Introduction
As a major method for ex situ conservation, storage of orthodox seed has commonly been adopted for the preservation of plant germplasm (Mira et al., Reference Mira, Estrelles and González-Benito2014). The improved orthodox seed viability equation demonstrated that considerable desiccation contribute to seed survival (Ellis and Roberts, Reference Ellis and Roberts1980). However, excessive dehydration may decrease seed vigour. It has been confirmed that ultra-dry storage, storing orthodox seeds at ambient temperature with moisture content <5%, has an optimum moisture content (Daniel, Reference Daniel2007). Storing seed beyond this limit provided no additional benefit to longevity or actually enhanced ageing rates (Vertucci et al., Reference Vertucci, Roos and Crane1994; Ellis and Hong, Reference Ellis and Hong2006). The values of optimum moisture content substantially vary among species for their variable degree of desiccation tolerance (Vertucci et al., Reference Vertucci, Roos and Crane1994; Ellis and Hong, Reference Ellis and Hong2006). What lead to the variation in optimum moisture content among different orthodox seeds?
In orthodox seeds, desiccation tolerance is a quantitative feature associated with the accumulation of reserves and various protective compounds through the seed-filling phase (Angelovici et al., Reference Angelovici, Galili, Fernie and Fait2010). Severe dehydration could destabilize protein and membrane structures and cause oxidative damage by overproduction of reactive oxygen species (Farrant and Moore, Reference Farrant and Moore2011; Sonia et al., Reference Sonia, Patricia, Joaquima, Eric, María and Blanca2014). Damage associated with the macromolecules is believed to be prevented by replacing water with solutes, which could substitute for the hydrogen bonds lost owing to dehydration (Hoekstra et al., Reference Hoekstra, Golovina and Buitink2001). These solutes include: (1) non-reducing sugars, disaccharides such as sucrose, and oligosaccharides such as raffinose and stachyose; (2) specific proteins, for example, the late embryogenesis abundant proteins and small heat-shock proteins (Hoekstra et al., Reference Hoekstra, Golovina and Buitink2001; Farrant and Moore, Reference Farrant and Moore2011). The desiccation-induced oxidative damage is considered to be prevented by the presence of antioxidants. In the dry state, only molecular antioxidants (e.g. glutathione, ascorbate and tocopherols) and amphiphilc antioxidants (e.g. arbutin and flavonoids) can alleviate oxidative damage caused by free radicals (Bailly, Reference Bailly2004; Petra et al., Reference Petra, Susanne, Angelika, Monika and Éva2014).
To contribute to the understanding of desiccation tolerance mechanisms and the variation in optimum moisture content among different orthodox seed lots, the role of protective substances and their impacts on desiccation tolerance needs to be analysed. So far, variations in optimum moisture content are assumed to be ascribed to differences in seed lipids (Zhang et al., Reference Zhang, Zhuo, Wang, Wu and Wang2010). Whether other reserves and protective substances contribute to the variation in the optimum moisture content of ultra-dry storage among species is not clear.
In the present study, 11 seed lots from six crops (Chinese cabbage, peanut, cucumber, pepper, maize and wheat) were chosen for their different lipids content. Previous results indicated that seeds of the six crops are suitable for ultra-dry storage. We determined: (1) the amounts of different reserves, putative protective substances and the free-radical scavenging activity (FRSA) in the 11 seed samples; (2) the optimum moisture content of ultra-dry stored whole seeds and non-lipid fraction in different seed lots; and (3) the relations between seed chemical compositions, including reserves and protective substances, the FRSA and the optimum moisture content of ultra-dry storage and non-lipid fraction among 11 crop seed samples, and hope to discover what protective substances, which act synergistically to determine the variation in optimum moisture content of ultra-dry storage among different crop seeds.
Materials and methods
Materials
The study was conducted with seeds of 11 seed lots from six crops: oilseed groundnut (Arachis hypogaea L. cv. Luhua 11 and cv. Huayu 22), cucumber (Cucumis sativus L. cv. Tang Shan Qiu Gua and cv. Lao Lai Shao), Chinese cabbage (Brassia pekinensis L. cv. LaiNong 50 and cv. Xiao Za 56), pepper (Capsicum annuum L. cv. Guan Jun Jiao and ChiFeng 1), and typical starchy seeds, maize (Zea mays L. cv. Zhengdan 958) and wheat (Triticum aestivum L.cv. Jimai 22 and cv. Lumai 21). All seeds were harvested from June to October 2013 and stored at −20°C before the experiments conducted in November 2013. The initial moisture content and germination percentage of the 11 seed lots were listed in Table 1. Part of the seeds was used to determine the reserves, putative protective substances and the FRSA. The other part of the seeds was used to determine the optimum moisture content of ultra-dry storage. All seeds were stored at −20°C before use.
Table 1. The initial seed moisture content and germination percentage, and the OMC for ultra-dry storage of different seeds lots stored at 65°C for a month, together with the equilibrium moisture of 13 and 23% RH of different seed lots at 20°C

IMC, initial moisture content; IGP, initial germination percentage; OMC, optimum moisture content. The values of IMC, 13 and 23% RH are the means of three replicates. The values of IGP are mean of four replicates.
The value after the ± is SD.
The moisture content corresponding to the maximum germination percentage in the curves of deterioration was regarded as the optimum moisture content of ultra-dry storage.
Determine the optimum moisture content of ultra-dry storage among different seed lots
To achieve ultra-dry seeds with different moisture contents, the seeds were packaged in nylon-bag and buried in silica gel (silica gel: seed = 10:1) at 20°C for a month. The silica gel was replaced and regenerated at 120°C daily. The weight of seeds at the required moisture content was calculated by using the following formula:
 $$\eqalign{{\rm Final}\;{\rm seed}\;{\rm weight} &= {\rm Initial}\;{\rm seed}\;{\rm weight} \cr & {\rm \times} \displaystyle{{{\rm (100} - {\rm Initial}\;{\rm \%} \;{\rm moisture}\;{\rm content)}} \over {{\rm (100} - {\rm Final}\;{\rm \%} \;{\rm moisture}\;{\rm contet)}}}}.$$
$$\eqalign{{\rm Final}\;{\rm seed}\;{\rm weight} &= {\rm Initial}\;{\rm seed}\;{\rm weight} \cr & {\rm \times} \displaystyle{{{\rm (100} - {\rm Initial}\;{\rm \%} \;{\rm moisture}\;{\rm content)}} \over {{\rm (100} - {\rm Final}\;{\rm \%} \;{\rm moisture}\;{\rm contet)}}}}.$$
During the drying period, seed samples were weighed regularly until their weight reached the calculated value. Seed moisture contents were determined by the low- (103 ± 2°C, 17 ± 1 h, groundnut, Chinese cabbage, pepper) and high-constant-temperature (130–133°C, maize 4 h, wheat 2 h, cucumber 1 h) oven methods described by the International Seed Testing Rules (ISTA, 2007) and calculated on a dry weight basis. The ultra-dried seeds were sealed in double-layer aluminium foil packages with air excluded as much as possible, and accelerated ageing at 65°C for 1 month in an oven (Ellis and Hong, Reference Ellis and Hong2006). Then the seed germination percentage was measured. To avoid imbibition damage, immediately before the germination tests, all seeds were humidified above saturated CaCl2 solutions [relative humidity (RH) 35%], then saturated NH4Cl solutions (RH 75%) and pure water (RH 100%) water at 20°C, respectively. Each step lasted 24 h (Ellis and Hong, Reference Ellis and Hong2006). Germination tests were carried out on top of moistened filter papers in plastic boxes 12 × 12 × 6 cm3 (pepper, cucumber, Chinese cabbage and wheat) with four replicates of 100 seeds each, or in sand in plastic boxes 13 × 19 × 12 cm3 (maize and groundnut) with four replicates of 50 seeds each. Environments of germination test and standard of seedling evaluation met the requirements of the International Seed Testing Rules (ISTA, 2007). To determine the optimum moisture content of ultra-dry storage, the quadratic polynomial models were established based on analysing the germination percentage of ultra-dry storage seeds with different moisture contents. The moisture content corresponding to the maximum germination percentage in the curves of deterioration was regarded as the optimum moisture content of ultra-dry storage. Seed moisture contents in equilibrium with about 13 and 23% RH were also determined by equilibrated over saturated LiCl solution and saturated CH3COOK solution, respectively, for 1 month.
Determine the amounts of putative protective substances in seeds
The amounts of reserves (starch, total protein and lipids) and some putative protective substances (non-reducing sugar, sucrose, raffinose, stachyose, tocopherol, ascorbic acid, flavonoids and arbutin) were measured by the PONY Test Company located in Beijing China (http://www.ponytest.com/). The contents of heat-stable protein and glutathione were analysed in Qingdao Agricultural University.
The starch content of seed was determined by enzymatic methods as described in AOAC (2000). Soluble sugars were extracted according to the procedures described by Black et al. (Reference Black, Corbineau, Grzesik, Guy and Côme1996) and the amount of non-reducing sugar, sucrose, raffinose and stachyose were analysed by high-performance liquid chromatography (HPLC) (Koster and Leopold, Reference Koster and Leopold1988).
Lipids content was determined by the Soxhlet extraction method (Horwitz, Reference Horwitz1975). The crude protein was determined by the micro Kjeldahl method described in AOAC (2000). Ascorbic acid was determined by titromateric method as described by Mazumdar and Majumder (Reference Mazumdar and Majumder2003). The HPLC method was used for quantitative analysis of arbutin (Thongchai et al., Reference Thongchai and Liawruangrath2007). Total flavonoids were determined following the method of Ordonez et al. (Reference Ordonez, Gomez, Vattuone and Isla2006). Tocopherol extraction, separation by HPLC and quantification were conducted following the procedures described by Goffman et al. (Reference Goffman, Velasco and Thies1999).
Soluble proteins were extracted following the procedures described by Farrant et al. (Reference Farrant, Berjak and Pammenter1992). Five hundred milligram seeds were ground in liquid nitrogen and the powders were incubated in hexane (1:10 w/v) at −20°C for 24 h and centrifuged (20,000 g at 4°C for 20 min) to remove fat. The fat-free powders were homogenized at 4°C in 2 ml extraction buffer containing 50 mM Tris–HCl, pH 8.0, 0.5 M NaCl, 10 mM β-mercaptoethanol and 1 mM phenylmethylsulphonyl fluoride and 10 mM leupeptinas protease inhibitors and then centrifuged (10,000 rpm at 4°C for 20 min) to obtain soluble proteins. To obtain heat-stable proteins, the supernatant was boiled for 10 min, cooled on ice for 15 min and centrifuged as described above. Protein concentration was measured according to Bradford (Reference Bradford1976).
Glutathione was determined by Glutathione Colorimetric Detection Kit (Jiancheng Co, Nanjing, China), which was based on Ellman's assay.
The FRSA of the seeds was determined by the method described by Blois (Reference Blois1958), based on the scavenging activity of the stable 1,1-diphenyl-2- picrylhydrazyl (DPPH) free radical. The powered seeds (0.5 g) was extracted with 5 ml 80% (v/v) methanol in a sealed bottle at 25°C for 5 h with occasional mixing, and then centrifuged at 5000 rpm for 15 min. Samples were diluted ten times. For each sample, 0.1 ml of the diluted seed extracts was mixed with 2.9 ml of a 0.1 mM DPPH solution in 80% (v/v) methanol. The mixture was incubated in the dark at 25°C for 20 min and the absorbance was measured at 515 nm. Methanol of 80% (v/v) was used to zero the spectrometer. The control was carried out with 80% (v/v) methanol instead of the tested sample, and methanol instead of DPPH was used as blank. The FRSA of the tested sample was calculated using the following formula:
Determine the relations between seed content, antioxidant activity and the optimum moisture content of ultra-dry storage
To determine the relationship between the seed chemical contents, the FRSA and the optimum moisture content, multiple stepwise regression analysis were conducted with SPSS software, using a significance level of 0.05. In the regression analysis, the difference in optimum moisture content was taken as the dependent variable and the difference in chemical substances and the FRSA were taken as independent variables. Variables were assumed to have normal distributions and germination percentages arcsine transformed prior to statistical analysis.
Results
Seeds of 11 varieties from six species were used in the present study and represent a wide range of species different in chemical contents. The chemical composition of the 11 seed lots are presented in Table 2. The composition of reserves (starch, protein and lipids) differed dramatically among different crop species. Maize and wheat are considered as typical starchy seed. They contained high level of starch (>600 g/kg) and low level of lipid and protein content. Maize cv. Zhengdan 958 has higher lipid content (46.0 g/kg) than that of wheat cv. Jimai 22 and cv. Lumai 21 (24.9 and 26.1 g/kg). As a typical oily seed, peanut cv. Luhua 11 and cv. Huayu 22 had the highest lipid content (501.5 and 492.4 g/kg) among the test samples and higher protein content (244.3 and 239.8 g/kg) than pepper cv. ChiFeng 1 and cv. Guan Jun Jiao (178.1 and 187.8 g/kg), wheat cv. Jimai 22 and cv. Lumai 21 (170.8 and 159.1 g/kg) and maize cv. Zhengdan 958 (189.7 g/kg). Chinese cabbage (cv. LaiNong 50 and cv. Xiao Za 56) and cucumber (cv. Tang Shan Qiu Gua and cv. Lao Lai Shao) had higher protein and lipid, thus had lower starch content. The pepper varieties cv. ChiFeng 1 and cv. Guan Jun Jiao had 182.5 and 183.4 g/kg starch, 178.1 and 187.8 g/kg protein and 217.2 and 138.1 g/kg lipid, respectively. The amounts of other putative protective chemicals [heat-stable protein (1.84–35.39 g/kg), GSH (0.02–0.49 g/kg), non-reducing sugar (13.21–53.87 g/kg), sucrose (3.60–50.90 g/kg), stachyose (3.86–48.55 g/kg), raffinose (0–5.30 g/kg), tocophernols (0.07–2.49 g/kg), ascorbic acid (0.01–0.09 g/kg), flavonoids (0.04–1.06 g/kg), arbutin (1.37–9.45 g/kg) and FRSA (2.17–49.39)] varied widely among species and even varieties.
Table 2. The amounts of protective substances, expressed as g/kg dry weight, and the free radical scavenging activity in different seed lots

GSH, Glutathione; FRSA, free-radical scavenging activity; The values are the means of three replicates.
The value after the ± is SD.
The curves of seed deterioration demonstrated that there was optimum moisture content for ultra-dry seed. The rate of seed deterioration increased when the storage moisture content was greater than or less than the optimum moisture content (Fig. 1). The value of the optimum moisture content of ultra-dry storage calculated from the first derivation of a fitted quadratic equation from different seed samples were listed in Table 2, together with the equilibrium moisture content of 13 and 23%RH at 20°C of different seed lots. Among the 11 seed lots, the starchy seeds (maize and wheat) possessed the highest optimum moisture contents of ultra-dry storage, and the oily seed (peanut) possessed the lowest. The same results were obtained from the equilibrium moisture content of 13 and 23% RH at 20°C.
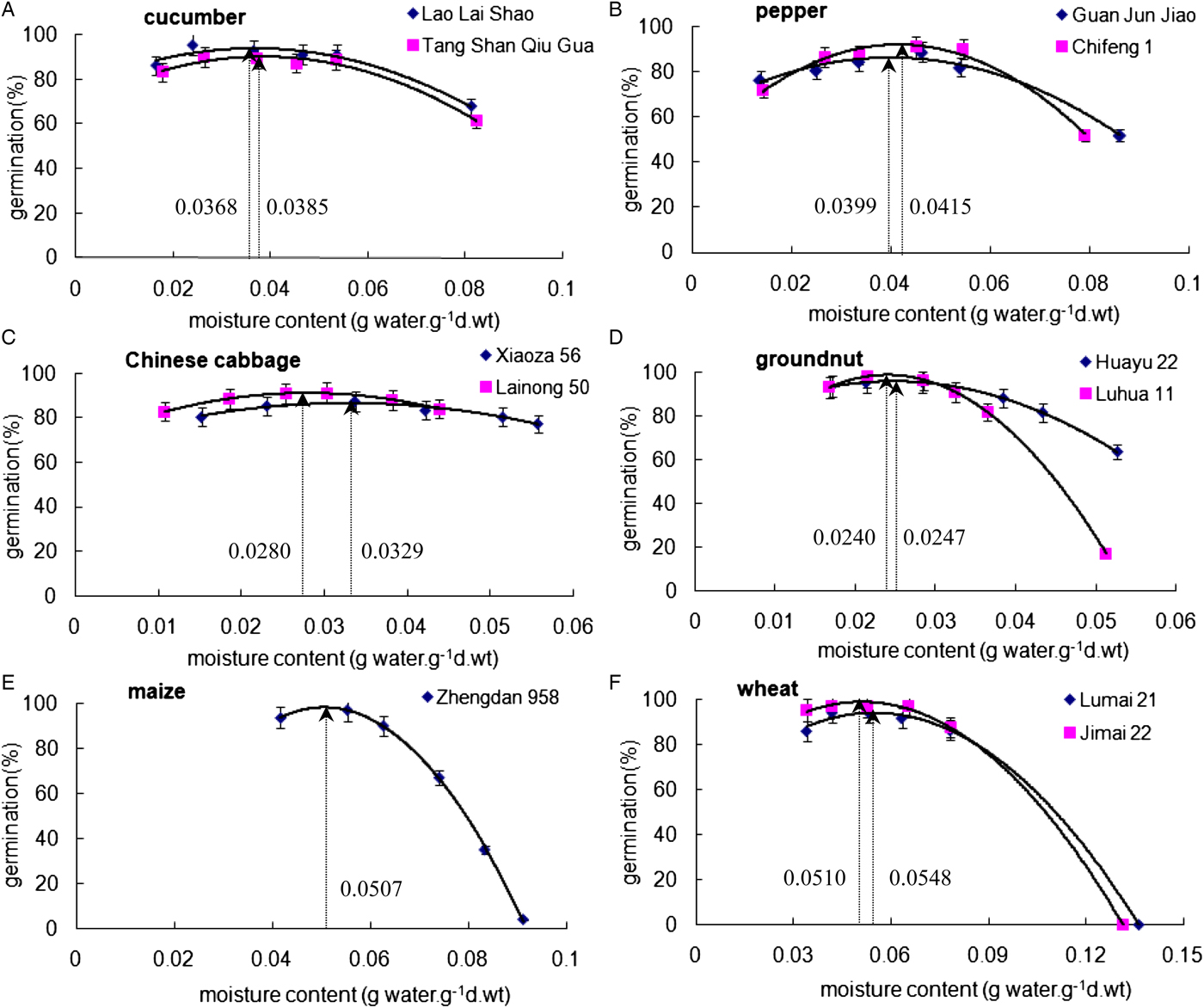
Fig. 1. Germination percentage of ultra-dry seeds of different seed lots and different initial moisture contents stored at 65°C for 1 month. Symbols represent mean germination percentages ± SD. Curves represent the best-fitting second-order polynomial equation. Arrows point to the optimum moisture content as determined from the maxima of the quadratic equation. Numbers indicate the optimum moisture content for each genotype.
Multiple linear regression analysis indicated that three (lipids, flavonoids and the FRSA) of the 14 independent variables were significantly correlated with the optimum moisture content of ultra-dry stored whole seeds (Table 3). They accounted for 97.7% of the total variation (Table 4). In order of contribution, lipids account for 90.0%, the FRSA and flavonoids accounted for 4.8 and 2.9% of total variation, respectively (Table 4). Multiple linear regression analysis also indicated that the FRSA and flavonoids were significantly negatively correlated with the optimum moisture content for non-lipid fraction of ultra-dry stored seeds (Table 5), and accounted for 74.1 and 15.3% of the total variation, respectively (Table 6).
Table 3. The best prediction model in predicting optimum moisture content of ultra-dry stored whole seeds based on the relationship between the optimum moisture content and different substances analysed by multiple stepwise linear regression analysis

GSH, Glutathione; FRSA, free-radical scavenging activity, SE, standard error.
Table 4. Relative contribution (partial and model R2) in predicting optimum moisture content of ultra-dry stored whole seeds by the stepwise regression analysis

FRSA, free-radical scavenging activity; SE, standard error.
a,bLetters denote P ≤ 0.05.
Table 5. The best prediction model in predicting optimum moisture content of non-lipid fraction ultra-dry stored seeds based on the relationship between the optimum moisture content of non-lipid fraction and different substances analysed by multiple stepwise linear regression analysis

FRSA, free-radical scavenging activity, SE, standard error.
Table 6. Relative contribution (partial and model R2) in predicting optimum moisture content of non-lipid fraction of ultra-dry stored seeds by the stepwise regression analysis

FRSA, free-radical scavenging activity, SE, standard error.
a,bLetters denote P ≤ 0.05.
Discussion
Orthodox seeds can survive desiccation tolerance to very low moisture contents, but there is an optimum moisture content for ultra-dry storage (Ellis et al., Reference Ellis, Hong and Roberts1988). Although the optimum moisture content varied greatly among species, they were all very similar, in terms of equilibrium RH or water activity. The value of the optimum moisture content is a matter of some debate. According to Ellis et al. (Reference Ellis, Hong and Roberts1989), maximum longevity is acquired when desiccation takes places at 10–13% RH at 20°C. For other authors, the value should be to 15–20% RH (Walters-Vertucci et al., Reference Walters-Vertucci, Crane and Vance1996). The debate results from temperature sensitivity of the optimum moisture content (Li et al., Reference Li, Qu, Yang and An2008). The researches of Ellis’ were carried out at 65°C, while others’ researches were carried out at 50°C. To determine the optimal moisture content of ultra-dry storage, the present study was carried out at 65°C, and the result indicated that the optimum moisture content of the 11 seed lots from six species (maize, wheat, pepper, cucumber, peanut and Chinese cabbage) were lower than 13% RH at 20°C (23% RH at 65°C), which is in agreement with Ellis et al. (Reference Ellis, Hong and Roberts1989, Reference Ellis, Hong and Roberts1990a, Reference Ellis, Hong, Roberts and Taob, Reference Farrant, Berjak and Pammenter1992) and Ellis and Hong (Reference Ellis and Hong2006).
The optimal seed moisture content is in agreement with 13% equilibrium moisture content at 20°C for six samples (Chinese cabbage, peanut and cucumber) out of the 11 samples but not for the pepper, maize and wheat samples. The difference in lipid content determination method may account for the deviation. In previous studies on the relationship between lipid content and the optimal moisture content of ultra-dry storage, the lipid content was extracted and quantified from the isolated embryos. In the present study, the lipid content was extracted and quantified from the whole seeds. Differences between the lipid content in the embryo and the whole seed may result in the deviation of the theoretical moisture content calculated by the oil content.
It is difficult to test the deterioration of ultra-dry seed at ambient temperatures, and we used high temperature (65°C ) to accelerate ageing in the present study (Ellis et al., Reference Ellis, Hong and Roberts1989, Reference Ellis, Hong and Roberts1990a, Reference Ellis, Hong, Roberts and Taob). Does present results are of considerable practical relevance to seed storage for genetic conservation, which are recommended to be stored at −20°C? Lipid auto-oxidation related to water activity has been regarded as a major cause for deterioration of stored seeds (Hendry, Reference Hendry1993). According to the theory of Clausius–Clapeyron, in hermetic storage the water activity for a given water content will be greater at higher temperatures, which means the optimal water activity moisture content for storage at −20°C might be higher than that for storage at 65°C. Previous researches also indicated that there is a negative logarithmic relationship between the optimum moisture content and storage temperature, the lower the temperature the higher the optimum moisture content of the ultra-dry stored seeds (Vertucci et al., Reference Vertucci, Roos and Crane1994; Ellis and Hong, Reference Ellis and Hong2006). Seed deterioration will accelerate when ultra-dry seeds stored at −20°C with sub-optimal moisture and water activity. However, it was found that the water remaining within seeds below the optimum moisture content has very little chemical potential and seed longevity is less sensitivity to temperature when seed moisture content below optimum moisture content (Roberts and Ellis, Reference Roberts and Ellis1989). When stored at such low temperature (−20°C), the increase in deterioration of ultra-dry seed with sub-optimal moisture and water activity would be minor, even not be quantified. Hence, the optimum moisture contents of ultra-dry seeds stored at −20°C have broader range and include the optimum moisture contents when they stored at 65°C.
McDonald (Reference McDonald2007) pointed out that seed reserves can prevent loss of tightly bound water necessary with sorption sites. Storage compounds such as proteins, carbohydrates and lipids have different hydration properties. Proteins possess both negative and positive charges and are ideal for hydrogen bonding with water. Starch only has hydroxyl groups for hydrogen bonding with water. Lipid has no hydrogen bonding and has no attraction for water. Thus, more water is attracted to proteins, then starch, and finally little to oil. Consequently, the chemical composition of seeds affects its water-holding capacity. The examples in Table 2 show that starchy seeds (maize and wheat) achieve a higher equilibrium moisture content value at the same RH than oily seed (peanut). When seed water activity decreases below the monolayer moisture content, lipid oxidation occurs rapidly (Labuza et al., Reference Labuza, Tannenabum and Karel1970). The present result indicated the optimum moisture content was significantly negatively correlated with seed lipid content and lipid had the highest effect on the optimum moisture content of ultra-dry storage, which agreed with previous findings (Ellis et al., Reference Ellis, Hong and Roberts1989, Reference Ellis, Hong and Roberts1990a, Reference Ellis, Hong, Roberts and Taob; Walters-Vertucci et al., Reference Walters-Vertucci, Crane and Vance1996; Zhang et al., Reference Zhang, Zhuo, Wang, Wu and Wang2010).
Under severe water stress conditions, disruption of electron transport chains results in overproduction of reactive oxygen species, which could cause damage to macromolecules and subcellular components (Boaretto et al., Reference Boaretto, Carvalho, Borgo, Creste, Landell, Mazzafera and Azevedo2014). Free-radical scavenging system is an important component of desiccation tolerance. Non-enzymic antioxidants are likely to confer protection during the desiccated state in seeds (Berjak, Reference Berjak2006). Flavonoids are abundant secondary metabolites present in most plant and accumulate in the embryos and testa both of monocots and dicots seeds. In monocots, flavonoids are also found in aleurone layer. Because of their various chemical structure and attached substituents, flavonoids have many functions in plants. There is evidence that flavonoids influence seed dormancy and germination (Koornneef, Reference Koornneef1981). Flavonoids also protect plant against biotic (e.g. herbivores and pathogens) and abiotic stresses (e.g. UV radiation and heat) due to their antioxidative properties (Mierziak et al., Reference Mierziak, Kostyn and Kulma2014). As amphiphilic molecules, flavonoids have a strong antioxidant function (Karimi et al., Reference Karimi, Mehrabanjoubani, Keshavarzian, Oskoueian, Jaafar and Abdolzadeh2014), and they may scavenge radical oxygen species and limit oxidant stress (Stevenson and Hurst, Reference Stevenson and Hurst2007). Testa flavonoids provide an antimicrobial quinones and insoluble polymers to reinforce the seed coat barrier to water and oxygen permeation and alleviate mechanical damage under biotic and abiotic stresses (Pourcel et al., Reference Pourcel, Routaboul, Cheynier, Lepiniec and Debeaujon2007; Stevenson and Hurst, Reference Stevenson and Hurst2007). In the embryo, flavonoids protect membranes by limiting lipid peroxidation (Treutter, Reference Treutter2006). The present study indicated that the flavonoids and the FRSA were significantly negatively correlated with the optimum moisture content of ultra-dry stored whole seeds and non-lipid fraction.
In the present study, impacts of seed composition on optimum moisture content of ultra-dry stored seed were investigated and showed significant negative correlations, which confirm the results in the current literature.
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (no. 30900121).









