Neonates with complex CHD, particularly those with a functional single ventricle, experience a high incidence of growth failure in the post-operative period following stage I palliation. Reference Pillo-Blocka, Miles and Beghetti1–Reference Anderson, Beekman and Eghtesady4 The growth failure in these infants may be related to insufficient nutritional intake, gastrointestinal malabsorption, or high energy expenditure. Reference Li, Zhang and Herridge5,Reference Peterson and Wetzel6 Clinicians are often reluctant to initiate and advance feedings in this population because of the increased risk of gastrointestinal complications, including necrotising enterocolitis, Reference Jeffries, Wells and Starnes7,Reference McElhinney, Hedrick and Bush8 and the high incidence of feeding intolerance and gastroesophageal reflux disease. Reference Cribbs, Heiss and Clabby9 Two groups of neonates have been identified for being at an increased risk of developing necrotising enterocolitis: premature infants and infants with single ventricle cardiac physiology, such as hypoplastic left heart syndrome. Reference Thompson and Bizzarro10,Reference Mahle, Spray and Wernovsky11 Thus, providing optimal nutritional support to critically ill infants with CHD is a significant clinical challenge, and published methods for initiating and advancing enteral feedings in infants with single ventricle physiology are limited. Reference Mahle, Spray and Wernovsky11–Reference Slicker, Hehir and Horsley15
Infants with single ventricle are at particular increased risk for developing necrotising enterocolitis because of the significant systemic-to-pulmonary run-off and baseline tissue hypoxia from cyanotic heart disease. It is unknown whether gut ischaemia or hypoxia is a primary or secondary factor in the development of necrotising enterocolitis; however, newer technology exists which may help to identify hypoxic conditions within tissue beds as it occurs in necrotising enterocolitis. Near-infrared spectroscopy is a non-invasive means of measuring regional tissue oxygenation. Thus, it may be possible to use near-infrared spectroscopy to measure tissue perfusion of the gut or other somatic tissue beds such as the kidney non-invasively in this high-risk patient population and perhaps predict those infants who may be at increased risk for feeding intolerance and necrotising enterocolitis. To our knowledge, there are no published enteral feeding programmes in infants with or without CHD that are guided by cerebral or somatic near-infrared spectroscopy values. Our primary hypothesis is that the initiation of a standardised feeding protocol for infants with single ventricle physiology after stage I palliation will result in a more rapid attainment of nutritional goals without an increase in the incidence of gastrointestinal co-morbidities and in fact will alert the clinician to possible feeding intolerance with the use of cerebral and somatic near-infrared spectroscopy in the advancement of feeds.
Materials and methods
Study population and design
The Medical City Children’s Hospital single ventricle standardised feeding protocol was implemented on a prospective cohort of consecutive patients with single ventricle physiology, most commonly hypoplastic left heart syndrome, following stage I palliation who were admitted to our pediatric cardiac ICU over an 18-month period of time (5/2014–10/2016). Inclusion criteria included all patients with evidence of adequate cardiac output for 24 hours (stable vital signs, low-dose inotropes defined as dopamine/dobutamine ≤ 7.5 mcg/kg/min, and evidence of good peripheral perfusion on physical exam). Excluded were patients with a prior diagnosis of necrotising enterocolitis, prior extracorporeal membrane oxygenation use, or initiation of the algorithm > 10 days post-operatively. Patients with < 36 weeks gestational age, < 2.5 kgs, and with other gastrointestinal co-morbidities could be enrolled at the discretion of the attending physician with modifications to the protocol if warranted. The decision to initiate enteral nutrition was discussed every morning on multi-disciplinary rounds, and when the multi-disciplinary team determined the patient was ready to feed, the infant was randomised to one of the two prospective feeding study groups: continuous feeding group with cerebro-somatic near-infrared spectroscopy feeding advancement criteria (protocol + near-infrared spectroscopy; n = 17) or continuous feeding group without cerebro-somatic near-infrared spectroscopy feeding advancement criteria (protocol − near-infrared spectroscopy; n = 16). Continuous data were collected via Vital Sync™ virtual patient monitoring platform (Medtronic). These data were compared with a similar cohort (retrospective control cohort) admitted to the cardiac ICU over an 18-month period (5/2010–9/2013) prior to implementation of the feeding protocol (n = 30).
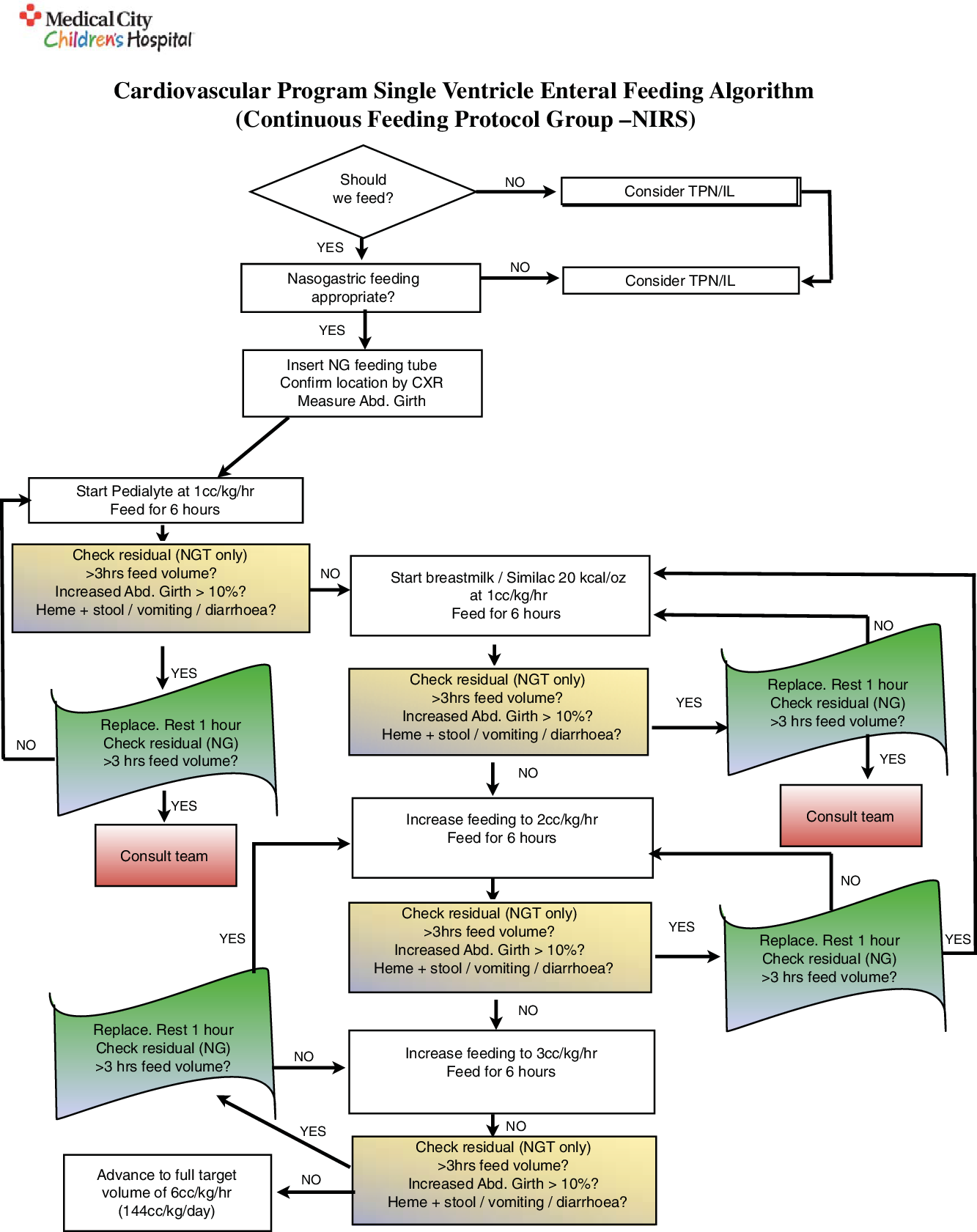
The Medical City Children’s Hospital single ventricle feeding protocol is shown in Figs 1 and 2. All patients who were randomised into a feeding protocol group had either a nasogastric tube or a nasoduodenal feeding tube inserted and confirmed via chest X-ray prior to the initiation of feeds. Cerebral near-infrared spectroscopy probes were placed on either side of the forehead to measure cerebral oxygen saturation, and splanchnic probes were placed on the abdomen in the midline below the umbilicus and above the symphysis pubis. Renal probes were placed on the posterior flank (T10-L2) and right or left of midline. These measurements were then combined as a ratio of somatic (renal) near-infrared spectroscopy saturation over cerebral near-infrared spectroscopy saturation (rSO2s/rSO2c) to produce a cerebro-somatic oxygenation ratio. For both feeding groups, if certain conditions were met (the gastric residual was ≤ 3 hours of feeds), the residuals did not contain blood, the stools did not contain blood, the abdominal girth was stable, there was no vomiting or diarrhoea, and the cerebro-somatic oxygenation ratio was ≥0.75 (protocol + near-infrared spectroscopy only), then feeds were increased every 6 hours to a goal volume of 6 cc/kg/hr (~144 kcal/kg/day), or to the maximum volume allowed given a fluid restriction.

Figure 1. Single ventricle enteral feeding protocol via continuous feeds with the use of cerebro-somatic near-infrared spectroscopy monitoring as a guideline for the advancement of feeds.
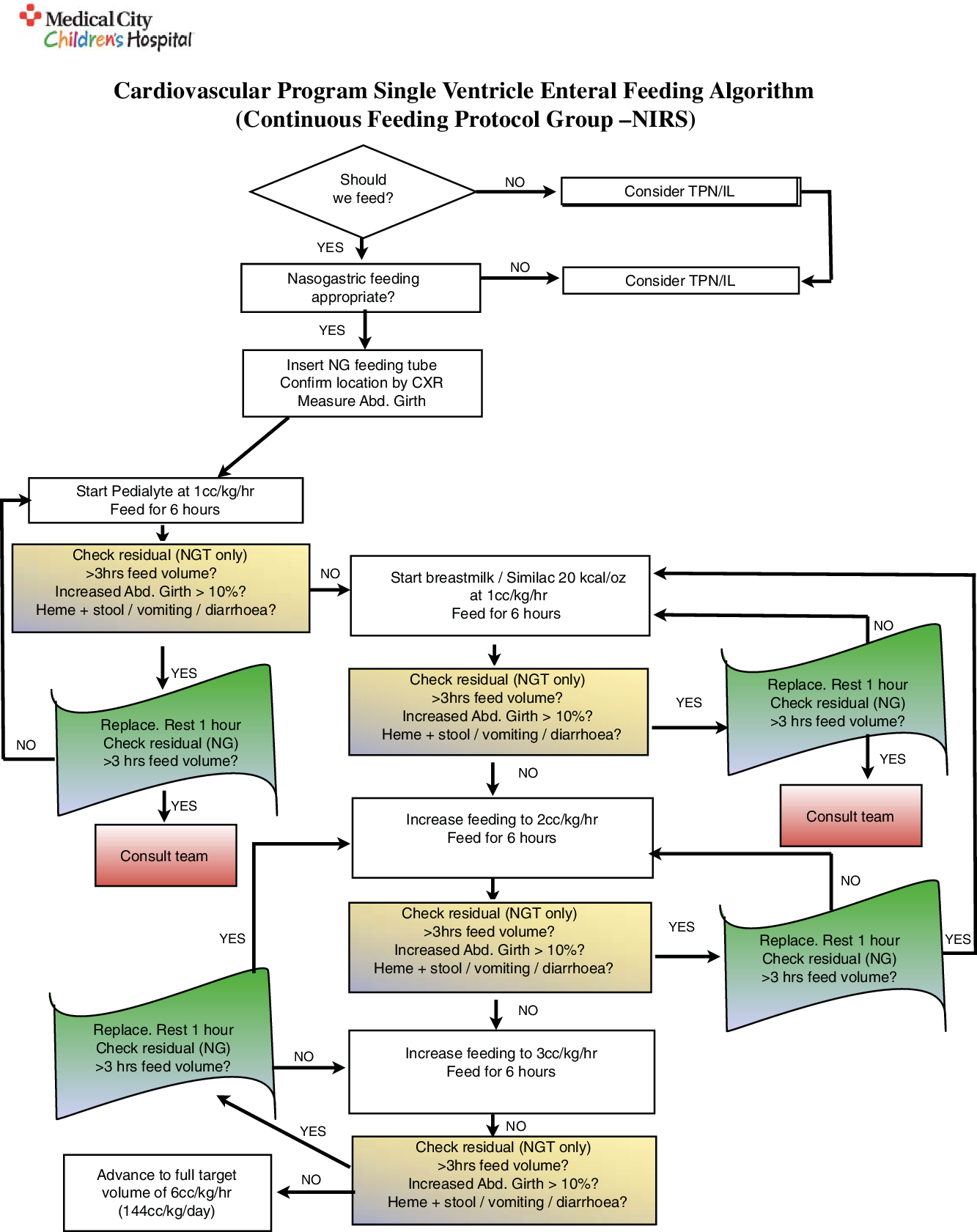
Figure 2. Single ventricle enteral feeding protocol via continuous feeds without the use of cerebro-somatic near-infrared spectroscopy monitoring as a guideline for the advancement of feeds.
Once goal volume feeds reached × 24 hours, the caloric density of feeds was increased by 4 kcal/oz every 24 hours to a maximum caloric density of 28 kcal/oz to reach a recommended daily allowance of calories equal to 130 kcal/kg/day (algorithm completed). Similac standard powder was used to fortify feeds and was the same in both cohorts. Once recommended daily allowance of calories was reached × 24 hours, 3 hours of feeding volume was consolidated to be given via nasogastric tube gravity q3 hours. If the patient was being fed via nasoduodenal tube, it was repositioned into the stomach and confirmed via chest X-ray prior to the initiation of bolus feeds. Consideration for oral feeds was assessed once at bolus nasogastric full volume feeds after evaluation of respiratory rate, suck/swallow coordination, and occupational therapy was consulted as needed. If the patient developed chylous chest tube drainage at any time during feeding advancement, the formula was changed to Enfaport. All patients were placed on intravenous Protonix post-operatively and converted to oral Prevacid when tolerating gastric feedings as per standard of care in our cardiac ICU. Prevacid was discontinued once the patient reached full volume gastric feedings if there were no clinical signs or symptoms of gastroesophageal reflux disease. If the patient developed feeding intolerance related to gastric dysmotility, they were initiated on Erythromycin as it is the current standard of care in our cardiac ICU.
Outcome measures
The primary goal was to safely achieve goal volume continuous drip feeds within 30 hours of initiation of the protocol, with full volume feeds defined as ~144 ml/kg per day or maximum volume allowed given fluid restriction, with close monitoring for feeding intolerance. The efficacy of the feeding protocol was evaluated by determining: the number of days it takes to reach goal volume of enteral feeds, defined as 144 ml/kg/day, or the maximum volume allowed given a fluid restriction; the number of days it takes to reach the goal caloric recommended daily allowance of enteral feeds, defined as 130 kcal/kg/day (protocol completed); the number of days it takes to establish consistent weight gain, defined as an average increase in weight of 20 grams per day for 3 consecutive days; the length of hospital stay; the percentage of feeds by mouth instead of via nasogastric feeding tube; and the cost differential associated with total parenteral nutrition use.
The safety of the feeding protocol was evaluated by monitoring patients for feeding intolerance, development of necrotising enterocolitis, and overall mortality. Feeding intolerance was defined as: increased abdominal girth > 10%, frank blood in stool, more than one haeme+ stool, vomiting more than once in 6 hours, continued diarrhoea, near-infrared spectroscopy cerebro-somatic odds ratio (rSO2s/rSO2c) value < 0.75 (protocol + near-infrared spectroscopy only), and feeding residuals associated with any of the above symptoms. Necrotising enterocolitis was defined using the Bell’s Staging Criteria which is commonly used to make the diagnosis of necrotising enterocolitis. Reference Thompson and Bizzarro10
Statistical analysis
Continuous data were summarised as median values with range and categorical data as number and percentage. Demographic information, anthropometric data before the implementation of the protocol, outcomes, and complication data for the protocol, and control groups were compared using Wilcoxon rank-sum test for continuous data and chi-square test or Fisher’s exact test for categorical data, as appropriate. Additionally, similar analyses were performed between the protocol + near-infrared spectroscopy and the protocol − near-infrared spectroscopy groups. A p value of <0.05 was considered statistically significant. All analyses were conducted using SAS (v9.2; Cary, North Carolina, United States of America) statistical software. The study was approved by the Institutional Review Board at Medical City Dallas Hospital, and all patients were enrolled after the consent was obtained.
Data Safety and Monitoring Board
A Data Safety and Monitoring Board monitored the study for complications related to the feeding protocol to include evidence of feeding intolerance as defined above and any incidence of necrotising enterocolitis as defined by Bell’s criteria as noted above. The Data Safety and Monitoring Board included two paediatric cardiologists/cardiac intensivists and a paediatric gastroenterologist. A Data Safety and Monitoring Board meeting was held when half of the study population had been enrolled and at the conclusion of enrolment for the study.
Results
The prospective feeding study cohort consisted of 33 consecutive patients admitted to our cardiac ICU following stage I palliation for single ventricle anatomy. These 33 patients were randomised to a prospective feeding study group: continuous feeding group with cerebro-somatic near-infrared spectroscopy feeding advancement criteria (protocol + near-infrared spectroscopy; n = 17) or continuous feeding group without cerebro-somatic near-infrared spectroscopy feeding advancement criteria (protocol − near-infrared spectroscopy; n = 16). These patients were compared with a similar retrospective control cohort of 30 consecutive patients admitted to the cardiac ICU from 5/2010 to 9/2013 prior to the implementation of the feeding algorithm. Pre-operative and operative demographics in both feeding and control cohorts were similar except for age at cardiac ICU admission (feeding cohort median 0.0 days [95% confidence interval 0.0–1.0] versus control cohort 5.0 days [95% confidence interval 1.0–6.0]; p < 0.0001) and use of pre-operative vasoactive infusions (feeding cohort 32/33 patients [97%] versus control cohort 18/30 [60%]; p = 0.003) (Table 1). The median time to achieve goal enteral nutrition volume (144 ml/kg/day) was significantly higher in the control compared to feeding cohort, but there were no differences seen in the median time to achieve goal enteral calories (130 kcal/kg/day) or the median time to achieve consistent weight gain (average increase in weight 20 grams/day for three consecutive days) between the cohorts. Interestingly, despite reaching goal enteral volume sooner in the feeding cohort, there was an increased duration of post-operative parenteral nutrition and subsequently, an increased cost of parenteral nutrition in the feeding cohort compared to the control cohort. There were no significant differences in enteral nutrition being held for feeding intolerance except the percent of patients with interruptions in enteral nutrition with the feeding study group having increased interruptions in 90% versus 70% of the control cohort. Among the feeding study group, there were four patients (12%) who had increased gastric residuals and were thus started on erythromycin to improve gastric motility as per the protocol (Table 2). The feeding cohort had significant improvements in discharge nutritional status (weight, difference in admit to discharge weight, difference in admit to discharge weight-for-age z score, volume enteral nutrition, caloric enteral nutrition) compared to the control cohort (Table 3). There were also significant improvements in the proportion of patients with a discharge weight-for-age z score in the “mild malnutrition” category (mean weight-for-age z-score ≥ -2) among the feeding cohort (75.8%) compared to the control cohort (43.3%); p = 0.009. There were no differences seen in post-operative necrotising enterocolitis or surgical mortality between cohorts; however, late mortality was higher in the control cohort (3/30; 10%) compared to the feeding cohort (2/33; 6.1%); p = 0.002.
Table 1. Demographics of feeding study and control cohorts

ACC = aortic cross-clamp; APGAR = Appearance, Pulse, Grimace, Activity, Respiration; CICU = cardiac ICU; CPB = cardiopulmonary bypass; HLHS = hypoplastic left heart syndrome; NW-BTS = Norwood operation with Blalock–Taussig shunt; NW-RV to PA = Norwood operation with right ventricle to pulmonary artery conduit; PA = pulmonary atresia; RACHS-1 Score = Risk Adjustment for Congenital Heart Surgery-1 score; RLFP = regional low-flow perfusion; TA = tricuspid atresia; TOF = tetralogy of Fallot
* Median, interquartile range (25th, 75th)
** Full range
Table 2. Nutrition variables in the feeding study and control cohorts.

EN = enteral nutrition; PN = parenteral nutrition; WAZ = weight-for-age z-score
* Median, interquartile range (25th, 75th)
Table 3. Outcomes of the feeding study and control cohorts.

CICU = cardiac ICU; EN = enteral nutrition; NEC = necrotising enterocolitis; WAZ = weight-for-age z score
* Median, interquartile range (25th, 75th)
Additional analyses were done to compare the two prospective feeding study groups with and without near-infrared spectroscopy monitoring as part of the feeding advancement criteria. There were no statistically significant differences between these groups based on demographic or nutritional variables, but there was a statistically significant difference found in the incidence of post-operative necrotising enterocolitis. No infants in the feeding group with near-infrared spectroscopy monitoring as advancement criteria developed post-operative necrotising enterocolitis, and 4 out of 16 (25%) in the control cohort without near-infrared spectroscopy as advancement criteria developed post-operative necrotising enterocolitis (p = 0.04). Notably, the feeding cohort without near-infrared spectroscopy as advancement criteria also had a longer length of cardiac ICU stay with a median of 48.5 days (interquartile range 37.5–62.0) versus 36.0 days (interquartile range 34.0–41.0) in the feeding cohort with near-infrared spectroscopy monitoring as advancement criteria; p = 0.03 (Supplementary Tables S1–S3).
Discussion
This single centre, prospective case series demonstrated and supports prior studies that the use of an enteral feeding protocol is a safe and effective means of initiating and advancing enteral nutrition in infants with functional single ventricle following stage I palliation, and resulted in improved nutrition delivery, weight gain, and nourishment status at discharge without an increased incidence of gastrointestinal co-morbidities. To our knowledge, this is the first published feeding advancement protocol to use near-infrared spectroscopy as part of the feeding advancement criteria for infants with CHD status post-cardiac surgery with a decreased incidence of necrotising enterocolitis in the near-infrared spectroscopy-based advancement cohort as well as a decreased length of the hospital stay.
Prior to initiation of the single ventricle feeding study protocol at the Medical City Children’s Hospital, infants with hypoplastic left heart syndrome and other forms of single ventricle following stage I palliation were fed by physician preference. Similar practices exist at most cardiac centres, and a recent publication across European pediatric intensive care units noted that 69% of them still did not have written guidelines for feeding and wide variation in practice existed in nutritional care, which reflects the absence of local protocols and guidelines. Reference Tume, Balmaks and da Cruz16 Despite recognition of the importance of perioperative nutrition, no consensus exists regarding optimal feeding strategies for neonates with CHD, and highly variable feeding management practices have also been found to exist across the United States of America cardiac centres. Reference Pasquali, Ohye and Lu17–Reference Hehir, Cooper and Walters20
Infants with hypoplastic left heart syndrome are known to be at risk for poor nutrition and growth failure. There is little published information regarding successful nutrition interventions for infants with single ventricle physiology. Feeding protocols have been shown to maximise the benefits and minimise the risks of enteral nutrition in the management of these critically ill patients. Although many feeding strategies have been proposed in neonates with hypoplastic left heart syndrome, including standardised feeding protocols and pre-emptive gastrostomy tube placement, none have resulted in a dramatic improvement in weight gain during the inter-stage period or become “standard care” to promote growth in this population. Because of this, there is significant centre variation in feeding practice and growth outcomes in these high-risk infants. The Norwood hospitalisation and early perioperative period is associated with a significant decrease in weight-for-age z score and failure to thrive for most patients. Reference Vogt, Manlhiot and Van Arsdell21–Reference Morgan, Young and McGuire23 Factors contributing to growth failure in single ventricle infants include inadequate calorie intake, high metabolic demands, gastrointestinal pathology, and genetic and extracardiac abnormalities. Despite the importance of weight gain, advancement to nutrition goals is often slow in the perioperative period. It is hampered by concerns for poor systemic output, the need for inotropic support, limitations of fluid intake, the risk of necrotising enterocolitis, and frequent interruptions in nutrient delivery. Post-operatively, there are concerns for cardiac and respiratory insufficiency, feeding intolerance, and laryngeal nerve injury. Additionally, several studies have examined the relationship between feeding practices and necrotising enterocolitis. The majority of the evidence on this topic has been inconclusive, and multiple meta-analyses have been unable to recommend a “best” feeding practice for the initiation and advancement of feeds in neonates at risk for necrotising enterocolitis. Reference Bombell and McGuire24–Reference Tortoriello, Stayer and Mott27 However, multiple studies have found that the initiation of a standardised feeding regimen reduces the risk of necrotisng enterocolitis. Reference Bombell and McGuire24–Reference Tortoriello, Stayer and Mott27
Investigators at the Boston Children’s Hospital published a feeding algorithm that advanced feedings in patients with hypoplastic left heart syndrome following stage I palliation to full volume feeds, defined as 108 kcal/kg/day, within 24 hours of starting feeds. The safety and the efficacy of the feeding algorithm were analysed using a prospective study. During the study, none of the patients fed using the standardised feeding regimen developed necrotising enterocolitis; however, there was an 11% incidence of necrotising enterocolitis in the control group. The authors were also able to demonstrate that implementing the feeding protocol enabled patients to achieve the recommended daily allowance of calories earlier in their post-operative course and also decrease the duration of total parental nutrition use. Reference Braudis, Curley and Beaupre13 Similarly, investigators at the Children’s Hospital Los Angeles implemented a standard feeding protocol in their infants with hypoplastic left heart syndrome and demonstrated a reduction in the incidence of necrotising enterocolitis and a reduction in the severity of necrotising enterocolitis as staged by Bell’s criteria. In their study, close attention to any symptoms of feeding intolerance and protocol design were followed to determine whether to continue feeds as opposed to leaving it up to physician preference. They postulated that the greatest advantage of a feeding protocol is the elimination of practice variation that normally occurs among physicians and nurse practitioners in the care of this fragile population of children. Reference Del Castillo, McCulley and Khemani14
Our feeding protocol is similar to the above-mentioned protocols but it is the first protocol to our knowledge to use near-infrared spectroscopy as part of the feeding advancement criteria. Through the use of location-specific non-invasive skin sensors, near-infrared spectroscopy takes advantage of the differential absorption of near-infrared spectroscopy light by oxyhaemoglobin and deoxyhaemoglobin. Measuring the attenuation of light at different wavelengths and different distances between the light source and detector, near-infrared spectroscopy provides a real-time estimation of deep-tissue oxygenation, which is expressed as a weighted average primarily of venous but also of arterial circulation. Reference Morgan, Young and McGuire25 Because impaired tissue oxygen delivery results in greater oxygen extraction and therefore in venous oxygen desaturation, the near-infrared spectroscopic signal can be used to detect regional tissue hypoxia. Near-infrared spectroscopy has been used extensively to monitor cerebral perfusion in neonates following cardiac surgery, and more recently reported uses of near-infrared spectroscopy have included the measurement of perfusion in other tissues, such as the kidneys, liver, and the lower abdomen. Reference Fortune, Wagstaff and Petros28–Reference Zabaneh, Cleary and Lieber32 The development of necrotising enterocolitis and other gastrointestinal morbidities in these infants may be related to the decreased regional tissue oxygen delivery that is secondary to decreased cardiac output or increased flow to the pulmonary vascular bed, increased oxygen extraction, or both. Decreased cardiac output is likely most significant in patients who have large systemic-to-pulmonary run-off lesions that may “steal” blood flow from the mesenteric tissue bed. Fortune et al investigated whether near-infrared spectroscopy could detect differences when applied to the abdomens of neonates with surgically proven splanchnic ischaemia in a prospective, observational cohort study. Reference Stapleton, Eble and Dickerson29 Forty neonates were studied: 10 with acute abdomens, including 4 with necrotising enterocolitis, and 29 controls with normal abdomens. Tissue oxygenation indexes of cerebral and splanchnic regions were measured using near-infrared spectroscopy and their relative values expressed as a cerebro-somatic oxygenation ratio. Median cerebro-somatic oxygenation ratio for the control group was 0.96 (interquartile range 0.83–1.02), whereas the acute abdomen group had significantly lower median cerebro-somatic oxygenation ratio value of 0.66 (0.45–0.69) (p < 0.001). The area under the receiver operating characteristic was 0.91 (95% confidence limits 0.78–1.00) for cerebro-somatic oxygenation ratio, and taking a boundary value of 0.75, intestinal ischemia was identified with a positive predictive value of 0.75 (0.43–0.95) and excluded with a negative predictive value of 0.96 (0.81–1.0). Using the cerebro-somatic oxygenation ratio was a better performance than using abdominal near-infrared spectroscopy alone. Reference Stapleton, Eble and Dickerson29–Reference Zabaneh, Cleary and Lieber32
We chose to model our feeding advancement criteria using cerebral and somatic near-infrared spectroscopy after the above protocol, Reference Stapleton, Eble and Dickerson29 and initially used splanchnic near-infrared spectroscopy as the somatic marker rather than renal near-infrared spectroscopy since we were advancing enteral feeds. However, after the first series of five “run-in” patients to test the feeding algorithm, we noted that splanchnic near-infrared spectroscopy in all patients was significantly lower than renal near-infrared spectroscopy post-operatively, and that feeds would rarely if ever have been advanced using the splanchnic near-infrared spectroscopy oxygenation alone. This is likely due to the post-operative issues surrounding these neonates with abdominal wall distension, ascites, bowel oedema, and other factors that are standard for most neonates following stage I palliation and cardiopulmonary bypass. We thus elected to use the renal near-infrared spectroscopy as a surrogate marker for splanchnic near-infrared spectroscopy to calculate the cerebro-somatic oxygenation ratio and determine feeding advancement criteria. In our feeding study cohort, we did identify an increased incidence of post-operative necrotising enterocolitis in the group without near-infrared spectroscopy monitoring as part of the feeding advancement criteria compared to those that incorporated the near-infrared spectroscopy values, and we think this may be an added benefit in this high-risk population to identify signs and symptoms of feeding intolerance before the development of necrotising enterocolitis. Of note, there were no infants in the feeding study cohort that used near-infrared spectroscopy as part of the advancement criteria who developed necrotising enterocolitis, and this likely contributed to the decreased length of cardiac ICU stay seen in this cohort compared to the non-near-infrared spectroscopy cohort despite having a longer duration of mechanical ventilation, although not statistically significant. These findings are promising and further study using near-infrared spectroscopy to guide and monitor feeding advancement and feeding intolerance in this high-risk population should be further studied.
The management of infants with complex heart disease consisting of functional single ventricle physiology is incredibly tenuous following stage I palliation. The risk of death for children with one variant of single ventricle, hypoplastic left heart syndrome, is highest between the 1st and 2nd palliative surgeries.11 Providing optimal nutrition to this patient population is crucial to optimise their chances for long-term survival and for reducing long-term morbidities.5 With this study, we have demonstrated that the implementation of a standardised feeding protocol in this high-risk population will enable us to reach full enteral feeds more quickly without increasing the risk of gastrointestinal co-morbidities, including necrotising enterocolitis. This would improve the health of our patients by meeting their metabolic and caloric needs at a time when good nutrition is essential to their recovery and survival. Publication of our findings further supports a feeding protocol in the post-operative feeding management of children with a single ventricle, hopefully leading to the prevention of death and long-term morbidity in this fragile patient population.
Limitations
The largest limitation of this study includes the retrospective nature of the review for the control group, although there were no other surgical changes that occurred during the study period with one cardiac surgeon performing all the surgical repairs during the entire study period. This study occurred over a 3-year period and the nature of the pre- and post-design may not reflect medical practice changes other than nutrition that occurred over time. One practice change that did occur during the study was the admission to the cardiac ICU within 24 hours after birth in the feeding cohort (median age 0 days) compared to the control cohort (median age 5 days), who were managed in the neonatal intensive care unit until either a day prior to their surgery or admitted directly from the operating room following stage I palliation repair. This likely also contributed to the increased use of pre-operative vasoactive infusions in the feeding cohort who were admitted sooner to the cardiac ICU and more likely to be initiated on milrinone pre-operatively. Despite this admission age difference, however, there was no significant difference in days to starting post-operative enteral nutrition between cohorts or pre-operative enteral nutrition between cohorts with the control cohort receiving pre-operative enteral nutrition in 18/30 (60%) and the feeding study cohort receiving pre-operative enteral nutrition in 22/33 (67%); p = 0.58. The algorithm was not tested on premature infants, patients supported on extracorporeal membrane oxygenation, or infants with a history of necrotising enterocolitis before the initiation of feedings. Challenges awaiting study in this area include the development of methods that will improve our ability to predict cardiac patients at highest risk of necrotising enterocolitis, such as non-invasive assessment of mesenteric perfusion using near-infrared spectroscopy, and means of detecting necrotising enterocolitis in its earliest stages, possibly allowing intervention before the disease progresses to its more fulminant forms.
Conclusion
We have demonstrated that our enteral feeding algorithm is a safe and effective means of initiating and advancing enteral nutrition in infants with a single ventricle following stage I palliation. The algorithm resulted in improved nutrition delivery as well as significant improvements in weight gain and nourishment status at discharge in infants undergoing stage I palliation without an increased incidence of gastrointestinal co-morbidities. Additionally, the use of somatic near-infrared spectroscopy as a marker of adequate systemic perfusion to help guide feeding advancement in this high-risk population may offer additional benefit and reduce post-operative morbidity and mortality by reducing the incidence of necrotising enterocolitis.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S104795112000253X
Acknowledgements
The authors would like to thank the faculty and staff within the Cardiac Intensive Care Unit at the Medical City Children’s Hospital for making this work possible.
Financial Support
This work was supported by a grant from Medtronic.
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Institutional Review Board at the Medical City Dallas Hospital. All patients were enrolled after written, informed consent was obtained.