Introduction
The Blue Lizard mine in Red Canyon, Utah, is now well known as a prolific source of new minerals and particularly for its many Na uranyl sulfates. Of the 22 new minerals described from this mine, all are sulfates, 16 are Na uranyl sulfates and three are uranyl sulfates that do not contain essential Na. The new mineral uranoclite, (UO2)2(OH)2Cl2(H2O)4, described herein, is the first new mineral described from the Blue Lizard mine that is not a sulfate. Notably, it also does not contain essential Na. Uranoclite is only the second known uranyl mineral containing essential Cl, the first being bluelizardite, Na7(UO2)(SO4)4Cl (Plášil et al., Reference Plášil, Kampf, Kasatkin and Marty2014), which was also first described from the Blue Lizard mine. Interestingly, Cl plays very different roles in the structures of these two minerals.
Uranoclite is named for its composition. It is the first uranyl chloride mineral that contains no other anions other than hydroxyl. The phase has previously been synthesised and its chemical name is di-μ-hydroxido-bis[diaquachloridodioxidouranium(VI)] (Huys et al., Reference Huys, Van Deun, Pattison, Van Meervelt and Van Hecke2010). The new mineral and name were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2020-074, Kampf et al., Reference Kampf, Plášil, Olds, Nash and Marty2021). The description is based on two cotype specimens, both micromounts, deposited in the collections of the Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007, USA, catalogue numbers 75101 and 75102.
Occurrence
Uranoclite was found in efflorescent crusts on mine walls underground in the Blue Lizard mine (37°33′26″N, 110°17′44″W), Red Canyon, White Canyon District, San Juan County, Utah, USA. The mine is ~72 km west of the town of Blanding, Utah, and ~22 km southeast of Good Hope Bay on Lake Powell. Detailed historical and geological information on the Blue Lizard mine is described elsewhere (e.g. Kampf et al., Reference Kampf, Plášil, Kasatkin, Marty and Cejka2015a), and is primarily derived from a report by Chenoweth (Reference Chenoweth1993). Abundant secondary uranium mineralisation in Red Canyon is associated with post-mining oxidation of asphaltum-rich sandstone beds laced with uraninite and sulfides in the damp underground environment. Uranoclite is a very rare mineral in the secondary mineral assemblages of the Blue Lizard mine. It occurs with gypsum on matrix comprised mostly of subhedral to euhedral, equant quartz crystals that are recrystallised counterparts of the original grains of the sandstone.
Morphology, physical properties and optical properties
Uranoclite occurs as tightly intergrown aggregates of irregular yellow crystals (Fig. 1). The streak is very pale yellow and the fluorescence is bright green–white under 405 nm ultraviolet light. Crystals are translucent with vitreous lustre. The tenacity is brittle and the fracture is irregular. The mineral is very soft, probably having a Mohs hardness of ~1½. Multiple cleavages are likely but are impossible to define because of the irregular shape of the crystals and their occurrence in intergrowths. The density could not be measured because the mineral is soluble in Clerici solution and there is insufficient material available for physical measurement. The calculated density based upon the empirical formula is 4.038 g⋅cm–3. The mineral is soluble in H2O at room temperature. The occurrence of the mineral in aggregates of small, poorly formed, translucent crystals made the measurement of optical properties impossible. The Gladstone–Dale relationship using k(UO3) = 0.118, as provided by (Mandarino, Reference Mandarino1976), predicts an average index of refraction of 1.660.

Fig. 1. Tightly intergrown uranoclite crystals with gypsum. The field of view is 1.14 mm across.
Raman spectroscopy
Raman spectroscopy was conducted on a Horiba XploRA PLUS using a 100× (0.9 NA) objective. The spectrum from 4000 to 60 cm–1 obtained using a 532 nm diode laser, 100 μm slit and 2400 gr/mm diffraction grating is shown in Fig. 2. The spectrum from 2000 to 60 cm–1 obtained using a 785 nm diode laser, 100 μm slit and 1800 gr/mm diffraction grating is shown in Fig. 3.
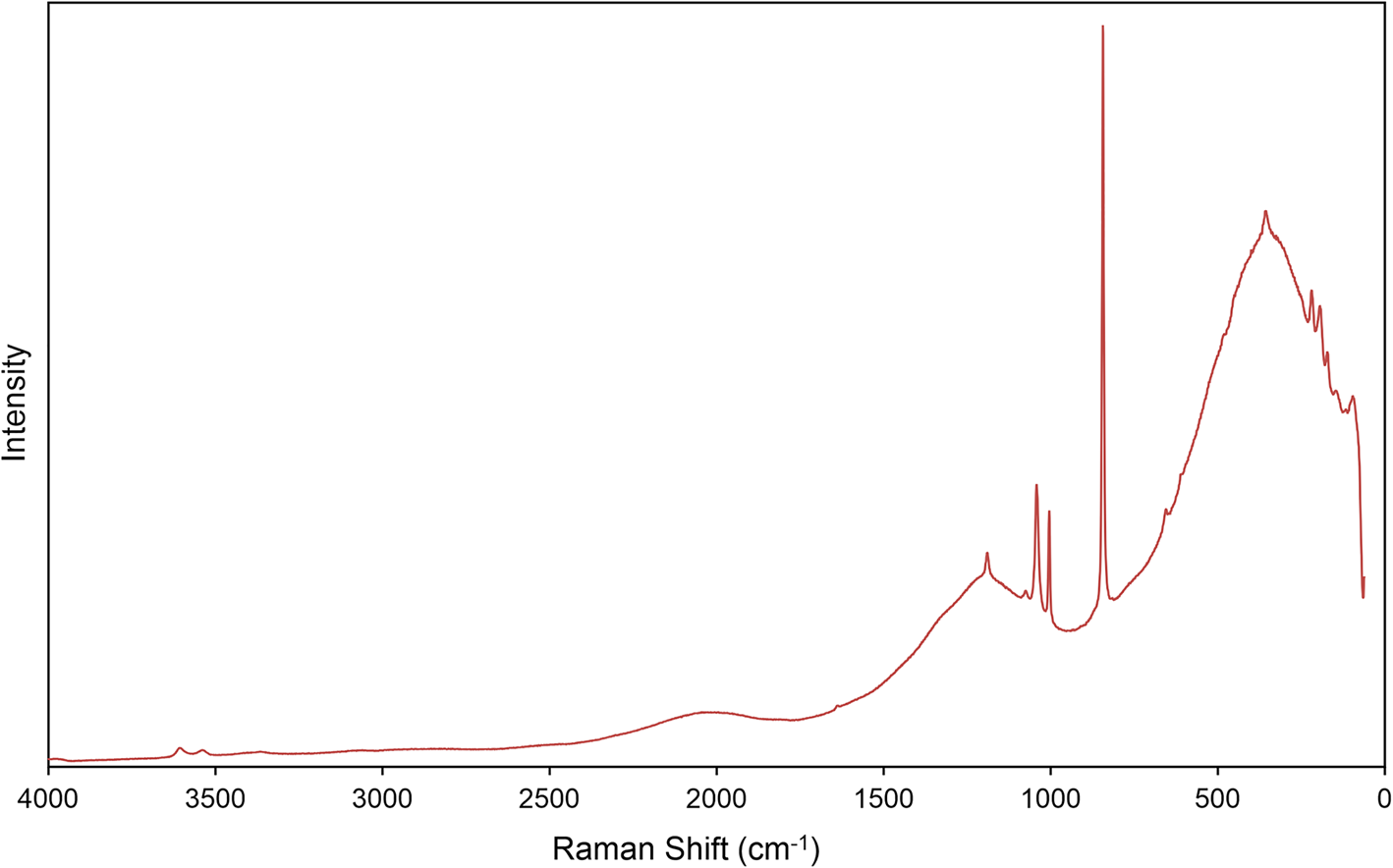
Fig. 2. The Raman spectrum of uranoclite recorded with a 532 nm laser. Because of significant fluorescence in the lower wavenumber portion of the spectrum, band assignments in this region are based on the spectrum recorded with a 785 nm laser (Fig. 3).
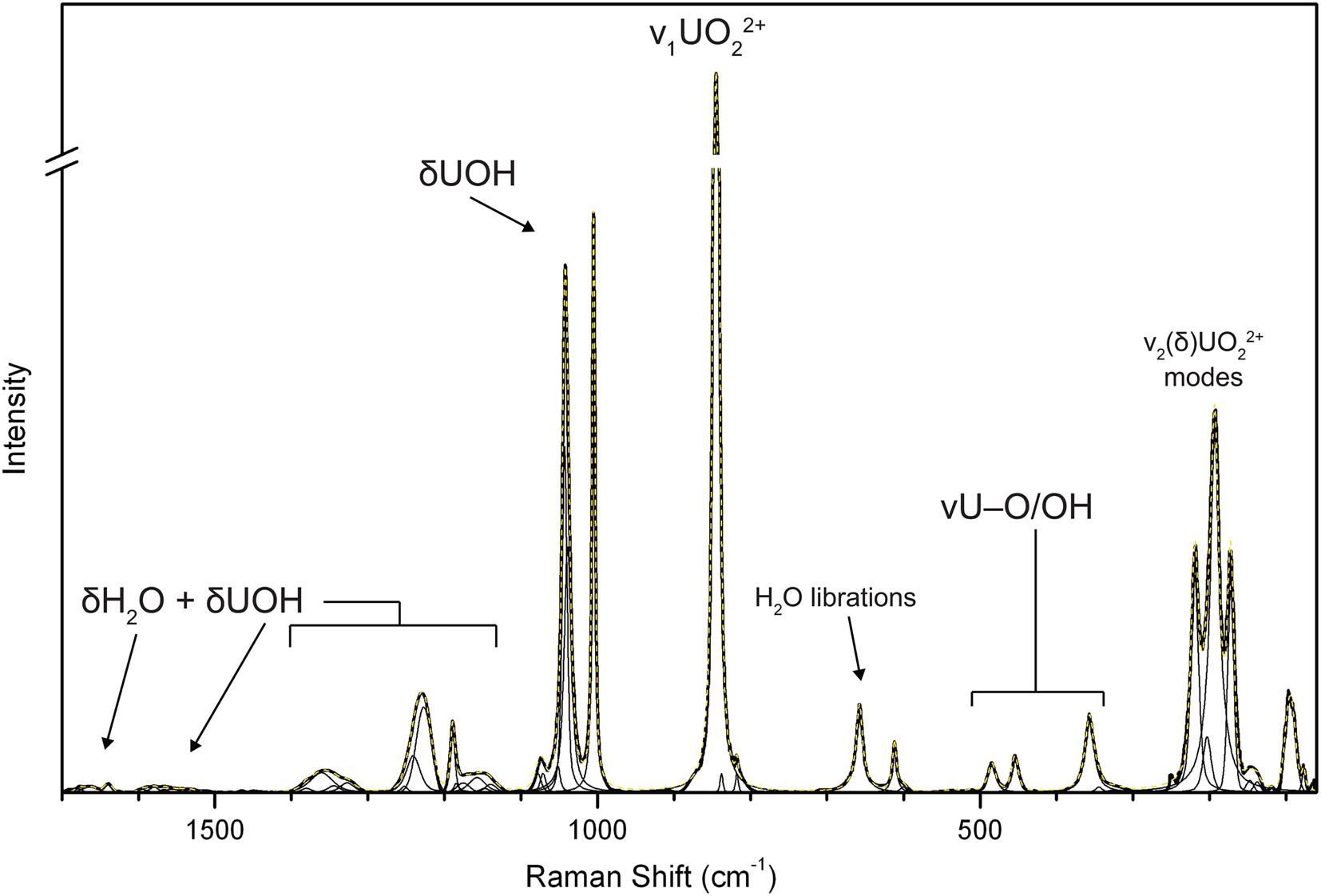
Fig. 3. The baseline corrected and fitted Raman spectrum of uranoclite recorded with a 785 nm laser.
As far as we know, no reliable vibrational spectroscopy assignments exist in the literature for synthetic uranyl hydroxide chloride phases, except those of Bullock and Parret (Reference Bullock and Parret1970), for instance. Thus, the following assignments are based primarily upon those for uranyl oxide hydroxide hydrate (UOH) minerals from Čejka (Reference Čejka, Burns and Ewing1999) and Frost et al. (Reference Frost, Čejka and Weier2007). All bands in the spectra were fitted using pseudo-Voigt peak profiles.
In the spectrum obtained using a 532 nm laser, three weak bands with centres at 3606, 3539 and 3366 cm–1 are assigned to ν (OH) stretching vibrations. Using the empirically derived equation of Libowitzky (Reference Libowitzky1999), the calculated O⋅⋅⋅O distances of the corresponding hydrogen bonds are between ~3.1 Å and ~2.8 Å, in reasonable agreement with refined O⋅⋅⋅O/Cl bond lengths determined from the structure refinement. We observed no bands related to ν (OH) stretching vibrations in the high wavenumber region of the spectrum using the 785 nm laser; however, a series of broad and very weak bands related to the ν2 (δ) bending vibrations of H2O groups are present from ~1680 to 1640 cm–1. The additional noisy, broad and very weak bands spanning from ~1530 to ~1600 cm–1 are possibly related to bending modes (δ) of U–OH bonds with overlap of ν2 (δ) H2O vibrations from the four crystallographically unique H2O groups in the structure. Strong fluorescence in the 532 nm spectrum prevented detailed analysis of the bands in this region and the remaining assignments have been made using the fitted bands of the 785 nm spectrum.
A complex series of bands between ~1400 and ~1000 cm–1 are assigned tentatively as bending vibrations (δ) U–OH of the bridging hydroxyl groups, possibly overlain with overtones or combination bands. The bands of highest intensity in this region are centred near 1226, 1189, and include a moderately intense and complex doublet near ~1040 and 1005 cm–1. These assignments are in line, for instance, with multiple bands observed in this range related to (δ) U–OH, librations of H2O and overtones, found in the calculated spectra of uranopilite (Colmenero et al., Reference Colmenero, Plášil, Timón and Čejka2020). Previously, these bands were generally assigned to stretching modes of SO4 tetrahedra.
The extremely weak band centred at 910 cm–1 is assigned to the ν3(UO2)2+ antisymmetric stretching vibration, while the ν1(UO2)2+ symmetric stretching vibration occurs as a very strong band at 845 cm–1. Bartlett and Cooney (Reference Bartlett and Cooney1989) provided an empirical relationship to determine the approximate U–OUr bond lengths from the band position assigned to the UO22+ stretching vibrations, which gives 1.76 Å (ν1) and 1.76 (ν3), in excellent agreement with the average U–OUr bond lengths from the X-ray data: 1.77 Å. Frost et al. (Reference Frost, Weier, Martens, Kloprogge and Kristóf2005) provide band positions for ν1(UO2)2+ in the synthetic analogue of uranoclite, stating: “Another example is [(UO2)2(OH)2Cl2(H2O)4], with two distinct uranium atoms in the unit cell and 847.5s, 850s, 852sh, 855vvw (Raman) and 851ms, sharp, 873sh, 883m cm–1 (IR)”; however, no further information is provided and we have located no studies examining the synthetic phase by Raman or IR. The reported band positions are comparable to those observed for uranoclite; however, despite the presence of two symmetrically distinct uranyl groups, the splitting of ν1(UO2)2 observed by Frost was not encountered in the spectrum of uranoclite.
A pair of weak bands at 657 and 611 probably arises due to libration modes of H2O groups, and those at 484 and 454 cm–1 to stretching modes of equatorial U–O bonds or librations of H2O as well. In other UOH minerals with uranyl hydroxide sheets, the bands near 400–350 cm–1 are related to various stretching modes of the equatorial OH and O atoms, and the band at 356 cm–1 is assigned as such. A distinct triplet of bands with fitted centres at 219, 193 and 172 cm–1 may be attributed to ν2(δ)(UO2)2+ bending vibrations and unassigned phonon modes.
Chemical composition
Electron probe microanalyses (6 points on 3 crystals) were performed at the University of Utah on a Cameca SX-100 electron microprobe with four wavelength dispersive spectrometers and using Probe for EPMA software. Analytical conditions were 15 kV accelerating voltage, 12 nA beam current and 10 μm beam diameter. Raw X-ray intensities were corrected for matrix effects with a ϕρ(z) algorithm (Pouchou and Pichoir, Reference Pouchou and Pichoir1991). No other elements were detected. Crystals took a poor polish, but there was minimal beam damage. Because insufficient material is available for a direct determination of H2O, it has been calculated based upon the known stoichiometry (U = 2 atoms per formula unit, O+Cl = 12 apfu). Analytical data are given in Table 1. The empirical formula is (UO2)2(OH)2.19Cl1.81(H2O)4. The ideal formula is (UO2)2(OH)2Cl2(H2O)4, which requires UO3 79.78, Cl 9.89, H2O 12.56, O = Cl –2.23, total 100 wt.%.
Table 1. Chemical composition of uranoclite.

*Based upon the known stoichiometry (U = 2 apfu, O+Cl = 12 apfu).
S.D. – standard deviation
X-ray crystallography
The small size, poor quality and intergrown nature of uranoclite crystals made a single-crystal X-ray diffraction study impossible. Powder X-ray diffraction (PXRD) data were recorded using a Rigaku R-Axis Rapid II curved imaging plate microdiffractometer with monochromatised MoKα radiation. A Gandolfi-like motion on the φ and ω axes was used to randomise the sample. The pattern provides an excellent match (Fig. 3) with that calculated from the structure of synthetic (UO2)2(OH)2Cl2(H2O)4 determined by Huys et al. (Reference Huys, Van Deun, Pattison, Van Meervelt and Van Hecke2010). Observed d values and intensities were derived by profile fitting using JADE Pro software (Materials Data, Inc.). Data are given in Supplementary Table S1.
Uranoclite is monoclinic with space group P21/n. The unit-cell parameters refined from the powder data using JADE Pro with whole pattern fitting are a = 10.763(8), b = 6.156(8), c = 17.798(8) Å, β = 95.656(15)° and V = 1173.5(18) Å3 (Z = 4).
Description of the structure
The crystal structure of uranoclite is presumed to be the same as that of synthetic (UO2)2(OH)2Cl2(H2O)4 (Huys et al., Reference Huys, Van Deun, Pattison, Van Meervelt and Van Hecke2010), as is clearly indicated by the close match between the PXRD of uranoclite and that calculated from the structure of the synthetic material. Some of the important details of the structure of synthetic (UO2)2(OH)2Cl2(H2O)4 and a bond-valence analysis, are provided in Tables 2 and 3. Henceforth, to simplify the wording, we will refer to this as the structure of uranoclite.
Table 2. Structure details for synthetic (UO2)2(OH)2Cl2(H2O)4 (Huys et al., Reference Huys, Van Deun, Pattison, Van Meervelt and Van Hecke2010).
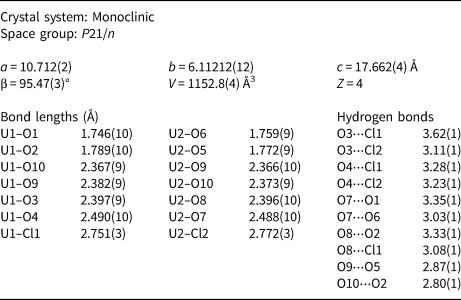
Table 3. Bond-valence analysis. Values are in valence units.
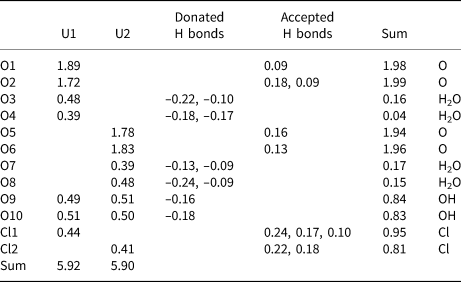
U6+–O bond-valence parameters are from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). U6+–Cl bond-valence parameters are from Zachariasen (Reference Zachariasen1978). Hydrogen-bond strengths for O–H⋅⋅⋅O bonds are based on O–O distances using the relation of Ferraris and Ivaldi (1988). Hydrogen-bond strengths for O–H⋅⋅⋅Cl bonds are based on H–Cl bond lengths using figure 2 in Malcherek and Schlüter (2007); note that H–Cl bond lengths were approximated by subtracting 1.0 Å from the O–Cl distance.
The most common coordination polyhedron for U6+ is a squat pentagonal bipyramid in which the apical vertices are the O atoms of the UO22+ uranyl group and the equatorial vertices are also O atoms that form longer bonds to U. In the structure of uranoclite, one of the equatorial vertices of each uranyl pentagonal bipyramid is a Cl anion. This is very unusual among uranyl phases and has not been reported previously in any mineral structures, although in nollmotzite (Plášil et al., Reference Plášil, Kampf, Škoda and Čejka2018), F occurs as a ligand in two different U6+ coordinations. The structural unit in uranoclite consists of two pentagonal bipyramids that share an equatorial OH–OH edge forming a dimer with the same formula as the mineral itself, (UO2)2(OH)2Cl2(H2O)4 (Fig. 4). The Cl anions in the dimer are in a trans configuration, possibly arranged so due to steric constraints of the large Cl anions. There is no interstitial complex in the structure; the dimers are linked to one another only by hydrogen bonding (Fig. 5).
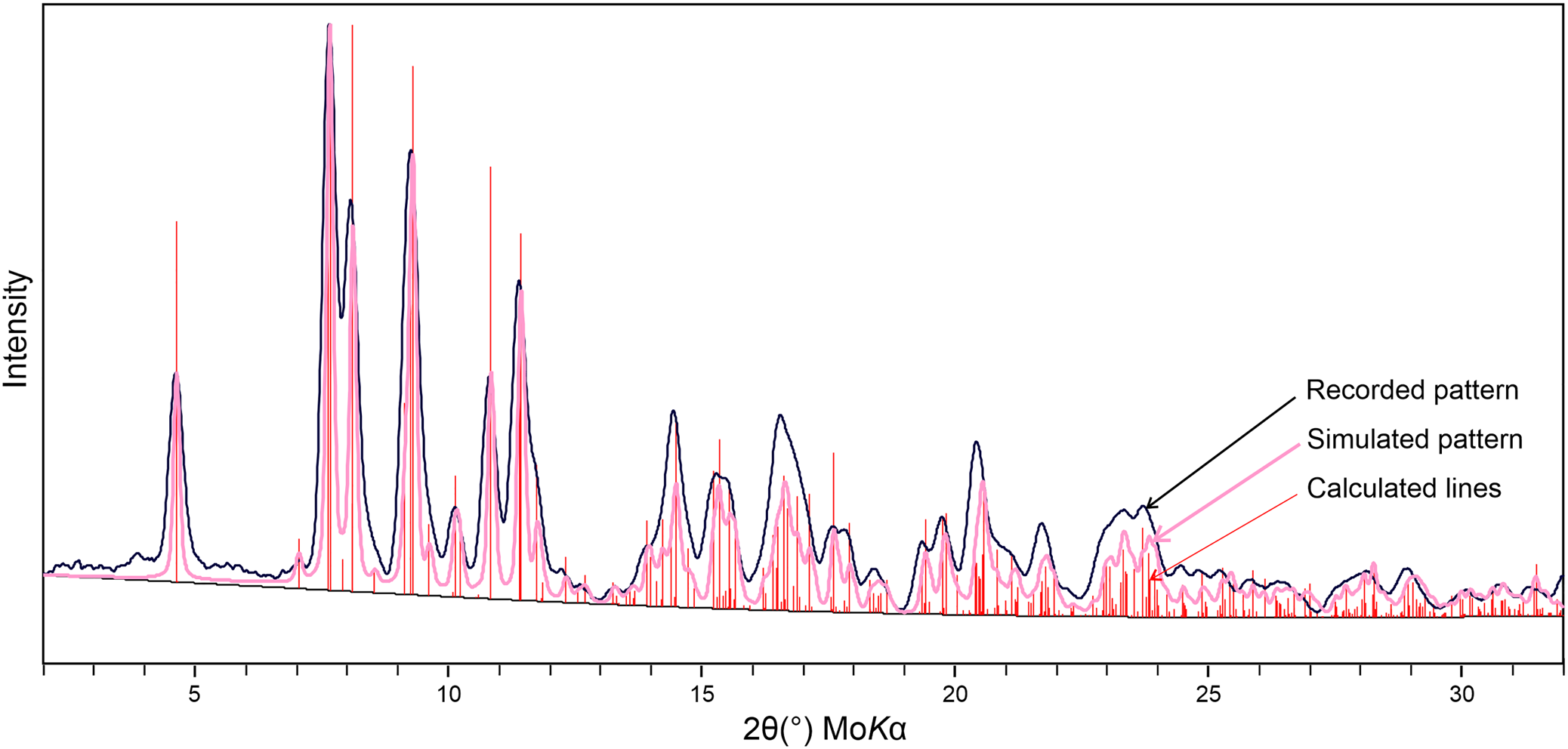
Fig. 4. Powder X-ray diffraction pattern for uranoclite compared with the lines and simulated pattern calculated from the structure of synthetic (UO2)2(OH)2Cl2(H2O)4 (Huys et al., Reference Huys, Van Deun, Pattison, Van Meervelt and Van Hecke2010).
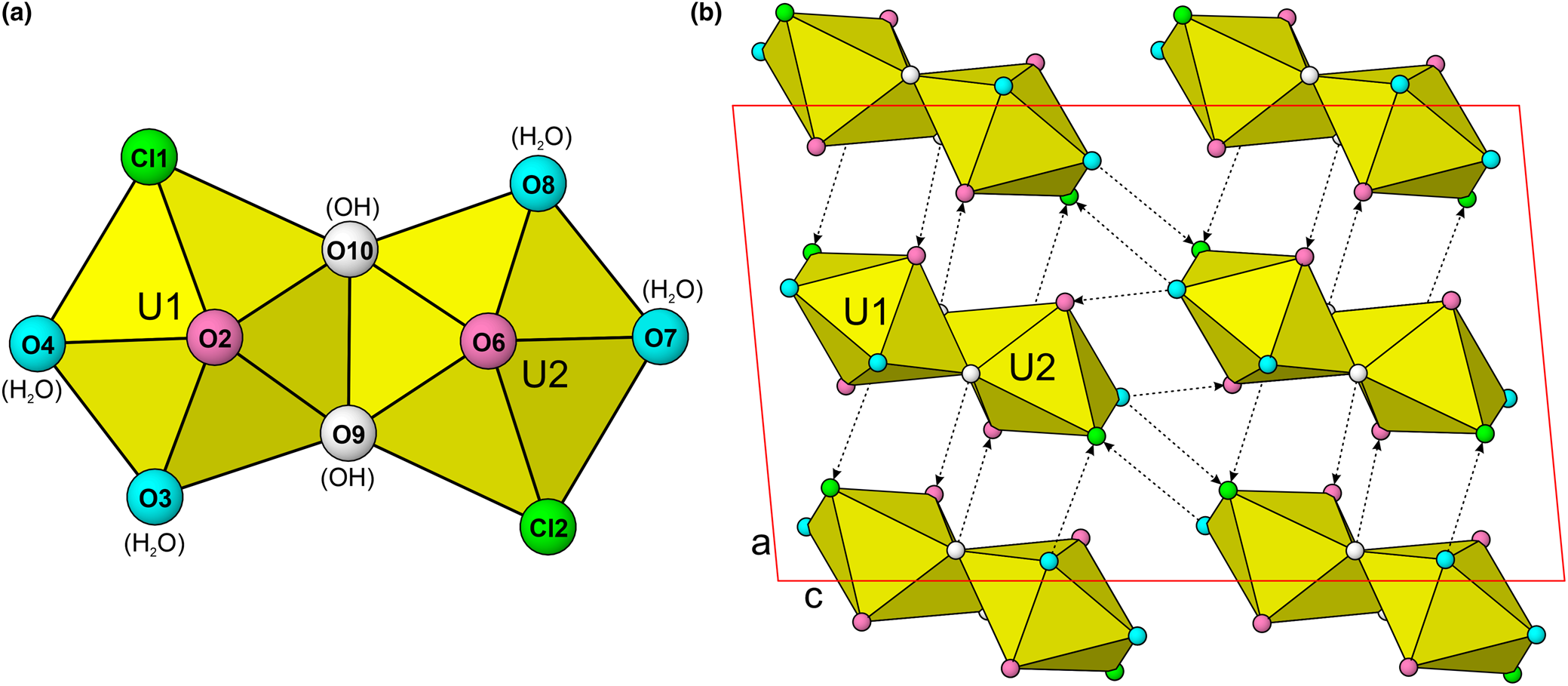
Fig. 5. (a) The [(UO2)2(OH)2Cl2(H2O)4] dimer in uranoclite and the synthetic analogue (Huys et al., Reference Huys, Van Deun, Pattison, Van Meervelt and Van Hecke2010). Note the colour coding of O, OH, H2O and Cl ligands. (b) The crystal structure of uranoclite viewed down [010]. Hydrogen bonds are shown as dashed lines with arrows indicating their direction (donor → receptor). The unit cell is outlined in red. Ligands are colour coded as indicated in (a). The drawing is based on the structure determination for synthetic (UO2)2(OH)2Cl2(H2O)4 (Huys et al., Reference Huys, Van Deun, Pattison, Van Meervelt and Van Hecke2010).
The cluster in the structure of uranoclite is of the U 2L 0-type of Lussier et al. (Reference Lussier, Lopez and Burns2016). While there are other mineral structures containing dimers composed of two edge-sharing Uφ7 pentagonal bipyramids, such dimers are generally linked by other polyhedra (e.g. SO4 tetrahedra) to form larger structural units, such as the sheets of phosphuranylite anion topology (Burns, Reference Burns2005) found in plášilite (Kampf et al., Reference Kampf, Kasatkin, Čejka and Marty2015b) and several other recently described Red Canyon minerals.
Acknowledgements
Sergey Aksenov and an anonymous reviewer are thanked for their constructive comments on the manuscript. We are grateful to retired miner Dan Shumway of Blanding, Utah, for advice and assistance in our collecting efforts in Red Canyon. Funding to JP was provided by the Czech Science Foundation (20-11949S). This study was also funded by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.33