INTRODUCTION
Recent reviews (Khan and Thulin, 1991; Poulin, 1992; Overstreet, 1993) indicate that pollutants in the surrounding macroenvironment directly influence the population dynamics, distribution and dispersal of fish ectoparasites, often leading to increased ectoparasitism (Khan and Thulin, 1991; Poulin, 1992), especially in situations such as aquaculture where the high densities of fish provide an ideal environment for direct fish-to-fish transmission (Möller, 1987). Metal contaminants of the aquatic environment such as zinc (Zn) influence host-parasite interactions either through harmful effects to the parasite (Overstreet, 1993; Soleng et al. 1999; Poléo et al. 2003) and/or through reduced host resistance to infection (Khan and Thulin, 1991; Poulin, 1992; Overstreet, 1997). In turn, parasites may reduce host tolerance to pollutants (Khan and Thulin, 1991; Overstreet, 1997).
In aquatic systems, most Zn is found in the sediment. In the water column, although Zn is almost entirely particulate and coupled with dissolved organic and inorganic compounds (Florence, Morrison and Stauber, 1992; Rozan et al. 2000), it may still be bioavailable (Gagnon and Saulnier, 2003). As an indispensable micronutrient, Zn is added as a growth-enhancing nutritional supplement in aquaculture (15 to 150 μg Zn/g in trout diet, Handy, 1996), but high quantities of unconsumed fish food result in Zn concentrations that exceed the Canadian Water Quality Guidelines (2001) of 30 μg Zn/l for the protection of aquatic life. In freshwater, Zn occurs at low concentrations (up to 20 μg/l) due to natural weathering of mineral deposits, but in industrialized areas waterborne Zn concentrations can increase far above 100 μg/l (Handy, 1996). Seawater collected from the open ocean contains low levels of total Zn (0·01–0·6 μg/l), but Zn concentrations in coastal waters polluted with industrial effluents have been reported to reach 30000 μg/l (Handy, 1996).
Excessive environmental Zn can seriously affect the survival of aquatic organisms such as fish (Widianarko et al. 2000, 2001). Although most estimates of the 96 h LC50 for fish are 1–10 mg Zn/l in soft water and 3-20 mg Zn/l in hard water, values range from 0·09 to 40 mg/l (Hogstrand and Wood, 1996). To date, toxicological studies on parasites have been performed on survival, activity, and encystment of larval digeneans, and results have varied depending on concentration, and the parasite species. Survival of miracidia and cercariae begins to be impaired at concentrations in the order of 100 μg/l (Asch and Dresden, 1977; Evans, 1982a,b; Morley, Crane and Lewis, 2001a,b, 2002). Exposure of Atlantic salmon to 50 to 400 μg Zn/l induced a significant decrease in the numbers of the ectoparasitic monogenean, Gyrodactylus salaris but had no apparent effect on the salmon (Poléo et al. 2003). Thus, it appears that gyrodactylids are more sensitive to Zn than their hosts, but very few studies have examined the concurrent effects of pollutants and parasites on fish.
The aim of the current research was to further explore the effects of waterborne Zn on the population dynamics of gyrodactylids on isolated fish, using Gyrodactylus turnbulli Harris, 1986 that lives on the skin and fins of the guppy, Poecilia reticulata (Peters) as our experimental model. Gyrodactylids are small (0·4–0·8 mm body length) epidermal feeders infecting many families of marine and freshwater teleost fish (Kearn, 1998; Cone, 1999). Their viviparous reproduction together with their direct fish-to-fish transmission makes them important pathogens in aquaculture (Johnsen and Jensen, 1991; Bakke et al. 1992; Cable, Harris and Tinsley, 1996; Cable et al. 2002a; Cable, Tinsley and Harris, 2002b) where they cause both direct mortality and indirect mortality through secondary bacterial or fungal infections (Kearn, 1998; Cone, 1999). On isolated fish, gyrodactylid numbers increase exponentially after an initial lag period. Some fish are able to control the infection and parasite numbers subsequently decline to zero. Other fish succumb to the infection and die (Scott, 1985). The phases of infection (initial lag, time to peak, time to recovery) have highly variable lengths depending on fish response (Scott and Robinson, 1984; Scott, 1985; Richards and Chubb, 1996, 1998), parasite virulence and the influence of various environmental factors (Scott and Nokes, 1984; Soleng et al. 1999; Poléo et al. 2003). Our study was designed to determine whether the phases of infection are influenced by the concentration of waterborne Zn.
MATERIALS AND METHODS
Host and parasite
The experiments were completed on isolated immature guppies (2 cm body length), naïve to Gyrodactylus infection, bred in our lab from a stock of adult guppies purchased through a local pet supplier. The fish were kept in transparent, covered, small plastic containers in an incubator at 25 °C and constant light cycling (16L[ratio ]8D), in 200 ml of artificial freshwater (Singh and Srinivastav, 1993) containing 0·123 g NaCl, 0·065 g Na2SO4, 0·004 g KCl, 0·117 g CaCl2 and 0·04 g MgCl2 per liter of distilled water, and adjusted to pH 7·6 with NaHCO3 (Fisher Scientific, Montreal, Canada). Various concentrations of Zn (0, 15, 30, 60, 120, 240 μg/l) were added as ZnCl2 with 98% purity (Anachemia Canada Inc., Montreal, Canada).
To confirm that Zn concentrations remained stable in the presence of fish that were fed Nutrafin Max Complete Flake Food (Rolf C. Hagen Inc., Montreal, Canada) once a day, we monitored Zn concentrations in our 200 ml containers at 0, 24 and 48 h. For each of the 6 Zn concentrations at each of the 3 time-points, a 50 ml sample from each of 3 replicate 200 ml containers was filtered, acidified to a pH of 2, and stored at 4 °C. Samples were analysed using inductively coupled plasma emission source in the chemistry laboratory of St Lawrence Centre, Environment Canada, Montreal, Canada. Within each prepared concentration, there was no significant difference in Zn concentration over the 48 h period (Table 1). Based on these results, artificial freshwater solutions were used for all experiments, they were replaced every 2 days, and the experimental fish were fed Nutrafin Max Complete Flake Food flakes once a day. As it was not possible to prepare artificial freshwater that contained less than 8 μg Zn/l, all measured concentrations were approximately 8 μg/l higher than the intended values (Table 1). The detected concentration of 8·31 μg/l in solutions to which no Zn was added was similar to the average obtained from our guppy breeding tanks (8·6±1·3 μg Zn/l). For convenience, we will refer to the amount of Zn added, rather than to the measured final concentrations.
Table 1. Resulting Zn concentrations, averaged from samples collected at 0, 24 and 48 h after isolated guppies were introduced into 200 ml of artificial freshwater to which various concentrations of Zn had been added (Fish were fed once at 24 h.)

Gyrodactylus turnbulli was originally obtained from infected guppies purchased from a local pet supplier, and was maintained in the laboratory by weekly addition of naïve fish into tanks with infected guppies. Experimental fish were anaesthetized for a maximum of 5 min in 50 ml of 0·02% tricaine methanesulfonate (Finquel MS222, Argent Chemical Laboratories, Washington) buffered to a neutral pH with NaHCO2, during infection and subsequent daily monitoring, using a dissecting microscope with a cold light source. Each experimental fish was infected by transfer of 3 G. turnbulli on a scale or small piece of fin from an infected donor to the caudal peduncle of a naïve recipient (Scott, 1982). All procedures were approved by a McGill University Animal Care Committee, in accordance with the Canadian Council on Animal Care Guidelines (1993).
Experimental protocol and outcome measures
Between 30 and 36 fish were randomly assigned to 1 of 6 solutions (0, 15, 30, 60, 120, 240 μg Zn/l). After 1 week pre-exposure to the respective Zn concentration, each fish was experimentally infected and then maintained in the same concentration of Zn until its death or until parasite numbers remained at zero for at least 3 consecutive days (defined as recovery). The number of parasites on each fish was recorded daily to follow the parasite population dynamics. Establishment (parasite numbers increased to at least 4 parasites within the first 5 days) was recorded for each of the Zn concentrations, and a minimum of 24 infected fish per concentration were then monitored. We recorded the following outcomes: (1) peak burden, (2) time to peak burden, (3) percentage mortality of fish, (4) survival time for fish that died, (5) duration of infection on recovered fish, (6) percentage of recovered fish with a long recovery period where parasite numbers remained low for more than 5 days after the rapid decline from peak burden, and (7) maximum intrinsic daily rate of increase of parasite population per fish, calculated as ln (N2/N1), where N1 and N2 are the parasite numbers on 2 consecutive days.
An additional 10 uninfected fish were maintained under identical conditions in each of the 6 Zn concentrations, and handled daily. Their survival was monitored for 45 days in order to determine whether fish mortality could be attributed to Zn exposure alone.
Statistical analysis
All categorical variables were analysed using χ2 and the binomial 95% confidence limits for percentages are reported (Rohlf and Sokal, 1981). For all continuous variables, the mean and S.E. are reported. In order to determine whether the pattern of parasite burdens over time on individual fish differed among Zn concentrations, a repeated measures ANOVA including Zn and time as the independent variables was used. The effect of Zn concentration was assessed using Kruskal-Wallis non-parametric ANOVA for peak burden, time to peak, survival time and time to recovery. In addition, linear and polynomial regression analyses were used to detect trends in outcome parameters with increasing Zn concentration. The maximum intrinsic daily rate of increase of the parasite population was compared among Zn concentrations using a 1-way ANOVA. Analyses were performed using SAS Version 9.1 software. The level of significance was established at P<0·05; statistics are reported only for significant effects.
RESULTS
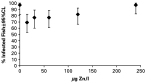
Of the 197 fish that were exposed to G. turnbulli, 34 fish did not retain their infection for more than 4 days, and therefore the parasite was not considered to have established on these fish. The overall number of fish on which the parasite successfully established differed significantly among waterborne Zn concentrations (χ2=14·19, D.F.=5, P=0·0144). Visual inspection of the data revealed that establishment was highest (97%) in fish exposed to 0 and 240 μg Zn/l and lowest (69%) in fish exposed to 15 μg Zn/l (Fig. 1).

Fig. 1. The effects of waterborne Zn concentration on Gyrodactylus turnbulli establishment ([lozf ]) (n=30, 36, 31, 35, 34, 31, respectively for concentrations ranging from 0 to 240 μg Zn/l). Isolated guppies were pre-exposed to various Zn concentrations for 1 week prior to infection with 3 parasites each.
Population dynamics on infected fish that recovered
A total of 69 fish became infected but then recovered. The average peak burden on these fish ranged from 10±2 to 37±5 parasites (Fig. 2A), and infections persisted between 18±2 and 29±7 days on average (Fig. 2C). Parasite numbers differed significantly with Zn concentration (F5,63=4·47, P=0·015) and with time (F68,1286=1·96, P<0·0001). In order to more fully explore these patterns, we compared the maximum intrinsic daily rate of increase of the parasite population, the peak parasite burden, the time to peak burden, the percentage of fish with a long recovery period and the duration of infection among Zn concentrations. Whereas the maximum intrinsic rate of the increase of the parasite population was unaffected by Zn concentration, both peak parasite burden (Fig. 2A; F1,68=3·80, P=0·0274) and time to peak (Fig. 2B; F1,68=6·05, P=0·0039) increased but then declined as Zn concentration increased and these two parameters were highly correlated (r69=0·43, P=0·0002). In contrast, the duration of the infection (Fig. 2C; F1,68=3·75, P=0·0571 – borderline significance) and the proportion of fish with an extended recovery period (Fig. 2D; F1,5=48·30, P=0·0023) continued to increase linearly with increasing Zn concentration. Together these data indicate that Zn had positive effects on parasite population growth over concentrations up to 60 or 120 μg Zn/l and that even the highest concentration allowed prolonged infection of fish.

Fig. 2. The effects of waterborne Zn concentration on infection parameters in guppies that recovered from an initial infection with 3 Gyrodactylus turnbulli (n=22, 12, 11, 10, 8, 6, respectively for concentrations ranging from 0 to 240 μg Zn/l). Data ([utri ]) and best fit linear or polynomial regression (—); (A) peak parasite burden, y=−0·0014x2+0·2761x+22·553; (B) time to peak parasite burden, y=−0·0004x2+0·116x+6·5098; (C) duration of infection, y=0·0429x+21·005; (D) percentage of fish with long recovery phase, y=0·1406x+18·6.
Effects of infection and Zn on guppy mortality
The percentage mortality among infected fish increased linearly (F1,5=8·26, P=0·045) as Zn concentration increased, from 24% in fish maintained at 0 μg Zn/l to 80% for fish kept in 240 μg Zn/l (Fig. 3A). Repeated measures ANOVA of the pattern of parasite numbers over time revealed significant Zn, time and Zn*time effects (F5,88=5·21, P=0·0003; F48,1508=9·40, P<0·0001, and F170,1508=1·48, P<0·0001 respectively). On average, the peak parasite burden in these fish ranged from 94±17 to 173±42 gyrodactylids per fish and the survival time ranged from 16±2 to 20±2 days, but neither parameter was significantly affected by Zn. The maximum intrinsic growth rate of the parasite population increased significantly as Zn concentration increased to 30 μg Zn/l, then declined (Fig. 3B; F2,93=3·76, P=0·0271).

Fig. 3. The effects of waterborne Zn and Gyrodactylus turnbulli infection on (A) fish mortality among (○) uninfected fish monitored for 45 days (n=10/ concentration) or ([lozf ]) fish infected with 3 G. turnbulli and then monitored until they recovered from infection or died (minimum of 24 fish per concentration); best fit linear regression for infected fish (—), y=0·1753x+43·914; and (B) maximum intrinsic rate of increase of the parasite on fish that died following infection ([lozf ]) (n=7, 13, 13, 17, 20, 24, respectively). Means with the same letter are not significantly different.
In order to determine whether the observed mortality was due to infection, to Zn, or to the combined effects of the two stressors, we compared survival between fish exposed only to Zn, and those exposed to Zn and infection. Mortality was significantly higher in infected fish compared with uninfected fish exposed to waterborne Zn, especially at the higher Zn concentrations (Fig. 3A; χ2=26·25, D.F.=11, P=0·0355). Uninfected fish experienced no mortality for the first 35 days of exposure, regardless of Zn concentration, and mortality after day 35 was independent of Zn concentration (Fig. 3A). We therefore concluded that the high mortality observed in infected fish was induced either by the parasite or by combined effects of parasite and Zn exposure, but not by Zn exposure alone.
DISCUSSION
In this study, fish were broadly divided into 3 categories, based on their response to infection. On some fish, the parasite was unable to successfully establish an infection. The proportion of fish falling into this category was highest at intermediate zinc concentrations. Successful establishment of gyrodactylid infections depends in large part on the parasite finding a suitable epidermal habitat on the fish. For example, gyrodactylids avoid areas rich in mucous cells (Buchmann and Bresciani, 1998), and also get trapped and sloughed off in excess mucous (Wells and Cone, 1990). We observed patches of mucous over the guppy epidermis in fish that had been pre-exposed to between 15 and 60 μg Zn/l, and suggest that the presence of mucous would have made the epidermis a less suitable habitat, thus explaining the reduced establishment success at intermediate concentrations of zinc.
The second category of fish were those on which the parasite successfully established and parasite numbers increased, but then declined to zero. Among these fish, the percentage of fish with a prolonged recovery period showed a linear increase in response to zinc concentration. One interpretation of these data is that the host was less able to control infection as zinc concentration increased, leading to longer recovery period. Interestingly, the most dominant host response to both gyrodactylid infection (Wells and Cone, 1990; Linderstrom and Buchmann, 2000) and to zinc exposure (Iger, Jenner and Wendelaar Bonga, 1994; Khunyakari, Tare and Sharma, 2001) is mucous production. Mucous and its associated complement and IL-1 are consistently reported as a requirement for killing of gyrodactylids (Buchmann, 1998, 1999; Harris, Soleng and Bakke, 1998; Buchmann and Bresciani, 1999). Mucous also greatly reduces underlying tissue exposure to zinc (Glover and Hogstrand, 2002) because of its high metal binding capacity (Handy, Eddy and Romain, 1989; Shephard, 1994), and this, together with the continual sloughing of both mucous and epithelial cells (Shephard, 1994; Glover and Hogstrand, 2002) prevents fish from accumulating zinc in tissues. Given that mucous production is central to control of infection and to protection against zinc, why then would fish be less able to control infection as zinc concentration increased? A variety of studies both on zinc (Iger et al. 1994) and on gyrodactylid infection (Wells and Cone, 1990) show that fish cannot continually produce mucous. An initial stress induces mucous production but this can only be maintained for a short period of time, after which mucous release stops until the mucous cells are repleted. This may help to explain the high establishment of the parasite at 240 μg Zn/l compared with intermediate zinc concentrations. In the case of more chronic stress, mucous production gradually declines, either because of exhaustion of mucous production capacity or because of a gradual acclimation to the stress which no longer induces a mucous production response (McGeer et al. 2000). We suggest that the combined demands of high zinc exposure and infection exhausted the ability of the guppies to continue to produce mucous, leading to prolonged infection in those fish that were eventually able to recover.
Although there was no zinc-dependent effect of the maximum intrinsic growth rate of the parasite population in fish that recovered, the peak parasite load and the time to peak burden both increased then declined as zinc concentration increased. A depletion of mucous production would account for the increase in parasite numbers and time to peak at the intermediate zinc concentrations, but doesn't explain why these parameters would decline at the higher zinc concentrations. Zinc toxicity would account for this observation, and has been hypothesized by Poléo et al. (2003). They reported a significant decrease in numbers of G. salaris on Atlantic salmon exposed to Zn in concentrations ranging from 50 to 400 μg Zn/l. Zinc toxicity in invertebrates has also been indicated by reduced longevity of free-living miracidia and cercariae when exposed to 100 μg Zn/l and higher (Asch and Dresden, 1977; Evans, 1982a,b; Morley et al. 2001a,b, 2002), and reduced reproduction in some terrestrial earthworms (Spurgeon and Hopkin 1996; Nursita, Singh and Lees, 2005). Our data from guppies exposed to high concentrations of zinc support the hypothesis that chronic exposure to zinc may be toxic to the parasite.
The third category of fish were those on which the infection became established, and increased unchecked eventually leading to the death of the guppies. The proportion of infected fish that died increased with increasing zinc concentration. Under natural conditions, fish are exposed to many stressors including various pollutants and a range of infectious organisms. The ability of fish to survive these stressors depends not only on their tolerance to the pollutants, but also on their ability to kill pathogens or limit their growth, and to repair tissue damage induced by the pathogens. We observed very little mortality in our uninfected fish, suggesting that chronic exposure for 45 days to concentrations up to 240 μg Zn/l was insufficient to cause mortality in the absence of infection. This was not surprising as guppies are known to survive well in waters highly polluted with heavy metals (Widianarko et al. 2000, 2001), with LC50 of 14·5±0·3 mg/l and 12·6±0·2 mg/l Zn after acute exposure for 24 and 48 h, respectively (Khunyakari et al. 2001). Whereas zinc alone had no impact on guppy survival, our study demonstrates that mortality on infected fish responded in a concentration-dependent manner to increasing concentrations of waterborne zinc. Gyrodactylids are known fish pathogens (Scott, 1985; Kearn, 1998; Cone, 1999) that browse the fish epidermis, ingesting both mucous and epidermal cells (Buchmann and Bresciani, 1998). Moreover, their opisthaptoral hooklets cause mechanical disruption of the epithelium that provides entry points for secondary bacterial infections (Cone, 1999). Scott (1985) reported 49% mortality among isolated guppies initially infected with 3 G. turnbulli, a value comparable to what we observed among fish exposed to 15 μg Zn/l, but much lower than that observed at higher zinc concentrations.
The increased mortality at higher concentrations could have been an indirect result of increased pathology due to higher parasite loads as zinc concentration increased. However, this is not consistent with our observations. First, among the fish that died, the maximum intrinsic growth rate of the parasite population did not increase, but rather declined at the higher zinc concentrations. Second, the peak parasite burden on fish that died was independent of zinc concentration. Alternatively, the increased mortality at higher zinc concentrations might have resulted from impaired host responses at the higher concentrations. This would allow the parasite population to increase to high levels, unchecked by the host response, perhaps because of exhaustion of the ability to produce mucous. A third possibility is that tissue damage caused by the parasites may have facilitated entry of zinc into host tissues by breaking the mucous barrier, thus increasing its toxic effect on the guppy, and perhaps reducing their ability to repair tissue damage induced by infection.
In summary, we suggest that the demands on mucous production induced by the 1-week pre-exposure to zinc prior to infection, together with subsequent infection-induced and zinc-induced production of mucous, exceeded the capacity of the host for sustained mucous release, allowing the parasite numbers to grow unchecked. Together, the pathogen and the pollutant reduced the percentage of fish that were able to survive the combined challenges in a zinc concentration-dependent manner. Although our data also suggest that chronic exposure to high zinc concentrations may be toxic to the parasite, results indicate that zinc affects guppy-gyrodactylid interactions on isolated fish in a way that is, overall, more detrimental to the host than to the parasite.
Funding for this research was provided by the Natural Sciences and Engineering Research Council (NSERC 3585). The first author also acknowledges an NSERC Postgraduate Scholarship. Research at the Institute of Parasitology is supported by a Regroupement Stratégique from FQRNT, a provincial funding agency. We extend special thanks to Lise Gougeon from St Lawrence Centre (Environment Canada) who kindly helped us with the software and technical expertize in assessing the waterborne Zn concentration in our experimental solutions.