Introduction
Usnea is a lichen-forming genus from the family Parmeliaceae (Lecanorales, Ascomycota), in which it forms a monophyletic lineage (Crespo et al. Reference Crespo, Lumbsch, Mattsson, Blanco, Divakar, Articus, Wiklund, Bawingan and Wedin2007) with the following synapomorphic characters: a fruticose thallus, branches with a cartilaginous central axis, and the presence of usnic acid in the cortex. This hyperdiverse genus comprises more than 350 species (Clerc Reference Clerc1998) that are widely distributed in polar, temperate and tropical regions. Since Motyka’s world monograph (1936, 1938), taxonomic revisions of the genus Usnea have been ongoing in Europe (Clerc Reference Clerc1987b , Reference Clerc2011; Halonen et al. Reference Halonen, Myllys, Ahti and Petrova1999), Macaronesia (Clerc Reference Clerc2006), North America (Halonen et al. Reference Halonen, Clerc, Goward, Brodo and Wulff1998; Herrera-Campos et al. Reference Herrera-Campos, Clerc and Nash1998, Reference Herrera-Campos, Nash and Garcia2001; Clerc Reference Clerc2008), East Africa (Swinscow & Krog Reference Swinscow and Krog1978, Reference Swinscow and Krog1979), Asia (Awasthi Reference Awasthi1986; Ohmura Reference Ohmura2001, Reference Ohmura2012; Ohmura et al. Reference Ohmura, Lin and Wang2010), Australia (Stevens Reference Stevens1999, Reference Stevens2004), and polar regions (Walker Reference Walker1985; Seymour et al. Reference Seymour, Crittenden, Wirtz, Øvstedal, Dyer and Lumbsch2007; Wirtz et al. Reference Wirtz, Printzen and Lumbsch2008, Reference Wirtz, Printzen and Lumbsch2012). Tropical regions harbour a rich diversity of species but remain particularly unexplored, yet being a potential source of new species to be discovered. Recently, taxonomic investigations have been taking place in South America (Rodriguez et al. Reference Rodriguez, Estrabou, Truong and Clerc2011; Truong et al. Reference Truong, Bungartz and Clerc2011, Reference Truong, Rodriguez and Clerc2013b ; Truong & Clerc Reference Truong and Clerc2012, Reference Truong and Clerc2013), contributing to a better understanding of this genus worldwide.
Many Usnea species show an extremely plastic morphology in response to environmental factors (Clerc Reference Clerc1998), which causes their circumscription to be particularly complex. Recent phylogenetic studies usually support the delimitation of Usnea species described from morphological and chemical characters (Ohmura Reference Ohmura2002, Reference Ohmura2008; Ohmura & Kanda Reference Ohmura and Kanda2004; Kelly et al. Reference Kelly, Hollingsworth, Coppins, Ellis, Harrold, Tosh and Yahr2011; Saag et al. Reference Saag, Tõrra, Saag, Del Prado and Randlane2011; Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ), but the presence of species complexes has also been detected (Seymour et al. Reference Seymour, Crittenden, Wirtz, Øvstedal, Dyer and Lumbsch2007; Wirtz et al. Reference Wirtz, Printzen and Lumbsch2008, Reference Wirtz, Printzen and Lumbsch2012; Lumbsch & Wirtz Reference Lumbsch and Wirtz2011; Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). Various species have wide distributions over several continents and most clades within the phylogeny of Usnea tend to show low geographic structuration (Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). In addition, phenotypic characters used to characterize species are often homoplasious in the phylogeny of Usnea and therefore inadequate to describe generic subdivisions (Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). As a result, Usnea species with a similar morphology or geographical distribution are not necessarily closely related.
The present work aims to complement the ongoing investigation of the genus Usnea in tropical South America by formally describing five new species previously delimited in our recent phylogenetic study (Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ), using morphological, chemical and ecological features combined with molecular data from the ITS rDNA, nuLSU, RPB1 and Mcm7 markers. In addition, one lectotypification is made and we report several new species records for the region.
Materials and Methods
Taxonomic studies
Herbarium specimens from B, CDS, COLO, G, H, LBL, SP and US were studied, in addition to material collected by the authors in Bolivia, Ecuador (including the Galapagos) and Peru. Collection sites are described in Truong et al. (Reference Truong, Bungartz and Clerc2011). Morphology of specimens was examined using a Leica MS5 stereomicroscope. Critical morphological characters commonly used in the taxonomy of Usnea include, among others: shape of branch and branch segments, shape of lateral branches at ramification, cortex surface such as foveoles, maculae, or pseudocyphellae, and morphology of vegetative reproductive structures (i.e. soralia and isidiomorphs) (Clerc Reference Clerc1987a , Reference Clerc1998, Reference Clerc2011; Herrera-Campos et al. Reference Herrera-Campos, Clerc and Nash1998; Ohmura Reference Ohmura2001). The cortex, medulla and central axis were measured in longitudinal sections of the largest branch above the trunk at ×40 magnification. The percentage thickness of the cortex, medulla, and axis compared to the total branch diameter (CMA) as well as the thickness ratio axis:medulla (A:M) were calculated according to Clerc (Reference Clerc1987b ) and Truong et al. (Reference Truong, Bungartz and Clerc2011). In the description of species, CMA values are given with their standard deviations and follow the categories described in Clerc (Reference Clerc2011). Chemical analyses were performed by thin-layer chromatography (TLC) following the method of Culberson & Ammann (Reference Culberson and Ammann1979), with solvent B modified according to Culberson & Johnson (Reference Culberson and Johnson1982).
Phylogenetic studies
The internal transcribed spacer (ITS) is widely used for successfully delimiting fungal species in phylogenetic studies (Kelly et al. Reference Kelly, Hollingsworth, Coppins, Ellis, Harrold, Tosh and Yahr2011; Schoch et al. Reference Schoch, Seifert, Huhndorf, Robert, Spouge, Levesque, Chen and Consortium2012), including in Usnea (Ohmura Reference Ohmura2002, Reference Ohmura2008; Ohmura & Kanda Reference Ohmura and Kanda2004; Wirtz et al. Reference Wirtz, Printzen and Lumbsch2008; Lumbsch & Wirtz Reference Lumbsch and Wirtz2011; Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). Sequences from the nuclear ribosomal regions ITS and nuLSU, as well as portions of the protein-coding genes Mcm7 and RPB1 were assembled in a data matrix of 40 specimens from 26 Usnea species (Table 1) from the Usnea clade described in Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). Three species from the Neuropogon clade served as outgroup (Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). The dataset mainly includes sequences used in Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ), in addition to new ITS and nuLSU sequences obtained from the newly described species U. aranea, U. rubriglabrata, U. subaranea and U. clerciana, following the procedure described in Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). Material from newly recorded species in South America was not included within the phylogeny, since these specimens mainly originate from herbarium collections and were not freshly collected by the authors.
Table 1 Specimen location, major chemotye and GenBank accession numbers for the species referred to in this study. Newly described species and sequences are in bold

Methods of alignment and tests for topological incongruence among markers are detailed in Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). Partition and substitution model selection were optimized using PartitionFinder 1.1.1 (Lanfear et al. Reference Lanfear, Calcott, Ho and Guindon2012) with the following parameters: branch lengths=linked; models=raxML (GTR+G, GTR+I+G); model selection=BIC; search=greedy. Two partitions and models were selected (GTR+G: ITS1, ITS2, MCM7–position3, RPB1–position3; GTR+I+G: 5.8S, LSU, MCM7–position1, MCM7–position2, RPB1–position1, RPB1–position2) and subjected to a Bayesian inference with a Monte Carlo Markov chain (B/MCMC) in MrBayes 3.2.3 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Hohna, Larget, Liu, Suchard and Huelsenbeck2012). We conducted three independent runs of 5 million generations, starting from a random tree. Parameters were estimated independently for each partition but sharing the same overall rate. Four chains were sampled every 1000 generations and temperature was set to 0·1 to improve mixing among chains. Convergence among runs was confirmed by 1) verifying that the average standard deviation of split frequencies was <0·01; and 2) plotting likelihood per generation for each run and identifying the effective sample size (ESS) for each parameter in Tracer v1.6 (Rambaut et al. Reference Rambaut, Suchard, Xie and Drummond2014). The majority-rule consensus tree was constructed by pooling trees sampled from all runs after discarding 25% as burn-in, with posterior probabilities (PP) as branch support. The data matrix with the same partition was analyzed using the GTRGAMMA model for maximum likelihood (ML) inference with 1000 bootstrap pseudoreplicates in RAxML 8.0.24 (Stamatakis Reference Stamatakis2014) on the CIPRES web portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010).
Results and Discussion
Taxonomic studies
Table 2 indicates the number of specimens studied for each species, their distribution per country and their altitudinal range. The newly described species U. aranea, U. rubriglabrata, U. subaranea and U. suglabrata are so far endemic to continental South America, whereas U. clerciana is endemic to the Galapagos. The distributions of U. dorogawensis and U. grandisora are extended to continental South America and new records from eastern South America (Argentina, Brazil or Venezuela) are reported for U. moreliana and U. subdasaea. CMA values and chemotypes of the newly described species are illustrated in Figure 1 and Table 3, respectively.

Fig. 1 The thickness of the cortex, medulla, and axis compared to the total branch diameter (CMA) expressed as a percentage and the thickness ratio axis:medulla (A:M) of the newly described species of Usnea. Standard deviations are shown as wide bars and range by vertical lines. The number of specimens measured is shown in parentheses.
Table 2 Distribution of new species and newly recorded species of Usnea in South America

Abbreviations for countries: ARG=Argentina, BOL=Bolivia, BRA=Brazil, COL=Colombia, ECU=Ecuador (continental), GAL=Galapagos, PER=Peru, VEN=Venezuela, SAM=continental South America; n=number of specimens studied; +=presence of species; newly described species are indicated in bold font.
Table 3 Major secondary metabolites and chemotypes detected in the medulla of new species of Usnea described in this study

Abbreviations for secondary metabolites: BAR=barbatic acid, PRO=protocetraric acid, PSO=psoromic acid, NOR=norstictic acid, SAL=salazinic acid, STI=stictic acid group (incl. constictic and menegazziaic acids), UU=unknown depside with RF classes A/B/C/D: 3/5–6/3–4/7, USN=usnic acid alone (detected in the cortex); n=number of specimens studied; +=presence constant within species; ±=presence variable among specimens within species.
Phylogenetic studies
The best ML tree (LnL=−7027·71) inferred from the multi-locus dataset is illustrated in Figure 2. It contains 22 highly supported internodes (bootstrap support BS>0·70). The B/MCMC majority-rule consensus tree (LnL=−7073·70) was almost identical to the ML tree, with 25 highly supported internodes (PP>0·95). Posterior probabilities are reported on the ML tree adjacent to BS values.

Fig. 2 Phylogram of the newly described species of Usnea based on maximum likelihood (ML) inference from the multi-locus dataset of ITS rDNA, nu LSU, Mcm7 and RPB1 genes revealing their position within the Usnea clade. Bootstrap supports (BS) are indicated above branches. Posterior probabilities (PP) from the Bayesian (B/MCMC) 50% majority-rule consensus tree are reported following the BS values. Thick branches indicate high support (BS≥70 and/or PP≥0·95). The Usnea clade is segregated into four clades USNEA–1 to USNEA–4 following Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ) (shaded in light grey). The newly described species U. aranea, U. rubriglabrata and U. subaranea cluster together with U. acanthella within the USNEA–2 clade (shaded in dark grey). Usnea glabrata and U. subglabrata belong to distinct lineages (black arrow heads). Sorediate and non-sorediate specimens of U. clerciana cluster within the same lineage (delimited with dashes). Abbreviations for chemotypes: NOR=norstictic acid, PRO=protocetraric acid, SAL=salazinic acid, STI=stictic acid, USN=usnic acid only (cortical). Newly described species are indicated in bold font. The Neuropogon clade was used as the outgroup.
As previously reported in Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ), the Usnea clade is segregated into four highly supported clades USNEA–1 to USNEA–4 (shaded in light grey in Fig. 2). The placement of the newly described Usnea species within these clades has been discussed extensively in Truong et al. (Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). Usnea aranea Truong & P. Clerc, U. rubriglabrata Truong & P. Clerc and U. subaranea Truong & P. Clerc cluster together with U. acanthella (I. M. Lamb) F. J. Walker within a small highly supported clade (USNEA–2, shaded in dark grey in Fig. 2), sister to the species from the section Usnea (i.e. U. florida (L.) F. H. Wigg. and relatives). All the species within this clade are endemic to the tropical Andes, in contrast to most clades within the phylogeny of Usnea that tend to show low geographic structuration, with several species occurring over different continents (Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). Concomitant patterns have been observed within the family Parmeliaceae, with truly widespread taxa but also lineages characterized by restricted geographical distributions (Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011; Amo de Paz et al. Reference Amo de Paz, Cubas, Crespo, Elix and Lumbsch2012; Leavitt et al. Reference Leavitt, Esslinger, Spribille, Divakar and Lumbsch2013). Usnea acanthella shares the morphology and habitat of neuropogonoid species (i.e. black cortical pigmentation and saxicolous substratum) but clusters outside of the Neuropogon clade (Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). It is the only neuropogonoid species that is absent from polar regions, and its phylogenetic placement correlates with its distribution as a tropical Andean endemic, together with the non-neuropogonoid U. aranea, U. rubriglabrata and U. subaranea. In addition, U. aranea and U. subaranea share similar ecological preferences with U. acanthella, often growing close by in exposed habitats, for example at high altitudes above the tree limit.
Following the species-pair concept of Poelt (Reference Poelt1970, Reference Poelt1972), Usnea taxa differing only in the presence/absence of soralia are traditionally considered as distinct species, providing that they show dissimilarities in their distribution patterns (Clerc Reference Clerc1998). The evolutionary significance of reproductive traits may vary between lineages in lichen-forming fungi (Scherrer et al. Reference Scherrer, Zippler and Honegger2005; Tehler et al. Reference Tehler, Irestedt, Bungartz and Wedin2009) and assumptions need to be corroborated with molecular data for each particular case. Sorediate Usnea species often have a wider distribution than non-sorediate fertile taxa (Herrera-Campos et al. Reference Herrera-Campos, Clerc and Nash1998), potentially correlated with increased dispersal abilities of vegetative propagules compared with ascospores. This does not necessarily mean that there isn’t any gene flow occurring among populations of sorediate and non-sorediate specimens. Recently, several phylogenetic studies demonstrated that various species-pairs in Usnea did not represent distinct monophyletic lineages and should be considered conspecific (Articus et al. Reference Articus, Mattsson, Tibell, Grube and Wedin2002; Seymour et al. Reference Seymour, Crittenden, Wirtz, Øvstedal, Dyer and Lumbsch2007; Wirtz et al. Reference Wirtz, Printzen and Lumbsch2012; Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). This seems to be the case for the newly described species U. clerciana, where sorediate and non-sorediate specimens belong to the same lineage based on their ITS and LSU sequences (delimited with dashed lines in Fig. 2).
New Species
Usnea aranea Truong & P. Clerc sp. nov.
MycoBank No.: MB 811375
Thallus stiff, branches inflated and constricted at ramification, papillae numerous, soralia minute and irregular, medulla loose and arachnoid.
Type: Bolivia, La Paz, Queara viejo, sendero hasta Muñamachay, 14·705556°S, 69·084306°W, 3379 m, bosque de neblina montano en transición con matorral, sobre ramas, 2007, Truong 2822 (LPB—holotype; G—isotype). % C/M/A: 6.5/34/19.5 Chemistry: usnic, stictic, constictic, menegazziaic acids, traces of norstictic acid.
(Fig. 3)

Fig. 3 Usnea aranea. A, holotype (above) and isotype (below); B, trunk blackish on the first mm below first ramification (Truong 286); C, section through branch (Truong 2822); D, branch slightly inflated and constricted at ramification (Truong 286); E, lichenicolous fungus forming a thin network on the surface of branches; fasciculate isidiofibrils bursting from pseudocyphellae (Cleef 3355); F, hemispherical papillae abundantly distributed on cortex surface; convex soralia (Truong 286); G, minute soralia aggregating in small clusters on terminal branches (Truong 286). Scales: A=1 cm; B–G=500 μm. In colour online.
Thallus erect-shrubby, rarely sub-pendulous, very stiff, with a greyish and somewhat translucid hue revealing clusters of algal cells visible in transparency, often abundantly infested by lichenicolous fungi typically forming a thin network on the branch surface (Fig. 3E); ramifications±anisotomic-dichotomous; trunk blackish on the first mm below first ramification (Fig. 3B), rarely brownish to concolorous; branches±tapering, segmented in slightly to strongly inflated segments, at least on basal branches (Fig. 3D); branch segments terete on terminal branches, slightly irregular or deformed on basal branches; lateral branches slightly to distinctly constricted at ramification, at least on basal branches (Fig. 3D); foveoles absent, rarely scarce on basal branches; maculae absent; pseudocyphellae minute, of irregular outline (young soralia?); papillae irregularly to abundantly distributed on basal and secondary branches (Fig. 3F), hemispherical, giving a coarse and rugulose aspect to the cortex surface; tubercles absent; fibrils slender, scattered to irregularly distributed; fibercles absent or scarce; soralia developing from the cortex ad initio, plane to slightly stipitate, becoming convex at maturity (Fig. 3F), remaining minute or slightly enlarged with irregular outline, aggregating in small clusters on terminal branches (Fig. 3G) and sometimes fusing in irregular ‘soralium’; isidiomorphs often abundant, growing into isidiofibrils bursting solitary or in fasciculate clusters from soralia or pseudocyphellae (Fig. 3E); cortex thin to moderately thin (5·5–8·0%), very rigid, shiny to vitreous in section, surface of cortex often becoming necrotic with a dark blue to blackish pigmentation on small areas; medulla moderately thick to thick (24·5–33·5%), loose but thick-‘arachnoid’ (Fig. 3C), often forming bundles of tight hyphae connecting cortex and axis or forming a thin and compact layer applied to the cortex; axis thin to moderately thin (21·5–35·5%), often pale orange pigmented, with an A:M ratio of 0·5–1·5.
Apothecia rare (n=4).
Pycnidia not seen.
Diagnostic notes. Usnea aranea is characterized by a stiff, erect-shrubby thallus with a rough cortex surface harbouring numerous papillae. Basal branches are inflated and constricted at ramification. Minute soralia aggregate in irregular clusters on terminal branches, with numerous isidiomorphs growing into isidiofibrils. The cortex is relatively thin but very rigid, with a characteristic greyish and somewhat translucid hue, and the medulla is loose and thick-‘arachnoid’.
Variation. Individuals with almost non-inflated and non-constricted branches may occur, for example in compact morphotypes growing at highly exposed sites. Basal part of trunk may lack black pigmentation and appear brownish or concolorous. Soralia of various sizes are visible on the branches, minute to slightly enlarging with irregular outline, always becoming convex at maturity. Isidiomorphs and isidiofibrils vary in abundance but are usually numerous. Several chemotypes were detected (Table 3), including an unidentified yellowish brown depside with Rf classes A/B/C/D: 3/5–6/3–4/7, which do not correspond to either lecanoric or gyrophoric acid.
Differentiation. See under U. subaranea. Usnea aranea resembles U. cornuta Körb. with branches inflated and constricted at ramification, irregular soralia aggregating in clusters, and the chemotype of stictic acid. Usnea cornuta differs in having a denser medulla with thin hyphae and the somewhat thinner axis (ratio A:M<1). In addition, the cortex is not as rigid as in U. aranea, resulting in a softer thallus, without a greyish translucid hue.
Ecology. Highlands of puna and páramo grasslands (i.e. shrubby páramo, páramo de pajonal, páramo de Frailejones, Polylepis stands or wooded ravines in páramo), transition between forest and páramo, primary and disturbed mountain cloud forests to the upper tree limit (Ceja andina), pastures or deforested zones of matorral in the vicinity of forest; corticolous on various trees and shrubs, occasionally saxicolous, rarely lignicolous on fence posts. This is a very common and abundant species on shrubs, at high altitudes or in exposed sites combining strong wind and high humidity from mist. It is closely related to the neuropogonoid species U. acanthella (I. M. Lamb.) F. J. Walker (Fig. 2). Both species are endemic to the tropical Andes and tend to grow in similarly exposed, high altitude habitats. They are often found close by in the same localities, where U. aranea is usually corticolous on shrubs but U. acanthella is saxicolous.
Distribution. So far endemic to the tropical Andes.
Selected specimens examined. Bolivia: Cochabamba: Prov. Ayopaya, comunidad de Saila Pata, 3350 m, 1997, Bach & Jimenez 911 (B). La Paz: Parque Nacional Alto Madidi, sendero hasta Muñamachay, 14·692306°S, 69·050111°W, 3008 m, 2007, Truong 2853 (G).—Colombia: Antioquia: 12 km al E de Sonsón hacia Nariño y Argelia, 2580 m, 1985, Churchill & Sastre de Jesús 12991 (B). Boyacá: páramos al NW de Belén, vereda San José de la Montaña, Alto de las Cruces y alrededores, 3800 m, 1972, Cleef 2302 (B). Caldas: Nevado del Ruiz, 3430 m, 1965, Merill King et al. 494 (US). Cauca: along road La Plata-Puracé, páramo 2 km W of Laguna de San Rafael, 3300 m, 1984, Aguirre & Sipman 5985 (B). Cundinamarca: páramo de Cruz Verde, km 16 of carretera Bogotá-Choachí, 3365 m, 1972, Cleef 2990 (B). Nariño: Munic. Pasto, páramo El Frailejonal, 8 km E de Mocondino, 3400 m, 1997, Ramírez et al. 10513 (B). Risaralda: Munic. Santa Rosa de Cabal, c. 500 m S of Finca La Sierra, 3750 m, 1984, Aguirre & Sipman 5478 (B).—Ecuador: Azuay: Parque Nacional Cajas, sector laguna Toreadora, sendero García Moreno, 2·783639°S, 79·209917°W, 3794 m, 2007, Truong 3188 (G). Imbabura: Reserva Ecológica Cotacachi-Cayapas, Laguna Piñán, 0·517611°N, 78·438333°W, 2786 m, 2007, Truong 3252 (G). Loja: Parque Nacional Podocarpus, sendero de las lagunas del Compadre, 4·115083°S, 79·165139°W, 3000 m, 2007, Truong 254 (G). Pichincha: Reserva de Vida Silvestre Pasochoa, sendero Los Pantzas, 0·443278°S, 78·494306°W, 3460 m, 2013, Truong 3943 (G). Tungurahua: Parque Nacional Llaganates, parte alta desde Pelileo, cerca de la entrada del parque, 1·082167°S, 78·411861°W, 3498 m, 2007, Truong 468 (G).—Peru: Cajamarca: Parque Nacional Cutervo, subiendo al cerro Tarros, 6·301389°S, 78·799778°W, 3075 m, 2007, Truong 1712 (G). Cusco: Santuario Histórico de Machu Picchu, Camino Inca, abra Runkuraquay, 13·226167°S, 72·508111°W, 3809 m, 2007, Truong 2006 (G). Huanuco: Lagunas Pichgacocha, bajando desde la primera laguna, 10·026833°S, 76·135611°W, 3751 m, 2007, Truong 761 (G). Pasco: Parque Nacional Yanachaga-Chemillén, trocha hasta la laguna San Alberto, 10·530833°S, 75·350556°W, 2835 m, 2007, Truong 2592 (G).—Venezuela: Mérida: Potreros de San Rafael, páramo de Las Coloradas, 2950 m, 1975, Hale & López-Figueiras 44400 (US).
Usnea clerciana Truong sp. nov.
MycoBank No.: MB 811376
Thallus erect-shrubby, branches inflated and constricted at ramification, tubercles elongated and eroded, soralia large and excavate, apothecia usually present when soralia are absent, medulla loose, reacting K+ (salazinic acid).
Type: Ecuador, Galapagos, Santa Cruz, above Mina Granillo, 0·618750°S, 90·365416ºW, 607 m, upper transition zone, branches of ferns at the top of a rock outcrop, 2008, Truong 1127 (CDS—holotype; G—isotype). %C/M/A: 6.5/36.0/15.0. Chemistry: usnic, salazinic, traces of protocetraric acids.
(Fig. 4)
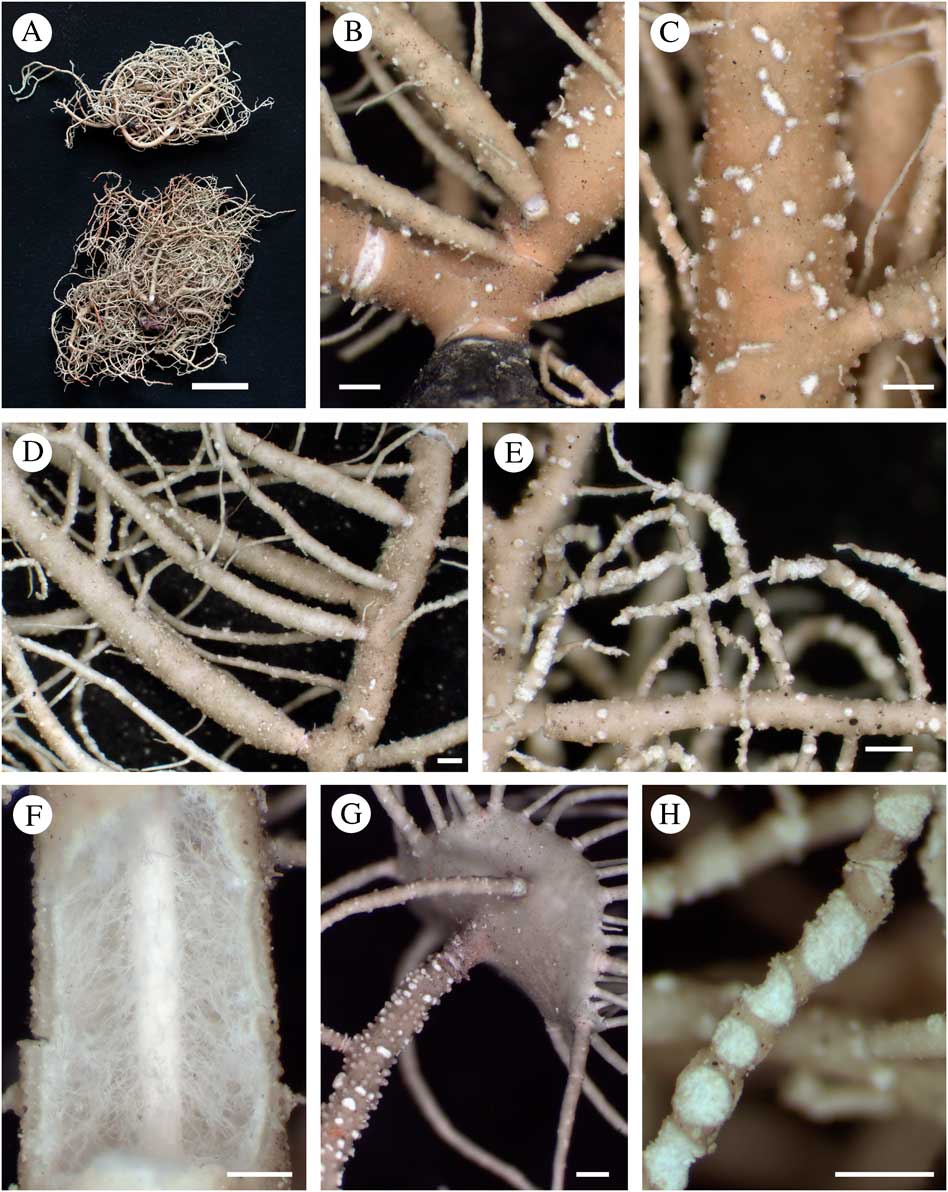
Fig. 4 Usnea clerciana. A, holotype (above) and isotype (below); B, trunk concolorous (Bungartz 7701C); C, elongated tubercles, eroded on top (Bungartz 7701C); D, branch inflated and constricted at ramification (Truong 1127); E, thin terminal branches with large and excavate soralia, eroding branch apices and exposing the central axis (Truong 1127); F, section through branch (Truong 1394); G, branch abundantly covered by tubercles and holding apothecia (Clerc 08-76); H, circular and excavate soralia delimited with a thin cortical margin (Truong 1127). Scales: A=1 cm; B–H=500 μm. In colour online.
Thallus erect-shrubby, relatively stiff; ramifications±anisotomic-dichotomous; trunk concolorous (Fig. 4B), rarely blackish or brownish on the first mm below first ramification; branches tapering to±irregular, segmented into slightly to strongly inflated segments, at least on basal branches (Fig. 4D); branch segments±terete to slightly flattened or deformed on basal branches; lateral branches slightly to strongly constricted at ramification towards base of thallus (Fig. 4D), terminal branches often thin (Fig. 4E) and not constricted; foveoles absent or scarce; maculae and pseudocyphellae absent; papillae scarce to abundantly distributed on basal and secondary branches; tubercles irregularly to abundantly distributed on basal and secondary branches, conical to verrucose, sometimes almost cylindrical, slightly elongated and usually eroding on top when fully developed (Fig. 4C & G); fibrils±thick and long, scattered to densely distributed; fibercles absent or scarce; soralia developing from the cortex ad initio or from tubercles, large and circular to elongated, delimited with a thin cortical margin, plane to strongly excavate (Fig. 4H), sometimes becoming slightly convex with age, often crowded on terminal branches and eroding the branch apices, exposing the central axis (Fig. 4E); isidiomorphs absent or scarce within soralia, sometimes few isidiomorphs bursting from tubercles on basal and secondary branches; cortex thin (4·0–5·5%), opaque and ±shiny in section; medulla thick (33·5–38·0%), loose with thin hyphae (Fig. 4F), somewhat denser towards the cortex; axis thin (15·0–22·0%), unpigmented, with an A:M ratio>0·75.
Apothecia often abundant, usually present when soralia are absent (n=38), rarely both soralia and apothecia present (n=1), pedicellate (Fig. 4G) or rarely sessile, with±thick and long cilia.
Pycnidia hemispherical on terminal branches, usually present only when apothecia or soralia are absent.
Etymology. This species is named in honour of Philippe Clerc for his major contribution to the study of the genus Usnea worldwide.
Diagnostic notes. Usnea clerciana is characterized by a stiff erect-shrubby thallus, basal branches inflated and constricted at ramification, and elongated eroded tubercles irregularly to densely distributed on branches. When present, large and circular soralia are often crowded on terminal branches, eroding the apices of branches. Numerous apothecia are usually present when soralia are lacking. The medulla is relatively loose, reacting K+ yellow turning red (salazinic acid).
Variation. Branch segments are±terete to deformed, with various degrees of branch inflation and constriction at ramification. Papillae and tubercles vary in abundance and in some specimens, especially when apothecia are present, a majority of tubercles may not erode, appearing verrucose to almost cylindrical. When present, soralia of all sizes may be visible on branches, but large and excavate soralia are always present. Sorediate and non-sorediate thalli often grow side by side and specimens with both soralia and apothecia were rarely encountered (n=1). Phylogenetic studies provided evidence that sorediate and non-sorediate specimens belong to the same lineage based on their ITS and LSU sequences (delimited with dashes in Fig. 2).
Differentiation. Usnea cirrosa Motyka, U. cornuta Körb. and U. fragilescens Lynge share with U. clerciana the inflated branches constricted at ramification, and the thick medulla with an A:M ratio<1. They differ from U. clerciana in the flaccid thallus, the terete branch segments, basal branches not irregular or deformed, and the absence or rarity of tubercles, remaining lower and never elongated. The non-sorediate U. cirrosa is distinguished by the denser medulla reacting K+ yellow (stictic) or K+ yellow turning red (salazinic acid) in South America. The sorediate U. fragilescens has circular to slightly excavate soralia that remain isolated (not crowded on terminal branches). Usnea cirrosa and U. fragilescens are abundant in continental South America but have not been encountered in the Galapagos so far. Usnea cornuta s. str. (according to Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ) is present in the Galapagos and differs by the minute soralia aggregating in irregular clusters, not circular or excavate, and the denser medulla, reacting K+ yellow (stictic) or K+ yellow turning red (salazinic acid). Our phylogenetic results indicate that U. clerciana and U. cornuta s. str. are potentially closely related (Fig. 2).
Ecology. Wide altitudinal range from the arid to the high altitude dry zone, most common in the transition zone; primarily corticolous on various trees and shrubs, including on cacti, rarely saxicolous or lignicolous.
Distribution. So far endemic to the Galapagos, where it is relatively common.
Selected specimens examined. Ecuador: Galapagos Islands: Isabela, volcán Darwin, 0·203194°S, 91·311472°W, 1286 m, 2007, Bungartz 7505 (CDS); Pinta, along trail going up the south-western slope to Las Pampas, 0·570417°N, 90·760611°W, 316 m, 2008, Nugra 577 (CDS); Pinzón, along the trail going up from Playa Escondida, 0·602778°S, 90·666944°W, 254 m, 2006, Aptroot 64129 (CDS); San Cristóbal, Cerro Colorado summit, 0·414556°S, 89·433194°W, 167 m, 2008, Truong 1413 (G); Santa Cruz, dirt road to Mina Granillo, 0·614611°S, 90·369944°W, 547 m, 2008, Clerc 08-174 (G); Santiago, along the trail from Bucanero to Jaboncillos, 0·176111°S, 90·815278°W, 225 m, 2006, Aptroot 65364 (CDS).
Usnea rubriglabrata Truong & P. Clerc sp. nov.
MycoBank No.: MB 811377
Thallus erect-shrubby, basal branches inflated and constricted at ramification, soralia large and excavate, reddish and maculate pigmentation on the cortex surface, medulla dense and reacting K− (protocetraric acid).
Type: Peru, Cajamarca, caretera 8 de Chachapoyas a Celendín, cerca del distrito de Las Palmas, 6·717972ºS, 77·844833°W, 2940 m, matorral arbóreo, sobre ramas, 2007, Truong 1877 (G—holotype). % C/M/A: 7.5/30.0/24.5. Chemistry: usnic and protocetraric acids.
(Fig. 5)
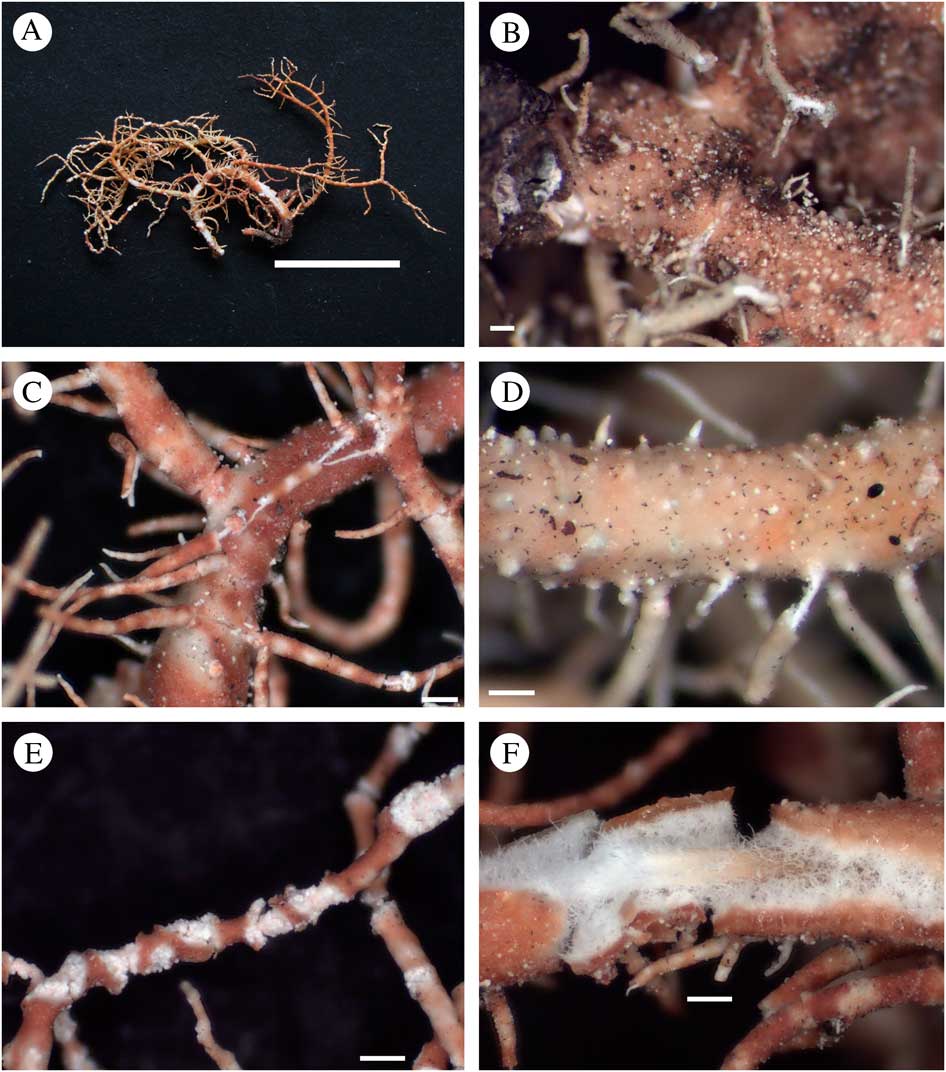
Fig. 5 Usnea rubriglabrata. A, holotype; B, trunk concolorous (Truong 1625); C, branch inflated and constricted at ramification; reddish and maculate cortex surface (Truong 1877); D, papillae on basal branches (Truong 1625); E, terminal branches thin and sinuous, with strongly excavate soralia (Truong 1877); F, section through branch (Truong 1877). Scales: A=1 cm; B–F=250 μm. In colour online.
Thallus erect-shrubby; ramifications±anisotomic-dichotomous; trunk concolorous (Fig. 5B), sometimes brownish on the first mm below first ramification; branches tapering to±irregular, segmented in slightly to strongly inflated segments on basal branches (Fig. 5C), terminal branches relatively thin and non-inflated, sinuous at the apices (Fig. 5E); branch segments±terete, rarely irregular on basal branches; lateral branches distinctly constricted at ramification on basal branches (Fig. 5C), terminal branches often not constricted; foveoles absent or scarce on basal branches; maculae and pseudocyphellae absent, although reddish pigmentation may look maculate; papillae and tubercles scarce to irregularly distributed on basal branches (Fig. 5D); fibrils slender, scattered to irregularly distributed; fibercles absent or scarce; soralia arising on terminal branches from the cortex ad initio, large and strongly excavate (Fig. 5E), delimited with a thin cortical margin; isidiomorphs absent with granular soredia instead; cortex thin to moderately thin (4·5–7·5%), shiny in section, with a reddish and maculate pigmentation on the cortex surface (Fig. 5C); medulla thick (30·0–36·0%) and dense, looser towards the axis (Fig. 5F); axis thin (17·0–29·5%), with an A:M ratio<1·0.
Apothecia and pycnidia not seen.
Diagnostic notes. Usnea rubriglabrata is characterized by the erect-shrubby thallus with reddish maculate pigmentation on the cortex surface. Basal branches are inflated and constricted at ramification, contrasting with the thin and sinuous terminal branches bearing large and excavate soralia. The medulla is dense, reacting K− (protocetraric acid). This species was described as a potential new species named Usnea sp. 1 in Truong et al. (Reference Truong, Bungartz and Clerc2011), but the small number of specimens available prevented us from describing it formally. Later, phylogenetic studies gave additional evidence for the validity of this taxon as a distinct species (Fig. 2).
Variation. The abundance of papillae, tubercles, fibrils and fibercles may vary among individuals. Isidiomorphs are usually absent, but may occur abundantly when the thallus is infected by parasitic fungi.
Differentiation. The red-pigmented cortex surface and the large excavate soralia make U. rubriglabrata readily distinguishable from any other Usnea species. In the Galapagos, U. dorogawensis Asahina has reddish orange subcortical pigmentation visible in section in the medulla as well as, a thinner cortex with a smoother cortex surface without papillae, and a looser medulla reacting K+ (lobaric with stictic or norstictic acids).
Ecology. Pastures or deforested zones of matorral in the vicinity of forest; corticolous.
Distribution. So far endemic to northern Peru; rare.
Selected specimens examined. Peru: Cajamarca: Parque Nacional Cutervo, cerca de San Andrés de Cutervo, sector alto pajonal, 6·221750°S, 78·742861°W, 2541 m, 2007, Truong 1625 (G); carretera 8 de Chachapoyas a Celendín, entre los distritos de Balsas y Celendín, 6·873917ºS, 78·100889°W, 2254 m, 2007, Truong 1894 (G).
Usnea subaranea Truong & P. Clerc sp. nov.
MycoBank No.: MB 811378
Similar to U. aranea but with large, circular and excavate soralia, without or with few isidiomorphs.
Type: Colombia, Cundinamarca, Parque Nacional Natural Chingaza, Munic. Fomeque, about halfway between Embalse de Chuza and Laguna Seca, 4·617°S, 74·733°W, 3200 m, subpáramo scrub on steep slope, epiphytic, 1988, Sipman & Aguirre 27435a (B—holotype; G—isotype). % C/M/A: 4.5/30.0/30.0. Chemistry: usnic, salazinic and norstictic acids.
(Fig. 6)

Fig. 6 Usnea subaranea. A, holotype (left) and isotype (right); B, branch slightly inflated and constricted at ramification; papillae abundant, giving a rugulose aspect to the cortex surface (Truong Reference Leavitt, Esslinger, Spribille, Divakar and Lumbsch2013); C, trunk blackish on the first mm below first ramification (Truong Reference Leavitt, Esslinger, Spribille, Divakar and Lumbsch2013); D, lichenicolous fungus forming a thin network on the branch surface (Truong 3229); E, section through branch (Sipman & Aguirre 27435a); F, terminal branches thin and sinuous, holding circular and isolated soralia (Truong 313); G, large and excavate soralia encircling thin terminal branches (Sipman & Aguirre 27435a). Scales: A=1 cm; B–G=500 μm. In colour online.
Thallus erect-shrubby, rarely subpendulous, very stiff, with a greyish and somewhat translucid hue revealing clusters of algal cells visible when transparent, often abundantly infested by lichenicolous fungi typically forming thin networks on the surface of branches (Fig. 6D); ramifications±anisotomic-dichotomous; trunk blackish on the first mm below first ramification (Fig. 6C), rarely brownish or concolorous; branches±tapering, basal branches segmented in slightly to strongly inflated segments (Fig. 6B), terminal branches often thin and non-inflated, with long and sinuous apices (Fig. 6F); branch segments terete on terminal branches to slightly irregular or deformed on basal branches; lateral branches slightly to distinctly constricted at ramification at least on basal branches; foveoles sometimes present on basal branches; maculae absent; pseudocyphellae absent or scarce, minute, of irregular outline; papillae irregularly to abundantly distributed on basal and secondary branches, hemispherical, giving a coarse and rugulose aspect to the cortex surface (Fig. 6B); tubercles absent; fibrils slender, scattered to irregularly distributed; fibercles absent or scarce; soralia arising on terminal branches from the cortex ad initio, circular, remaining isolated (Fig. 6F) and delimited with a thin cortical margin, plane to excavate, sometimes encircling thin terminal branches (Fig. 6G); isidiomorphs absent or scarce within soralia with granular soredia instead, rarely few to numerous isidiomorphs bursting from pseudocyphellae or fibercles on basal and secondary branches, but not on terminal branches, and growing into isidiofibrils; cortex thin to moderately thin (4·0–6·5%), very rigid, shiny to vitreous in section; medulla thick (28·5–36·0%), loose but thick-‘arachnoid’ (Fig. 6E), often forming bundles of tight hyphae connecting cortex and axis or forming a thin and compact layer applied to the cortex; axis thin to moderately thin (18·5–30·5%), often pale orange pigmented, with an A:M ratio usually >1·0 (rarely to 1·5).
Apothecia rare (n=1).
Pycnidia not seen.
Diagnostic notes. Usnea subaranea is characterized by a stiff, erect-shrubby thallus with a rough cortex surface harbouring numerous papillae. Basal branches are inflated and constricted at ramification. Circular and excavate soralia remain isolated and delimited with a thin cortical margin, with granular soredia instead of isidiomorphs. The cortex is relatively thin but very rigid, with a characteristic greyish and somewhat translucid hue, and the medulla is loose and thick-‘arachnoid’.
Variation. Individuals with almost non-inflated and non-constricted basal branches may occur, for example in compact morphotypes growing at highly exposed sites. Basal part of trunk may lack black pigmentation and appear brownish or concolorous. Several chemotypes were detected (Table 3), including an unidentified yellowish brown depside with Rf classes A/B/C/D: 3/5–6/3–4/7, which does not correspond to either lecanoric or gyrophoric acid.
Differentiation. See under U. subglabrata. Apart from soralia, U. aranea has a similar morphology and branch section. It differs in the non-enlarging soralia of irregular outline, capitate at maturity, not circular or excavate, often aggregating in clusters with numerous isidiomorphs. In U. subaranea, isidiomorphs are usually absent from soralia with granular soredia instead, but occasionally grow from pseudocyphellae on basal branches (i.e. in thalli infested by parasitic fungi). Both species share several chemotypes (Table 3), yet the most frequent chemotype in U. subaranea (salazinic acid) is absent in U. aranea. Phylogenetic studies provided evidence that these two species are closely related (Fig. 2). Intermediate morphs may occur between the two species and the presence of hybrids is not excluded.
Ecology. Highlands of puna and páramo grasslands (i.e. shrubby páramo, páramo de pajonal, páramo de Frailejones, Polylepis stands or wooded ravines in páramo), transition between forest and páramo, primary and disturbed mountain cloud forests to the upper tree limit (Ceja andina), Pinus plantations, pastures or deforested zones of matorral in the vicinity of forest; corticolous on various trees and shrubs, occasionally saxicolous, rarely lignicolous on fence posts. This is a very common and abundant species on shrubs, at high altitudes or in exposed sites combining strong wind and high humidity from mist. It is closely related to U. aranea and the neuropogonoid species U. acanthella (I. M. Lamb.) F. J. Walker (Fig. 2). The three species are endemic to the tropical Andes and share similar ecological preferences, often growing close by in exposed habitats, for example at high altitudes.
Distribution. So far endemic to the tropical Andes.
Selected specimens examined. Bolivia: Cochabamba: Parque Nacional Carrasco, Sehuencas, alrededores del puente sobre río Fuertemayu, 17·496528°S, 65·272500°W, 2183 m, 2007, Truong 3124 (G). La Paz: Reserva Faunística Ulla Ulla, saliendo de Pelechuco, 14·818750°S, 69·063722°W, 3499 m, 2007, Truong 2898 (G).—Colombia: Antioquia: c. 10 km NW of Medellín, hilltop with transmission tower along the road to San Félix, 3000 m, 1986, Sipman et al. 34273 (B). Boyaca: Páramo de la Rusia, along road Charala-Duitama, 3500 m, 1988, Sipman & Aguirre 27679 (B). Cauca: along road La Plata-Puracé, páramo 2 km W of Laguna de San Rafael, 3300 m, 1984, Aguirre & Sipman 5985 (B). Cundinamarca: Laguna Seca in páramo Alto between Cogua and San Cayetano, 3450 m, 1975, Florschütz 4467 (B). Risaralda: Pereira, Nevado de Santa Isabel, vertiente NWW, 4150 m, 1980, Boekhout 159 (B).—Ecuador: Azuay: Cuenca, sendero hasta el cerro Cajas desde el puente de los Dos Chorreras, 2·740306ºS, 79·153889ºW, 3796 m, 2007, Truong 397(G). Imbabura: Reserva Ecológica Cotacachi-Cayapas, laguna Cuicotcha, Islote Verovi, 0·301194°N, 78·362750°W, 3068 m, 2007, Truong 3304 (G). Loja: Parque Nacional Podocarpus, sendero de las lagunas del Compadre, 4·115083°S, 79·165139°W, 3000 m, 2007, Truong 257 (G). Pichincha: Reserva de Vida Silvestre Pasochoa, sendero Los Pantzas, 0·443278°S, 78·494306°W, 3547 m, 2013, Truong 4197 (G). Tungurahua: Parque Nacional Llaganates, ruta desde Pelileo, a lo lado del río Talata, 1·081889°S, 78·410528°W, 3567 m, 2007, Truong 453 (G).—Peru: Cajamarca: Caretera 8 de Chachapoyas a Celendín, cerca del districto Las Palmas, 6·717972°S, 77·844833°W, 2940 m, 2007, Truong 1878 (G). Cusco: Centro de Investigación Wayqecha, Trocha Picaflor, 13·173972°S, 71·587944°ºW, 2892 m, 2007, Truong 2119 (G). Huanuco: Bosque de Carpish, 9·715306°S, 76·094167°W, 2675 m, 2007, Truong 2794 (G). Pasco: Parque Nacional Yanachaga-Chemillén, trocha hasta la ambra Esperanza, 10·540250°S, 75·356250°W, 2482 m, 2007, Truong 2495 (G).—Venezuela: Bolívar: Cerro Guaiquinima, near west end of upper plateau, 1500 m, 1990, Sipman 27246b (B). Mérida: vers la laguna de Los Patos, 3700 m, 1989, Grundlehner (G).
Usnea subglabrata Truong & P. Clerc sp. nov.
MycoBank No.: MB 811379
Thallus small erect-shrubby, branches inflated and constricted at ramification, low papillae often abundant, soralia strongly excavate without isidiomorphs, medulla loose with thin hyphae, reacting K+ yellow-orange (stictic acid).
Type: Bolivia, La Paz, ruta nueva hasta Coroico, 1 km después de Unduavi, 16·291639°S, 67·860556°W, 3132 m, bosque de neblina montano en transición con matorral, sobre ramas de árboles pequeños, 2007, Truong 2978 (LBP—holotype; G—isotype). %C/M/A: 5.0/31.5/27.0. Chemistry: usnic, stictic, constictic, menegazziaic, barbatic, traces of norstictic acids.
(Fig. 7)

Fig. 7 Usnea subglabrata. A, holotype (left) and isotype (right); B, branch distinctly inflated and constricted at ramification (Truong 304); C, trunk blackish on the first mm below first ramification (Truong 2978); D, papillae abundant on basal branches, giving a rugulose aspect to the cortex surface (Truong 304); E, section through branch (Truong 304); F & G, strongly excavate soralia becoming larger than the branch diameter (Truong 2409). Scales: A=1 cm; B–G=500 μm. In colour online.
Thallus erect-shrubby, usually of small size (1–3 cm); ramifications±anisotomic-dichotomous; trunk blackish on the first mm below first ramification (Fig. 7C), rarely brownish or concolorous; branches tapering to±irregular, with thick apices, slightly to strongly inflated on basal branches (Fig. 7B); branch segments slightly to strongly irregular or deformed on basal branches,±terete on terminal branches; lateral branches slightly to distinctly constricted at ramification at least on basal branches (Fig. 7B); foveoles often present on basal branches; maculae and pseudocyphellae absent; papillae irregularly to abundantly distributed on basal and secondary branches, hemispherical, giving a coarse and rugulose aspect to the cortex surface (Fig. 7D); tubercles absent; fibrils slender, scattered to irregularly distributed; fibercles absent or scarce; soralia arising on terminal branches from the cortex ad initio, circular, isolated and delimited with a thin cortical margin, strongly excavate at maturity, becoming larger than the branch diameter or encircling thin terminal branches and exposing the axis (Fig. 7F & G); isidiomorphs usually absent with granular soredia instead; cortex thin (3·5–5·5%), shiny to vitreous in section; medulla thick (32·5–39·0%), loose with thin hyphae (Fig 7E); axis thin (14·0–24·0%) with an A:M ratio<0·75.
Apothecia and pycnidia not seen.
Diagnostic notes. Usnea subglabrata is characterized by a small erect-shrubby thallus with basal branches inflated and constricted at ramification, and short terminal branches with thick apices, holding strongly excavate soralia, bearing granular soredia instead of isidiomorphs. The cortex is thin and shiny, with a rugose cortex surface irregularly covered by papillae. The medulla is loose with thin hyphae, reacting K+ yellow (stictic±barbatic acids), although the reaction is often faint and may take a few minutes to become visible.
Variation. The degree of inflation and constriction of branches at ramification may vary among individuals, as well as the number of papillae on the cortex surface. Basal part of the trunk may lack black pigmentation and appear brownish or concolorous. Isidiomorphs are usually absent, but may occur abundantly when the thallus is infected by parasitic fungi.
Differentiation. Usnea subaranea has a similar morphology of soralia and specimens with short and compact thalli may be difficult to distinguish from U. subglabrata. In U. subaranea the thallus is usually stiffer from the rigid cortex, with a typically greyish shade. Terminal branches tend to be thin and sinuous towards the apices, while they are short and thick in U. subglabrata. Soralia usually remain circular, rarely exceeding the branch diameter in size or torn apart as in U. subglabrata. The medulla is loose with thick-‘arachnoid’ hyphae, which are thin in U. subglabrata. Both species share one chemotype (stictic acid), but with different accompanying substances; barbatic acid in U. subglabrata and an unidentified yellowish brown depside in U. subaranea. In the temperate zone, U. glabrata (Ach.) Vain. has a very similar morphology, with a short and shrubby thallus, inflated branches and strongly excavate soralia. It differs mainly in the smooth cortex surface without apparent papillae. The medulla of U. glabrata usually reacts K− (protocetraric acid), but the chemotypes of salazinic or stictic acids have also been detected, as in U. subglabrata (Herrera-Campos et al. Reference Herrera-Campos, Nash and Garcia2001; Clerc Reference Clerc2011). Our phylogenetic study revealed that these two taxa belong to distinct lineages within the Usnea clade–3 (black triangles in Fig. 2).
Ecology. Relatively open sites within primary or disturbed mountain cloud forests to the upper tree limit (Ceja andina), highlands of puna and páramo grasslands (i.e. shrubby páramo, páramo de pajonal, Polylepis stands or wooded ravines in páramo), Pinus and Eucalyptus plantations, pastures with forest relics or deforested zones of matorral in the vicinity of forest; primarily corticolous on various trees and shrubs, rarely saxicolous or lignicolous on fence posts. This is a small but often abundant species, with a wide ecological and altitudinal range.
Distribution. So far endemic to continental South America.
Selected specimens examined. Bolivia: Cochabamba: Cerca de Corani, alrededores de la laguna, 17·227278°S, 65·889528°W, 3173 m, 2007, Truong 587 (G). La Paz: Parque Nacional Cotapata, ruta antigua hasta Coroico, 16·288194°S, 67·808417°W, 2664 m, 2007, Truong 3907 (G).—Brazil: Rio de Janeiro: Parque Nacional do Itatiáia, km 11–12 on Planalto road to Agulhas Negras, 22·383°S, 44·683°W, 2400 m, 1993, Marcelli et al. 25155 (H).—Colombia: Antioquia: Munic. Guarne, Piedras Blancas, along the road to Santa Helena, 2300 m, 1986, Sipman et al. 33946 (B). Boyaca: Munic. Cucaita, c. 10 km W of Tunja along road to Villa de Leíva, 2950 m, 1986, Sipman & Reyes 34467 (B). Cundinamarca: Mosquera, Alto de Mondoñedo, 4·675833°N, 74·289333°W 2903 m, 1998, Pinzón & Linares 912 (B). Huila: Munic. La Plata, Vereda La Candelaria, volcán Merenberg, 2600 m, 1984, Aguirre & Sipman 5842 (B). Nariño: Munic. Piedrancha, along road from San Isidro to Reserva Natural La Planada, 1700 m, 1986, Sipman et al. 33482 (B).—Ecuador: Imbabura: Reserva Ecológica Cotacachi-Cayapas, laguna Cuicotcha, islote Veroti, 0·301194°N, 78·362750°W, 3068 m, Truong 3296 (G). Loja: Loja, antena militar arriba de San Lucas, 3·679361°S, 79·237889°W, 3217 m, 2007, Truong 222 (G). Pichincha: Quito, comunidad de Llano Grande, 2585 m, 2007, Araguillin (G). Tungurahua: Parque Nacional Llaganates, ruta desde Pelileo, cerca de la entrada, 1·082167°S, 78·411861°W, 3498 m, 2007, Truong 470 (G).—Peru: Cajamarca: Parque Nacional Cutervo, cerro Tarros, 6·295083°S, 78·796111°W, 3207 m, 2007, Truong 1721 (G). Cusco: Santuario Histórico de Machu Picchu, km 82 cerca de Wayllabamba-bajo, 13·242444°S, 72·428083°W, 2700 m, 2007, Truong 1919 (G). Huanuco: Bosque de Carpish, 9·698917°S, 76·086472°W, 2473 m, 2007, Truong 2765 (G).—Venezuela: Bolívar: Auyan Tepui, 1996, Schultz 24026b (private hb.). Mérida: Potreros de San Rafael, páramo de Las Coloradas, 2950 m, 1976, Hale 46782 (US).
New Records
Usnea dorogawensis Asahina
Journal of Japanese Botany 28: 228 (1953); type: Japan, Honshu, Yamato Prov., Dorogawa, Amakawa-mura, Yoshino-gun, 1952, Togashi (TNS!—lectotype). % C/M/A: 4.0/37.5/16.0. Chemistry: usnic, lobaric, norstictic, stictic and constictic acids, unidentified US6 (Ohmura Reference Ohmura2001).
Detailed descriptions and illustrations are available in Ohmura (Reference Ohmura2001) and Truong et al. (Reference Truong, Bungartz and Clerc2011). This species is characterized by a short erect-shrubby thallus, branches inflated and constricted at ramification, large and excavate soralia without isidiomorphs, a smooth cortex surface without papillae, and a loose medulla with orange subcortical pigmentation. Lobaric acid was detected in the medulla of all specimens, together with norstictic acid (major) in the Galapagos, or with stictic (major) and norstictic (traces) acids in the tropical Andes.
Distribution. Newly reported for continental South America in the tropical Andes, occurring in the upper part of the montane cloud forest (Ceja andina) or in deforested zones of matorral in the vicinity of forest; previously reported from Japan (Ohmura Reference Ohmura2001) and the Galapagos (Truong et al. Reference Truong, Bungartz and Clerc2011). This species has been rarely reported but could be overlooked in the field.
Selected specimens examined. Colombia: Antioquia: Piedras Blancas, c. 10 km S of Medellín, along the road to Santa Helena, 2300 m, 1986, Sipman et al. 33945 (B).—Peru: Huanuco: Bosque de Carpish, 9·703333°S, 76·088944°W, 2557 m, 2007, Truong 3913 (G).
Usnea grandisora Truong & P. Clerc
Bryologist 114: 490 (2011); type: Ecuador, Galapagos, Santa Cruz, above Mina Granillo, 607 m, Truong 1122 (CDS!—holotype; G! UPS!—isotypes). % C/M/A: 11.0/20.5/38.0. Chemistry: usnic, salazinic, galbinic and norstictic acids (Truong et al. Reference Truong, Bungartz and Clerc2011).
Detailed description and illustrations are available in Truong et al. (Reference Truong, Bungartz and Clerc2011). This species is characterized by an erect-shrubby thallus, branches irregularly covered by fibrils and isidiofibrils, large and excavate soralia crowded on terminal branches, a relatively thick and shiny cortex in section, and a dense medulla with orange subcortical pigmentation.
Distribution. Newly reported for continental South America in Venezuela, where it is possibly rare and overlooked; previously reported from the Galapagos (Truong et al. Reference Truong, Bungartz and Clerc2011).
Selected specimen examined. Venezuela: Mérida: La Carbonera, El Pedregal, 1950 m, 1989, Grundlehner 51281E (G).
Usnea moreliana Motyka
Lich. Gen. Usnea Stud. Monogr. Pars Syst 2: 584 (1938); type: Mexico, Morelia, Cerro San Miguel, 1910, Brouard 137 (LBL!—lectotype and isolectotype selected here). % C/M/A: 6.0/32.0/24.5. Chemistry: usnic acid and unidentified triterpenoids UT6.
Usnea rubricornuta Truong & P. Clerc syn. nov., Bryologist 114: 494 (2011); type: Bolivia, Cochabamba, Parque Nacional Carrasco, Sehuencas, ruta hasta Monte Puncu, 2500 m, 2007, Truong 3132 (LPB!—holotype; G! UPS!—isotypes). %C/M/A: 8.0/32.0/20.5. Chemistry: usnic acid and unidentified triterpenoids UT6 (Truong et al. Reference Truong, Bungartz and Clerc2011).
(Fig. 8)
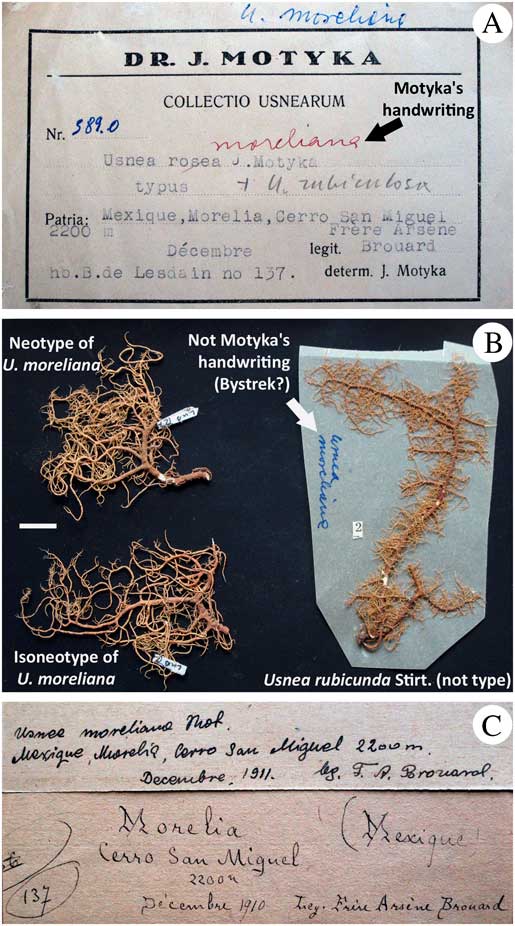
Fig. 8 Lectotypification of U. moreliana based on a paratype specimen found in LBL. A, identification of U. moreliana by Motyka; B, three specimens inside the packet, two of them corresponding to U. moreliana and one corresponding to U. rubicunda (right); C, original label. Scale=1 cm. In colour online.
Lectotypification. Motyka (Reference Motyka1938) indicated that the holotype of U. moreliana was deposited in B, but this specimen appears to be missing. In LBL, we found a paratype of U. moreliana that was cited in Motyka’s description. This paratype included heterogeneous material from three specimens (Fig. 8B): one specimen corresponded to U. rubicunda Stirt., with the reddish cortex and the compact medulla reacting K+ red (salazinic and norstictic acids), with an A:M ratio>2·0. The two other specimens represented fragments of thalli lacking trunk, that we identified as U. rubricornuta from the reddish cortex and the dense medulla reacting K− (unidentified triterpenoids UT6), with a ratio A:M<2·0 (see in Truong et al. Reference Truong, Bungartz and Clerc2011 for a detailed description of this species). These two specimens have almost non-inflated and non-constricted branches, characters that appear to be variable in this species (Truong et al. Reference Truong, Bungartz and Clerc2011). Motyka’s description mentioned the reddish cortex of U. moreliana, but for unknown reasons he didn’t place this species in subsection Rubigineae of his monograph, together with other species with a reddish cortex. Because of this, the name was overlooked when describing U. rubricornuta and it was only when we discovered the paratype of U. moreliana in LBL that we realized it corresponded to the same taxon. These two specimens fit perfectly with Motyka’s description that mentions the reddish cortex, branches slightly constricted at ramification, the minute soralia and the dense medulla reacting K−, corresponding to the presence of triterpenoids. We therefore selected one of the two individuals (LUB127) as the lectotype of U. moreliana and the second fragment (LUB128) was considered as an isolectotype.
Distribution. Newly reported here for eastern South America in Brazil and Argentina, in relatively open sites within primary forest or in Pinus plantations; previously reported from Mexico and the tropical Andes (Motyka Reference Motyka1936; Truong et al. Reference Truong, Bungartz and Clerc2011). It is a common and often abundant species in tropical South America.
Selected specimens examined. Argentina: Misiones: San Ignacio, Gisela, 50 m, 1953, Montes (LBL).—Brazil: Parana: Res. Biológica de Sapitanduva, 50 m, 1991, Hatschbach 55396B (B). São Paulo: Serra de Paranapiacaba, 80 km südwestlich von São Paulo, am rio Juquiá, 700 m, 1978, Kalb & Plöbst (G); Serra da Mantiqueira, Campos do Jordão, 150 km nordöstlich von São Paulo, 1700 m, 1978, Kalb & Plöbst (G); zwischen Guapira und Apiaí, 800 m, 1980, Kalb (G).
Usnea subcornuta Stirt.
Scott. Nat. 6: 107 (1881); type: Portugal, Madeira, near Funchal, Payne (BM!—holotype). % C/M/A: 8.5/23.0/37.0. Chemistry: usnic acid and unknown yellow substances (Clerc Reference Clerc1987b ).
Detailed description and illustrations are available in Truong et al. (Reference Truong, Bungartz and Clerc2011). This species is characterized by an erect-shrubby thallus, branches inflated and constricted at ramification, slender fibrils scattered on branches, minute soralia aggregating in irregular clusters on terminal branches, and a dense medulla with orange subcortical pigmentation. Recent studies demonstrated that specimens identified as U. subcornuta were clearly polyphyletic in the phylogeny of Usnea (Truong et al. Reference Truong, Divakar, Yahr, Crespo and Clerc2013a ). Morphological delimitation will need careful re-examination in the light of molecular data, but the paucity of material available makes it a difficult task.
Distribution. Newly reported here for eastern South America in Venezuela and Brazil; previously reported from south-western Europe, North Africa, Western Asia (Clerc Reference Clerc1987b ; Fos & Clerc Reference Fos and Clerc2000) and the tropical Andes (Truong et al. Reference Truong, Bungartz and Clerc2011). It is a rare but possibly overlooked species with a scattered distribution.
Selected specimens examined. Brazil: São Paulo: Ilha de São Sebastião, 130 km östlich von São Paulo, 780 m, 1978, Kalb & Plöbst (G).—Venezuela: Bolívar: Auyan Tepui, 1996, Schultz 24026a (private hb.).
Usnea subdasaea Truong & P. Clerc
Bryologist 114: 499 (2011); type: Ecuador, Galapagos, Isabela, road to Sierra Negra crater, close to la Esperanza, 306 m, Truong 1194 (CDS!—holotype; G! UPS!—isotypes). %C/M/A: 5.0/30.0/30.0. Chemistry: usnic, salazinic, galbinic and norstictic acids (Truong et al. Reference Truong, Bungartz and Clerc2011).
Detailed description and illustrations are available in Truong et al. (Reference Truong, Bungartz and Clerc2011). This species is characterized by an erect-shrubby thallus, irregular branches±inflated and constricted at ramification, spinulous fibrils and isidiofibrils irregularly distributed on branches, minute soralia aggregating in irregular clusters on terminal branches, a thin and shiny cortex in section, and a dense medulla with orange subcortical pigmentation.
Distribution. Newly reported here for Venezuela and Brazil; it is a South American endemic previously reported from the tropical Andes and the Galapagos (Truong et al. Reference Truong, Bungartz and Clerc2011).
Selected specimens examined. Brazil: Minas Gerais: Munic. de Catas Altas, Parque natural do Caraça, Tanque Grande, 20·102806°S, 43·494000°W, 1275 m, 2006, Spielmann et al. 2358 (SP).—Venezuela: Mérida: La Carbonera, El Pedregal, 1950 m, 1989, Grundlehner 73281F (G).
Fieldwork was supported by the Conservatoire et Jardin botaniques de la Ville de Genève (G), an ‘Augustin Lombard’ grant from the Société de Physique et d’Histoire Naturelle de Genève and a grant from the Amazon Conservation Association. The authors collaborated with the herbaria CDS, LPB, QCNE, USM and the Wayqecha Biological Station (Peru). F. Bungartz, M. Herrera-Campos, C. Aldana Munguia, M. F. Collaguazo, A. Cuba Villena, Y. Manami, A. Ramírez-Ordaya, P. Rodriguez, L. Salcedo Valdez and A. X. Shuguli participated in the field work. Curators of the cited herbaria kindly sent specimens on loan. Laboratory work was supported by a grant from the Basler Stiftung für Biologische Forschung and was carried out in G and in the Facultad de Farmacia of the Universidad Complutense of Madrid. Sequences generated in Madrid were supported by the Spanish Ministerio de Ciencia e Innovación funds (CGL2010-21646) and a Ramón y Cajal start-up grant (RYC02007-01576). P. K. Divakar supervised laboratory work in Madrid and gave valuable advice on the analysis of data.













