,000 × g for 10 min. The nonaqueous (upper) phase was removed and retained. An additional 500 µl of hexane was added to the remaining aqueous phase, and the procedure was repeated. The collected nonaqueous phases were combined, dried under nitrogen flow, dissolved in 1 ml of methanol, and filtered (0.4 mm). Samples (200 µl) were injected into a high-performance liquid chromatography column equipped with a C18 column (Inertsil ODS-3, Millipore-Sigma, St Louis, MO 63178). The flow rate was 1 ml min−1 and the mobile phase was 50:46:4 v/v/v methanol:acetonitrile:methylene chloride. UV fluorescence was measured at 295 nm. Calibration curves were generated and used to determine α-tocopherol concentrations, and tocoperinol (100 µM) was added as an internal standard before sample extraction. Two subsamples were taken per treatment plate, and treatment plates were replicated in time (n = 6).
Major Phytosterol Measurement
Arabidopsis thaliana plants were grown on agar plates containing DMSO or methiozolin (1 µM) as described earlier. At 72 HAT, whole seedlings (˜20 mg fresh weight) were placed in Eppendorf tubes, frozen in liquid nitrogen, and stored at −80 C until used. Treatment plates were repeated three times in time. To quantify the change in major plant sterol content, β-sitosterol, stigmasterol, and campesterol levels were determined by grinding ˜20 mg of plant tissue in liquid nitrogen, which was followed by saponification and extraction as described by Jiang et al. (Reference Jiang, Kempinski and Chappell2016). Briefly, 5 ml of ethanol:33% potassium hydroxide 94:6) and 250 μl of 200 g L−1 ascorbic acid (made fresh) was added to the homogenized tissue. The mixture was vortexed and incubated at 50 C for 1 h and then cooled on ice for 10 min. Next, 7.5 ml of water and 7.5 ml of hexane were added, and sterols were extracted by shaking at room temperature for ˜20 min. The phases were separated by briefly centrifuging, and the hexane (upper) phase was collected. The sample was partitioned once more with 7.5 ml of hexane, and the hexane phases were pooled. The hexane was dried to completion with nitrogen gas, and samples were derivatized using a 1:1 mixture of pyridine and MSTFA (N-Methyl-N-trimethylsilyltrifluoroacetamide) + 1% TMCS (2, 2, 2-Trifluoro-N-methyl-N-(trimethylsilyl)-actamide, Chlorotrimethylsilane; Thermo-Fisher, Grand Island, NY 14072) at 50 C for 1 h (total volume 50 μl). Samples were analyzed on an Agilent 7890 gas chromatography system (Agilent Technologies, Santa Clara, CA 95050; HP-5MS column, 30 m by 0.25 mm, 0.25 μm film, 250 C inlet temperature; oven temperature was 200 C for 1 min, then increased at 10 C min−1 to 270 C, then increased at 3 C min−1 to 320C, then held at 320 C for 10 min; 0.9 ml min−1 He flow rate) connected to a mass spectrometer (run in positive ionization mode, 70 eV, scanning 50 to 500 amu).
Physiological Assays
All physiological assays were carried out as previously described (Grossmann Reference Grossmann2005; Grossmann et al. Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012). Assays performed included cell suspensions of heterotrophic false baby’s breath (Galium mollugo L.), photoautotrophic green algae [Scenedesmus obliquus (Turpin) Kützing], L. aequinoctialis plants, and germinating gardencress pepperweed (Lepidium sativum L.) seedlings. Effects on the Hill reaction in isolated wheat (Triticum aestivum L.) chloroplasts, carbon assimilation in G. mollugo plants, O2 consumption in heterotrophic G. mollugo cell suspensions, uncoupler activity and accumulation of reactive oxygen species (ROS) in L. aequinoctialis root tissue, and toluidine-blue staining of L. sativum hypocotyls for the detection of inhibition of very-long-chain fatty-acid (VLCFA) synthesis were also tested. Additionally, A. thaliana seedling morphology, chlorophyll fluorescence, and adenosine triphosphate (ATP) content were evaluated. In brief, for the A. thaliana assay, sterilized seeds (MC24 ecotype) were stratified overnight at 4 C in 48-well plates containing 250 µl half-strength MS including Gamborg’s B5 vitamins (Millipore-Sigma) containing 2.5 µl of acetone (as solvent control) or 2.5 µl of the respective compound, resulting in the following concentrations 0.001, 0.01, and 0.1 mM. The 48-well plates were sealed with micropore tape and grown for 7 d in constant light at 22 C under 75% humidity. Growth inhibition and symptoms were evaluated manually. ATP content was measured with the ATP Determination Kits (A22066, Thermo Fisher Scientific, Grand Island, NY 14072) according to the manufacturer’s instructions using L. aequinoctialis extracts. Results were normalized to fresh weight.
Measurement of Fatty Acids
Preparation, treatment, and sampling of L. aequinoctialis plants for the measurement of free fatty acids (FFAs) was performed as described in Grossmann et al. (Reference Grossmann, Niggeweg, Christiansen, Looser and Ehrhardt2010). Samples were taken at 6, 24, and 48 HAT, and three independent replicates were individually sampled per treatment and time point. Controls were treated with the same final concentration of acetone and sampled at each time point. Measurement and analysis of the samples were performed as described in Campe et al. (Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018).
For measurement of fatty acids in A. thaliana plants were grown in liquid culture (MS media without agar) in the presence or absence of methiozolin (1 µM). Whole seedlings were harvested at 72 HAT, dried, weighed, placed in Eppendorf tubes, frozen in liquid nitrogen, and stored at −80 C until used. Treatments were repeated three times over time. Seed coats were removed before weighing. Fatty acids were extracted using a modified Bligh and Dyer technique (Iverson et al. Reference Iverson, Lang and Cooper2001). Briefly, ˜20 mg of tissue was added to an Eppendorf tube and mixed in a solution of 320 μl ice-cold chloroform together with 640 μl of ice-cold of methanol containing butylated hydroxytoluene (50 μg ml−1) with occasional vortexing for 20 min. Afterward, 300 μl of water was added, and the sample was centrifuged for 5 min at 2,000 × g. All of the upper (aqueous) phase was removed, and both phases were retained. The aqueous phase was centrifuged again to recover the residual organic phase. Finally, the aqueous phase was discarded. The combined organic phases were dried under a stream of nitrogen and stored at −80 C until used. For gas chromatography–flame ionization detector (GC-FID) analysis, 0.5 M sodium methoxide (1 ml) was added to each Eppendorf tube, and the tube was shaken for 10 min. Afterward, 1 ml of isooctane containing 0.001% BHT was added. Then, 200 µl from the upper layer was extracted and transferred to a vial that already contained 1 ml of isooctane. Samples were run on a Varian CP-3800 gas chromatograph equipped with a with an Agilent Varian 25 m by 0.25 mm ID fused silica column (Chrompack, CP=Select CB for Fatty Acid Methyl Esters [FAME]), with a film thickness of 0.25 µm. The temperature program ran from 90 C to 250 C, with 25 C ramp rate for an 8-min runtime with a constant column flow mode of 0.9 ml min−1 utilizing a splitless injection. Quantification was performed using an FID, and peaks were quantified using Star Chromatography Workstation v. 6.00 (Agilent Technologies). Peak area for each fatty acid was measured and expressed as relative percentage of the total FAMEs area.
FAT Expression and Purification
Expression and purification of used FAT proteins were performed as described in Campe et al. (Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018).
Fluorescence-based Thermal Shift Assay (FTSA)
The protocol for this assay was modified and optimized from that of Layton and Hellinga (Reference Layton and Hellinga2010), as outlined in Campe et al. (Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018).
Molecular Modeling
The BASF internal X-ray structure for the cinmethylin-FAT A complex and the docking program GOLD (Jones et al. Reference Jones, Willett, Glen, Leach and Taylor1997) were used to generate methiozolin arrangements in the cinmethylin binding site. Local minimization of the highest-scoring pose using the Molecular Operating Environment (MOE v. 2019.01; Anonymous 2019a) resulted in the binding arrangement presented in this paper.
Statistical Analysis
Before analysis, all data were checked for basic ANOVA assumptions. There were no run by treatment interactions in any experiment, so data were combined over experimental runs. Means were separated using a Tukey’s or Dunnett’s test and were considered significantly different at an alpha value of <0.05. Statistical analysis of FFA in L. aequinoctialis was performed as described in Campe et al. (Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018).
Results and Discussion
Effect of Methiozolin on Plant Growth
Methiozolin is phytotoxic to a number of grass and sedge weeds (Grossmann et al., Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012; Hwang et al. Reference Hwang, Kim, Jeon, Hong, Song and Cho2005; Lee et al. Reference Lee, Koo, Hwang, Hwang, Jeon and Kim2007; Ryu et al. Reference Ryu, Kim, Jeon, Song, Kim, Lee, Kim and Hong2002), but its activity on broadleaves is not as well documented. In this study, A. thaliana, P. annua (Figure 1A–D), and soybean [Glycine max (L.) Merr.] (data not shown) responded similarly to methiozolin, with growth being severely inhibited at rates exceeding 100 nM. Our rate range is consistent with rates used by other authors to inhibit growth in grass species and L. aequinoctialis (Grossman et al. Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012; Lee et al. Reference Lee, Koo, Hwang, Hwang, Jeon and Kim2007; Venner Reference Venner2015). In all species, meristematic growth was more sensitive to methiozolin than cotyledon tissue (i.e., the hypocotyl of dicots or coleoptiles of grasses). For light-grown plants, methiozolin GR50 values were 8 nM for A. thaliana root growth, 12 nM for P. annua root growth, and 26 nM for P. annua shoots. In contrast, dark-grown plants required approximately a 1,000-fold increase in methiozolin concentration before methiozolin inhibited elongation of A. thaliana hypocotyls (Figure 1B and D). At the highest tested rate, 100 µM, methiozolin did not inhibit hypocotyl elongation more than 30%, so a GR50 could not be determined. Seedlings treated with >1 µM methiozolin exhibited abnormal skotomorphogenesis, mainly loss of the apical hook and cotyledon expansion (Figure 1B).

Figure 1. Photographs and a graph showing the symptomology and dose responses of methiozolin-treated Arabidopsis thaliana and Poa annua seedlings at 7 and 14 d after treatment (DAT). (A) Photograph showing symptomology of A. thaliana seedlings treated with methiozolin at 7 and 14 DAT. Within a time point, the rates from left to right are: 0, 5 nM, 50 nM, 500 nM, 5 μM, 50 μM. Caret (^) highlights the purpling on the underside of cotyledons at 500 nM. Asterisk (*): These two plants exhibit the types of symptoms seen at 50 µM at 14 DAT. (B) Photograph of A. thaliana seedling hypocotyl growth in the dark at 7 DAT and treated with methiozolin at (left to right) 0, 1, 5, 10, 25, 50, and 100 µM. (C) Photograph of root and shoot growth of light-grown P. annua seedlings at 14 DAT. The seedlings were treated with (from left to right) 0, 10 nM, 25 nM, 37.5 nM, 75 nM, 100 nM, and 500 nM methiozolin (D) Methiozolin dose response curves for A. thaliana and P. annua and the calculated rate that reduces root, shoot, or hypocotyl growth by 50% (GR50).(E–H) Close-up images of the apical meristems from A. thaliana plants treated with (E) dimethyl sulfoxide (DMSO) control, (F) 500 nM, (G) 5 µM, and (H) 50 µM methiozolin at 14 DAT. Scale bars: (A–C) 0.2 cm; (E–H) 0.2 mm.
Methiozolin injury symptomology observed in A. thaliana seedlings was primarily a shortening of the primary root length with no apparent swelling at 7 DAT (Figure 1A). At 14 DAT, lateral roots were present in untreated plants, partially stunted in plants treated with 5 nM, and absent in in seedlings treated with rates above 50 nM. Lateral roots were present in seedlings treated with 50 nM methiozolin, but they did not elongate, resulting in knotty root phenotype. Shoot tissue and the apical meristem of light-grown A. thaliana treated seedlings also exhibited unique concentration-dependent symptomologies (Figure 1A). At 7 DAT, emergence of true leaves was increasingly delayed at rates above 10 nM and was completely arrested above 500 nM. Chlorophyll content of A. thaliana seedlings at 7 DAT was only reduced (41%) at the highest rate tested (50 µM) (Table 1). At 14 DAT, methiozolin significantly reduced chlorophyll content at all concentrations above 5 nM to a maximum of 82%. At rates between 50 nM and 5 µM, shoot tissue and, especially, the abaxial side of cotyledons developed a purple tinge (^ in Figure 1A), and as rates exceeded 500 nM, plants were more chlorotic. A bleached seedling morphology was observed at the highest rate tested (50 µM), but only one-third of the seedlings exhibited this response (Figure 1A, * marks the two bleached seedlings observed on the 50 µM plates at 14 DAT). The apical meristem of seedlings treated with 500 nM, while severely chlorotic, still appeared to be meristem-like (Figure 1F). However, as the methiozolin rate increased to 5 µM, the meristem appeared to be an undifferentiated mass of chlorotic cells (Figure 1G) and at 50 µM was necrotic (Figure 1H).
Table 1. Effect of increasing methiozolin rate on chlorophyll content in the shoot tissue of Arabidopsis thaliana seedlings at 7 and 14 d after treatment (DAT).

a Means in a column not followed by the same letter are significantly different (P < 0.05).
Is Methiozolin a CBI? Root Cell Length Measurement
To determine whether methiozolin-treated seedlings exhibited symptoms characteristic of CBI inhibitors, such as loss of anisotropic cell expansion, we examined and measured the length of root cells of A. thaliana seedlings treated with or without methiozolin at 100 nM. A treatment with indaziflam (2 nM), a known CBI (Brabham et al. Reference Brabham, Lei, Gu, Stork, Barrett and Debolt2014), was included as a positive control. This indaziflam rate inhibits A. thaliana root growth comparable to 100 nM methiozolin based on root GR50 values (Brabham et al. Reference Brabham, Lei, Gu, Stork, Barrett and Debolt2014). To measure root cell lengths, we used confocal microscopy to capture fluorescence from treated transgenic A. thaliana plants expressing a red fluorescently (RFP) labeled aquaporin called plasma membrane intrinsic protein 2 (PIP2). This protein is plasma membrane bound and can be used to outline the plasma membrane of cells and thus the length of cell. In these images, there was no apparent change in root cell morphology between the control plants (Figure 2A) and those treated with methiozolin (Figure 2C). In contrast, the root cells from plants treated with indaziflam (Figure 2B) showed swelling and loss of anisotropic growth as expected from treatment with a CBI. Methiozolin also did not significantly reduce the length of root cells in the treated zone after division at 3 DAT (Figure 2D). Indaziflam, meanwhile, reduced cell length by 65% compared with both methiozolin-treated and control plants. These results strongly indicate that methiozolin is not a direct inhibitor of cellulose biosynthesis.

Figure 2. Methiozolin effects on Arabidopsis thaliana root cell lengths. (A–C). Representative confocal microscopy images of root cell lengths in control and treated transgenic A. thaliana plants expressing a red fluorescently labeled aquaporin (plasma membrane intrinsic protein 2) at 3 d after treatment. (A) dimethyl sulfoxide control, (B) 2 nM of a known cellulose biosynthesis inhibitor (indaziflam), (C) and methiozolin at 100 nM (D). Bar chart depicting the mean (± 1 standard error) cell length of control and treated plants. In total, 60 cells (6 cells plant−1) were measured for each treatment and means were separated using Tukey’s test. Different letters between treatments indicate a significant difference at an alpha value of 0.05. Scale bars: 20 µm.
Is Methiozolin a TAT Inhibitor? Metabolite Supplementation Study
It was proposed that methiozolin prevents the conversion of tyrosine to 4-hydroxyphenyl-pyruvate (4-HPP) by inhibition of TATs (Grossmann et al. Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012). This, in turn, would inhibit homogentisate biosynthesis and the subsequent tocopherol and prenylquinone biosynthesis pathways. Thus, TAT inhibition would produce similar physiological effects as HPPD inhibition. Based on this, we conducted a metabolite complementation study to determine whether additions of chorismate, tyrosine + phenylalanine, 4-HPP, or homogentisate (all at 10 µM) could lessen the phytotoxic effects of methiozolin. We used methiozolin concentrations of 5 and 50 nM, which approximate the GR50 values for roots and shoots, respectively. Tyrosine was not applied alone, because it is involved in feedback inhibitory loops (Huang et al. Reference Huang, Tohge, Lytovchenko, Fernie and Jander2010). In a preliminary experiment, we demonstrated that adding 10 µM homogentisate could partially counteract mesotrione (20 nM, a known HPPD inhibitor) phytotoxicity to A. thaliana (Supplementary Figure 1). At 14 DAT, mesotrione alone, mesotrione plus 4-HPP, or mesotrione plus homogentisate reduced A. thaliana shoot weights by 89%, 77%, and 56%, respectively, in comparison to the control (data not shown).
The addition of chorismate or tyrosine plus phenylalanine slightly reduced (7% and 11%, respectively) methiozolin inhibition of root growth at 7 DAT compared with the control (no supplementation, 41% inhibition of root growth) (Table 2). Neither 4-HPP nor homogentisate reduced the effect of methiozolin on root growth. None of the supplements reduced the effect of methiozolin on shoot growth at 14 DAT (Figure 3A; Table 2). If methiozolin was preventing the formation of homogentisate because of inhibition of the TAT responsible for 4-HPP biosynthesis, then addition of homogentisate should have at least reduced methiozolin toxicity, as it did for mesotrione.
Table 2. Effect of tyrosine aminotransferase (TAT) pathway metabolites on phytotoxic effects of methiozolin at 7 and 14 d after treatment (DAT).

a All metabolites were added at 10 μM.
b 5 nM is the ˜GR50 of methiozolin treated roots, and 50 nM is the ˜GR80 value of shoots.
c Means were separated using Tukey’s test, and those followed by the same letter within a column are not significantly different at an alpha of >0.05.

Figure 3. Effects of supplements on methiozolin injury to Arabidopsis thaliana. (A). Supplements in proposed pathway (chorismate, tyrosine + phenylalanine [Y+F], 4-HPP, and homogentisate at 10 µM) did not lessen the phytotoxic effects of 50 nM methiozolin at 14 d after treatment (DAT). Shoots are representatives of the seedlings’ response with each combination. (B) Methiozolin, a proposed tocopherol and plastoquinone inhibitor, does not inhibit alpha-tocopherol synthesis at 7 or 14 DAT. Means (± 1 standard error) within a time period were separated using Tukey’s tests and a different lowercase letter (7 DAT comparisons) or uppercase letter (14 DAT comparisons) indicates a significant difference at an alpha value of 0.05. Scale bar: (A) 0.2 cm.
Is Methiozolin a TAT Inhibitor? α-Tocopherol Content
Another way to examine whether methiozolin is inhibiting TATs is to measure the level of metabolites downstream of 4-HPP or homogentisate biosynthesis in the prenylquinone or tocopherol pathways. We measured the α-tocopherol content of A. thaliana seedlings treated with methiozolin at 5, 50, or 500 nM at 7 and 14 DAT (Figure 3B). The α-tocopherol content in shoot tissue of untreated plants at 7 and 14 DAT averaged 3 to 4 μg g−1. These A. thaliana α-tocopherol levels are consistent with those previously reported for young plants growing under ideal conditions (Porfirova et al. Reference Porfirova, Bergmuller, Tropf, Lemke and Dormann2002; Riewe et al. Reference Riewe, Koohi, Lisec, Pfeiffer, Lippman, Schmeichel, Willmitzer and Altmann2012). Methiozolin had no effect on α-tocopherol content at 7 DAT and actually increased the levels 2.6-fold at 14 DAT in both the 50 and 500 nM treatments (Figure 3B). The increase in α-tocopherol levels may be a response to the stress caused by methiozolin injury (Holländer-Czytko 2005; Porfirova et al. Reference Porfirova, Bergmuller, Tropf, Lemke and Dormann2002). It certainly shows the formation of α-tocopherol is not inhibited by methiozolin and, consequently, methiozolin is not a direct inhibitor of the TAT for 4-HPP synthesis.
Is Methiozolin an Mevalonic Acid Biosynthesis Inhibitor? Comparison of Methiozolin Injury Symptoms to Those Produced by Lovastatin
A guilt by association approach was next used to generate leads for the MOA of methiozolin. To do this, we conducted an extensive search of the A. thaliana literature for chemicals and/or genetic mutations that produced symptomologies similar to those we observed with methiozolin. Using this approach, we identified inhibition of enzymes in the mevalonic acid (MVA) pathway as a potential methiozolin MOA. Specifically, methiozolin could be acting as a statin mimic (Kobayashi et al. Reference Kobayashi, Suzuki, Tang, Nagata, Ohyama, Seki, Kiuchi, Kaneko, Nakazawa, Matsui, Matsumoto, Yoshida and Muranaka2007; Rodriguez-Concepcion et al. Reference Rodriguez-Concepcion, Fores, Martinex-Garcia, Gonzalez, Phillipis, Ferrer and Boronat2004; Soto et al. Reference Soto, Stritzler, Lisi, Alleva, Pagano, Ardila, Mozzicafreddo, Cuccioloni, Angeletti and Ayub2011; Suzuki et al. Reference Suzuki, Kamide, Nagata, Seki, Ohyama, Kato, Masuda, Sato, Kato, Tabata, Yoshida and Muranaka2004). The MVA pathway produces cytosolic-available isoprenoid units required for synthesizing membrane-essential phytosterols and other important compounds (Vranova et al. Reference Vranova, Coman and Gruissem2013). Statins inhibit an early step in the MVA pathway by targeting 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase). This enzyme is responsible for the conversion of HMG-CoA to mevalonate (Istvan and Deisenhofer Reference Istvan and Deisenhofer2001; Vranova et al. Reference Vranova, Coman and Gruissem2013). We conducted a side-by-side dose–response experiment with methiozolin and lovastatin to compare their symptomologies (Figure 4A). At 7 and 14 DAT, symptoms exhibited by A. thaliana seedlings treated with methiozolin or lovastatin were nearly indistinguishable. One difference we did note was the presence of adventitious roots in seedlings treated with lower lovastatin rates but not in those treated with methiozolin. Regardless, the remarkable similarities between these two compounds warranted further study.
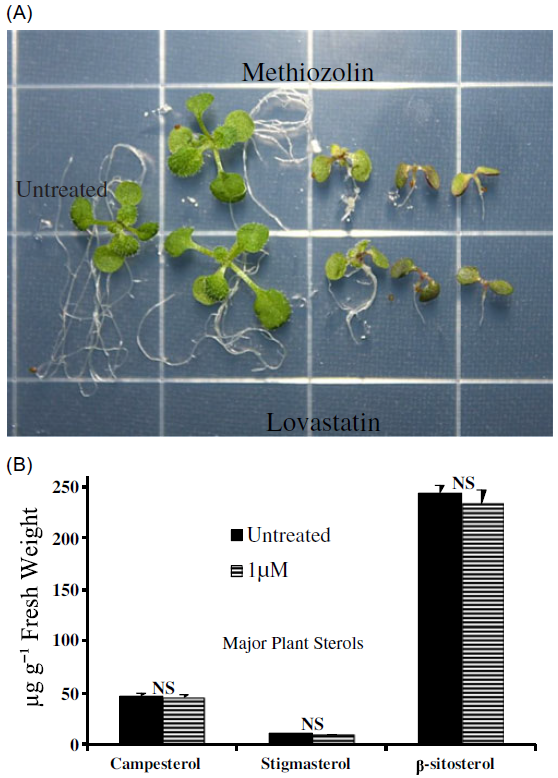
Figure 4. Methiozolin was hypothesized to be a mevalonic acid pathway (MVA) inhibitor. (A) The characteristic symptomology of Arabidopsis thaliana seedlings treated with increasing rates (left to right) of methiozolin and the MVA inhibitor, lovastatin, at 14 d after treatment (DAT). Notice the rate-dependent similarity between treatments. (B) Comparison of major phytosterol content in Arabidopsis seedlings treated with or without methiozolin (1 µM) at 3 DAT.
Is Methiozolin an MVA Biosynthesis Inhibitor? Metabolite Supplementation Study
Assuming methiozolin inhibits an early step in the MVA pathway, we tried to overcome the phytotoxic effects of methiozolin by supplementing treated A. thaliana seedlings with 20 µM farnesol or squalene. Lovastatin (1 µM) served as a positive control, and this concentration gave symptomologies similar to methiozolin at 50 nM. We expected, at best, farnesol or squalene would partially alleviate the phytotoxicity of lovastatin. This is because several enzymatic steps and important metabolites exist between the biosynthesis of mevalonate and farnesyl diphosate (farnesol) and subsequently squalene (Vranova et al. Reference Vranova, Coman and Gruissem2013). At 7 DAT, lovastatin reduced root length by 88% in comparison to the control (data not shown). The addition of 20 µM farnesol, but not squalene, partially (18%) reversed the lovastatin inhibition of root growth. However, the addition of neither farnesol nor squalene could rescue methiozolin-treated plants (data not shown).
Is Methiozolin an MVA Biosynthesis Inhibitor? Major Phytosterol Measurement
As an alternative approach to test whether methiozolin is an inhibitor of the MVA pathway, we measured phytosterol content in A. thaliana seedlings treated with or without methiozolin (1 µM) 3 DAT (Figure 4B). Phytosterols are essential components of cell membranes, and the major sterols found in most plants and A. thaliana are β-sitosterol, stigmasterol, and the brassinosteroid precursor campesterol (Guo et al. Reference Guo, Venkatramesh and Nes1995; Hartmann Reference Hartmann1998; Schaller Reference Schaller2003). There were no significant differences between treated and untreated seedlings for any of the major sterols measured. Thus, we found no evidence that methiozolin inhibits the MVA pathway, despite the similarities in the symptoms caused by it and the known HMG-CoA reductase inhibitor lovastatin.
Do Methiozolin and Cinmethylin Result in Similar Physiological Profiles?
Both Grossman et al. (Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012) and Campe et al. (Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018) described the physiological profile of cinmethylin phytotoxicity. The primary characteristic of cinmethylin toxicity is strong growth inhibition in assays with high cell division activity such as G. mollugo cell suspension cultures and L. aequinoctialis. Growth of photoautotrophic algae and germination of dicotyledonous L. sativum seeds were, in contrast, only slightly affected. Comparing the responses to cinmethylin with those of methiozolin in the same assays (Figure 5), there is a high degree of similarity but with minor differences. Methiozolin showed higher activity on algae and weaker activity on A. thaliana than cinmethylin. Methiozolin also had no activity on CO2 assimilation and ATP content, while cinmethylin reduced these slightly (13% to 14%) at the highest concentration (0.1 mM) tested.

Figure 5. Effect of cinmethylin and methiozolin on heterotrophic Gallium mollugo cell suspensions, green algae Scenedesmus obliquus, Lemna aequinoctialis plants, Arabidopsis. thaliana seedling morphology, germination of cress (Lepidium sativum L.) under dark/light conditions, toluidine-blue (TB) staining of cress hypocotyls for the detection of inhibition of very-long-chain fatty-acid (VLCFA) synthesis, the Hill reaction in isolated Triticum aestivum chloroplasts, CO2 assimilation in G. mollugo plants, oxygen consumption (respiration) in heterotrophic G. mollugo cell suspensions, uncoupler activity and accumulation of reactive oxygen species (ROS) in L. aequinoctialis root tissue, chlorophyll fluorescence, and ATP content. Uncoupler activity and ROS accumulation are shown as effect compared with control, while values of all other assays are expressed as percentage of inhibition. Letters indicate physiological symptoms observed: necrosis (N), chlorosis (C), reduced leaf growth (H), root growth inhibition (I), intensified leaf pigmentation (WR) and promotive effect (M).
Do Methiozolin and Cinmethylin Affect FFAs Similarly?
Given the similarities in the physiological profiles between methiozolin and cinmethylin (Figure 5), we tested whether methiozolin treatment disrupts A. thaliana fatty-acid metabolism by examining the change in global fatty-acid composition (14:0 to 26:0) of seedlings treated with methiozolin at 1 µM (Figure 6). The fatty-acid levels of 16:1, 16:3, 17:0, 19:0, 20:4, 20:5, 22:5, and 22:6 were less than 1% of the total fatty acids, and 14:0, 14:1, 16:2, 20:3, 22:4, and 26:0 were not detected (data not shown). Otherwise, the fatty-acid profiles of seedlings with or without methiozolin treatment were similar, and no statistical differences existed between any fatty acids from the two treatments. However, the following needs to be considered: (1) the fatty-acid turnover in A. thaliana was shown to be extremely low (less than 5% d−1; Bao et al. Reference Bao, Focke, Pollard and Ohlrogge2000; Bonaventure et al. Reference Bonaventure, Bao, Ohlrogge and Pollard2004); (2) the influence of fatty acids derived from seed storage lipids rather than from de novo synthesis in the seedlings was not determined; and (3) the ratio between meristematic and differentiated cells, as well as the low ratio between the levels of FFA versus membrane lipids (Sánchez-Martin et al. Reference Sánchez-Martín, Canales, Tweed, Lee, Rubiales, Gómez-Cadenas, Arbona, Mur and Prats2004), might mask the effects of methiozolin. In addition, even under environmental stress such as drought, the lipid content and class distribution in A. thaliana leaves is relatively stable (Gigon et al. Reference Gigon, Matos, Laffray, Zuily-Fodil and Pham-Thi2004). Therefore, we also analyzed the FFA pool after methiozolin treatment. In Lemna, we compared the effects of methiozolin with the effects caused by cinmethylin treatment already described in Campe et al. (Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018). Both cinmethylin and methiozolin reduce C14:0, but the inhibition by cinmethylin occurs sooner and at a lower concentration (Figure 7). Cinmethylin affects C16:0 but, in contrast, methiozolin has little effect on C16:0. Both cinmethylin and methiozolin similarly reduce C18:1 and C18:2, but methiozolin has little effect on C18:3 compared with cinmethylin. Explaining the observed differences in FFA composition caused by methiozolin and cinmethylin would be a potential area for further research. Both cinmethylin and methiozolin show only slight effects on VLFCAs. The effects of methiozolin and cinmethylin on L. aequinoctialis FAs are fundamentally different from those caused by ACCase inhibitors and VLFCA inhibitors (Campe et al. Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018).

Figure 6. The prominent fatty acids of Arabidopsis thaliana seedlings untreated or treated with methiozolin at 1 µM. Fatty-acid content is presented as a percentage of the total fatty-acid profile from seedlings within a treatment. The fatty-acid levels of 14:0, 14:1, 16:1, 16:2, 16:3, 17:0, 19:0, 20:3, 20:4, 20:5, 22:4, 22:5, 22:6, and 26:0 were less than 1% of the total fatty-acid profile or not detected. The fatty-acid content was compared with the untreated percentage using Dunnett’s test.
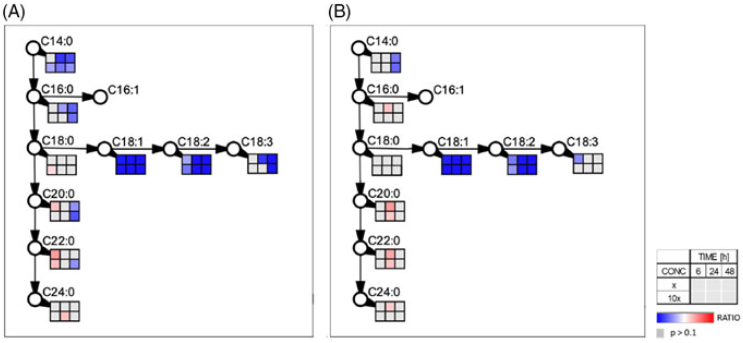
Figure 7. Effect of (A) cinmethylin (data published in Campe et al. [2008]) and (B) methiozolin on the composition of free fatty acids (FFAs) in Lemna aequinoctialis plants. Boxes indicate accumulation (red) or depletion (blue) of FFAs at three different time points (6, 24, and 48 h after treatment) and two herbicide rates (1 and 10 µM) relative to untreated control. Values were calculated based on three biological replicates.
Does Methiozolin Bind to FAT?
Campe et al. (Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018) used a combination of cellular Target Profiling™, FAT crystallization and cinmethylin binding, and an FTSA to identify FAT enzymes as the target site for cinmethylin binding and inhibition of fatty-acid synthesis. As shown in Figure 8, we determined the melting point for A. thaliana FAT A and L. aequinoctialis FAT A in the absence and presence of methiozolin. As expected, in the presence of cinmethylin, the melting temperature (Tm) of both enzymes significantly increased in the FTSA. Using cinmethylin concentrations between 2.5- to 40-fold of the enzyme concentrations for A. thaliana FAT A, the delta Tm ranged between 6.6 C and 7.6 C, and for L. aequinoctialis FAT A between 6.9 C and 7.5 C. Comparably, in the FTSA assay, methiozolin also increased the melting temperature for both the A. thaliana and L. aequinoctialis FAT A enzymes. Using methiozolin concentrations between 2.5- to 40-fold the enzyme concentrations for A. thaliana FAT A, the delta Tm ranged between 4.5 C to 7.1 C, and for L. aequinoctialis FAT A between 5.6 C and 7.4 C. Thus, as with cinmethylin, methiozolin binds to both tested FAT A variants in vitro. Finally, using protein modeling, we compared the binding mode of methiozolin with the cinmethylin binding mode using the L. aequinoctialis FAT A-cinmethylin co-crystal structure as a basis (Figure 9). The cinmethylin binding site of FAT A can be divided into three subsites. The first part is a deep hydrophobic pocket (left-hand side in figure), where the o-methyltolyl part of cinmethylin binds tightly, forming favorable hydrophobic contacts, and CH-pi interactions between the neighboring phenylalanines and a cysteine. In the center, a prominent arginine side chain forms a basic interaction site, where the linker ether oxygen and the oxygen in the bridged ring system of cinmethylin act as hydrogen bond acceptors. Two tryptophan side chains form a hydrophobic cage at the entrance, accommodating the cineol part of cinmethylin. It forms interaction patterns similar to those in the deep site of the pocket. In general, cinmethylin shows several favorable hydrophobic contacts with FAT A and a tight complementary shape fit to the FAT A binding pocket. The binding mode of methiozolin to FAT is very similar to that of cinmethylin. The 2,6-difluorobenzyl binds to the deeper site of the pocket, the ether oxygen and the isoxazole form hydrogen-bonding interactions with the arginine, and the thiophene binds to the hydrophobic entrance of the pocket. Moreover, the thiophene of methiozolin forms a pi-pi stacking with a tryptophan and CH-pi contact with the backbone of a nearby cysteine.
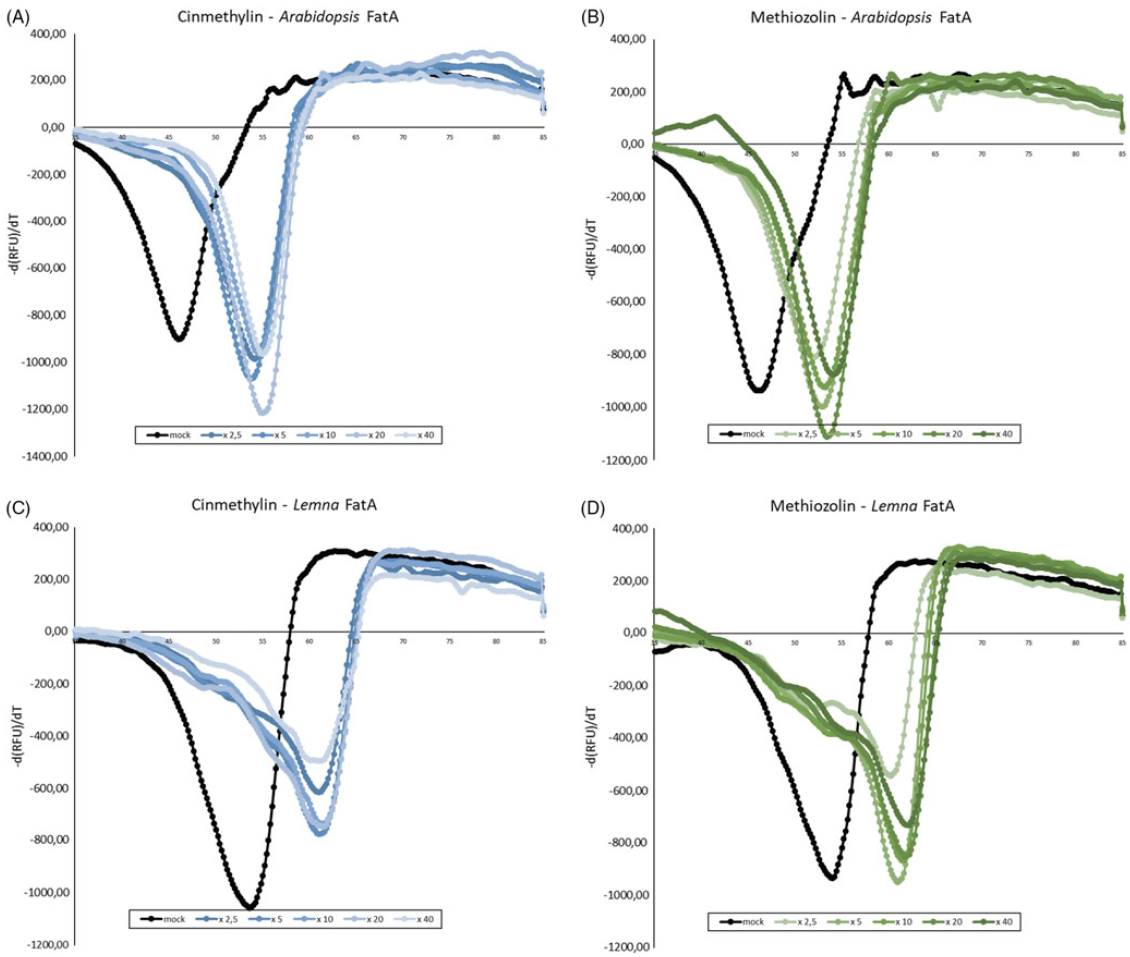
Figure 8. Negative first derivatives of relative fluorescence unit (RFU) data over temperature for binding studies of Arabidopsis thaliana fatty-acid thioesterase (FAT) A and Lemna aequinoctialis FAT A with cinmethylin (blue lines) and methiozolin (green lines) via fluorescence-based thermal shift assay. Functional enzyme concentration was 2.5 µM and inhibitor concentrations of 2.5-, 10-, 20- and 40-fold enzyme concentrations were applied. dimethyl sulfoxide (DMSO) control samples (black lines) show a Tm value of 47.67 C for A. thaliana FAT A and 53.32 for L. aequinoctialis FAT A. Upon addition of cinmethylin (A and C) or methiozolin (B and D), considerable shifts in Tm occurred for both herbicides, indicating binding events. The assay was repeated independently with similar results.

Figure 9. In-house X-ray crystal structure of cinmethylin in fatty-acid thioesterase (FAT) A. Cinmethylin is represented as sticks with carbon atoms colored in green and oxygen atoms in red. The protein is shown with secondary structure elements: beta sheets in yellow, alpha-helical structures in red, and loops in blue and gray. Intermolecular interactions are indicated. CH-pi interactions are highlighted by dotted lines in light green, hydrogen bonds in light blue. Amino acids nearby are shown, with the important arginine in stick representation (white carbons). The proposed binding mode of methiozolin to FAT A is represented as sticks with carbon atoms colored in cyan, oxygen in red, nitrogen in blue, and sulfur in yellow.
Methiozolin is an interesting new herbicide with well-documented efficacy on P. annua in several warm- and cool-season turfgrasses and with an unknown MOA (Askew and McNulty Reference Askew and McNulty2014; Hoisington et al. Reference Hoisington, Flessner, Schiavon, McElroy and Baird2014; Koo et al. Reference Koo, Hwang, Jeon, Kim, Lim, Lee and Cho2014; Venner Reference Venner2015). In this paper, we show methiozolin has activity on the broadleaves A. thaliana and soybean (data not shown) in addition to P. annua and thus may have broad-spectrum activity. The most characteristic methiozolin symptomology observed in all species was a cease in meristematic growth. At nonlethal rates, the shoot symptomology differed between P. annua and A. thaliana. At 14 DAT, A. thaliana was mildly chlorotic (˜30% chlorophyll loss) and this, although not quantified, was not obvious in P. annua. The A. thaliana symptomology is similar to that described by Grossmann et al. (Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012), in which methiozolin-treated (200 nM) L. aequinoctialis exhibited photobleaching in newly formed fronds followed by the tissue becoming greenish with a touch of brown and a necrotic meristem.
Is Methiozolin Activity Dependent on Light or Proliferating Tissue?
Interestingly, we found the activity of methiozolin varied in the presence or absence of light. In the absence of light, it took a nearly 1,000-fold increase in methiozolin rate to have any inhibitory effect on dark-grown etiolated hypocotyls when compared with light-grown roots (Figure 1B and D). Even at our highest tested rate (100 µM), hypocotyl length was not reduced by more than 30%. Two major scenarios explain the reduced activity of methiozolin in the dark: (1) light is required for phytotoxicity; or (2) methiozolin has little activity on cells as they elongate, because hypocotyl growth is solely depend on cell elongation and not on cell division (Gendreau et al. Reference Gendreau, Traas, Desnos, Grandjean, Caboche and Hofte1997). A second point could be that Fatty-acid synthesis is reduced in the dark, and the effect of methiozolin is reduced under this condition. Fatty-acid synthesis is negligible in A. thaliana leaves in the dark (Bao et al. 2001).
Our data would indicate the primary methiozolin MOA works on proliferating tissue and does not require light for phytotoxicity. In our CBI MOA experiment, we found methiozolin at 100 nM had little to no effect on the cell length of light-grown PIP2:RFP A. thaliana roots at 3 DAT. Other researchers have found methiozolin can significantly reduce P. annua panicle number in a dose-dependent manner, but it has only a minor (˜20%) effect on panicle length (Askew and McNulty Reference Askew and McNulty2014; Koo et al. Reference Koo, Hwang, Jeon, Kim, Lim, Lee and Cho2014). However, we cannot exclude the possibility that light may be important for indirect methiozolin phytotoxic effects or could act as a synergist.
The Inhibitory Mechanism of Methiozolin
Two MOAs were proposed for methiozolin. First, Grossmann and colleagues (Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012) followed a logical order of progression from photobleached L. aequinoctialis to supplementation with 4-HPP, but not homogentisate, to conclude that methiozolin and related compounds target TATs. Venner (Reference Venner2015) also reported that 4-HPP could slightly (6%) reduce symptoms of methiozolin injury to L. aequinoctialis, but it had no effect on methiozolin injury to P. annua, A. stolonifera, or perennial ryegrass (Lolium perenne L.). We also could not rescue methiozolin-treated A. thaliana seedlings with supplementation with up- or downstream metabolites to HPPD. In contrast, we could partially rescue mesotrione-treated A. thaliana with exogenous homogentisate. In addition, methiozolin did not affect α-tocopherol levels at 7 DAT, and plants actually had increased α-tocopherol concentration at 14 DAT. Our data do not support TAT inhibition as an MOA for methiozolin in A. thaliana. Later work by Campe et al. (Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018) showed that effects on TAT occurred later than other effects for cinmethylin and, presumably, for methiozolin.
Second, Lee et al. (Reference Lee, Koo, Hwang, Hwang, Jeon and Kim2007) showed decreased 14C-glucose incorporation into cell wall fractions such as cellulose and hemicelluose. However, since the time lag was longer than the growth response, whether methiozolin inhibits cell wall biosynthesis directly remained unknown. Our data show that methiozolin does not directly inhibit cell wall biosynthesis. Analysis of confocal microscopy images of fluorescently labeled A. thaliana root cells revealed methiozolin had a negligible effect on cellular anisotropic growth and cell length. Consistent with our results, Venner (Reference Venner2015) found methiozolin did not inhibit the incorporation of [13C]glucose into cell wall materials of P. annua, A. stolonifera, L. perenne, and Kentucky bluegrass (Poa pratensis L.) at 72 h after the beginning of treatment.
During the preparation of this article, another possible MOA for methiozolin appeared in the literature. The MOA of cinmethylin was recently identified as inhibition of lipid synthesis. Campe et al. (Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018) elegantly showed that cinmethylin disrupts cell membranes by preventing the export of saturated and unsaturated C16 or C18 fatty acids from plastids, via FATs, to the endoplasmic reticulum for later incorporation into lipids. Interestingly, Grossman et al. (Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012) suggested that methiozolin, cinmethylin, and other 5-benzyloxymethyl-1,2 isoxazolines (ISO1) would share an MOA. This was because they share a 1,4 cineole-like structural motif and have a similar “phytotoxic fingerprint” based on physiological bioassays and metabolomics. It is also clear that the inhibitory mechanism of all these compounds disrupts the ability of meristematic tissue to maintain a proliferative state (Baum et al. Reference Baum, Karanastasis and Rost1998; Campe et al. Reference Campe, Hollenbach, Kammerer, Hendriks, Hoffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018; El-Deek and Hess Reference El-Deek and Hess1986; Grossmann et al. Reference Grossmann, Hutzler, Tresch, Christiansen, Looser and Ehrhardt2012; Lee et al. Reference Lee, Koo, Hwang, Hwang, Jeon and Kim2007). Thus, together with the observed similarities in the extended physiological profile presented here, one could assume the molecular targets of methiozolin are FAT proteins. Even though we found that methiozolin did not quantitatively or qualitatively disrupt global fatty-acid composition at 3 DAT in A. thaliana, the FFA profiles after methiozolin or cinmethylin treatment of L. aequinoctialis were comparable, supporting FAT enzymes as a common target for both herbicides. Furthermore, using FTSA, we showed that methiozolin binds directly to both A. thaliana FAT A and L. aequinoctialis FAT A proteins, plus, using the L. aequinoctialis FAT A-cinmethylin co-crystal structure as a basis, the protein model suggested a binding mode for methiozolin to FAT A similar to that of cinmethylin. Taken together, the data presented here do not support various physiological processes such as TAT, cellulose synthesis, and chlorophyll biosynthesis as the target site of methiozolin. Instead, they indicate that FAT proteins are the herbicidal targets for methiozolin, and the consequent inhibition of fatty acid synthesis is the MOA for methiozolin. The identical symptomology of methiozolin and lovastatin was an interesting observation and may warrant further research.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2020.87
Acknowledgments
This research received no specific grant from any funding agency or the commercial or not-for-profit sectors. No conflicts of interest have been declared.













