Published online by Cambridge University Press: 06 May 2004
It has been demonstrated that the surface lipophilicity of the plant-parasitic nematode Globodera rostochiensis decreases when infective larvae are exposed to the phytohormones indole-3-acetic acid (auxin) or kinetin (cytokinin). In the present study, it was shown that inhibition of phospholipase C (PLC) or phosphatidylinositol 3 kinase (PI3-kinase) reversed the effect of phytohormones on surface lipophilicity. The signalling pathway(s) involved in surface modification were investigated using ‘caged’ signalling molecules and stimulators or inhibitors of different signalling enzymes. Photolysis of the ‘caged’ signalling molecules, NPE-caged Ins 1,4,5-P3, NITR-5/AM or caged-cAMP to liberate IP3, Ca2+ or cAMP respectively, decreased the surface lipophilicity. Activation of adenylate cyclase also decreased the surface lipophilicity. In contrast, inhibition of PI3-kinase using Wortmannin, LY-294002 or Quercetin, and inhibition of PLC using U-73122 all increased the surface lipophilicity. Two possible signalling pathways involved in phytohormone-induced surface modification are proposed.
Activation of infective larvae of animal-parasitic nematodes is accompanied by changes in the lipophilicity of their surfaces (Proudfoot et al. 1993a,b). It was suggested that these surface changes might be a sign of developmental progression, or an adaptation for entry in the host's tissue environment. Plant-parasitic nematodes have also been shown to respond to host stimuli including root diffusates and phytohormones (Akhkha et al. 2002). In the case of G. rostochiensis-infective larvae (J2s), phytohormones were found to trigger a decrease in the surface lipophilicity; this was suggested to be important for the nematode to cope with the host environment. How the surface changes take place, their biological significance and what signalling pathways are involved in these changes, is unknown. In previous work (Modha, Kusel & Kennedy, 1995), we demonstrated the involvement of the secondary messengers cAMP (cyclic adenosine monophosphate), IP3 (inositol triphosphate) and Ca2+ (calcium ions) in the control of activation-induced changes to the surface of infective larvae of the animal-parasitic nematode Trichinella spiralis. This was investigated using membrane-permeant photo-activable ‘caged’ signalling molecules to alter intracellular levels of cAMP, IP3 and Ca2+. Similar methods were used in the present work to identify signalling molecules and signalling pathways that are behind surface lipophilicity changes triggered by the phytohormones auxin (indole-acetic acid) and kinetin (6-furfurylamino purine) in the G. rostochiensis J2s.
G. rostochiensis cysts were washed extensively in distilled water. Hatched J2s were obtained by incubating cysts in distilled water for 4 days followed by exposure to potato root diffusate (PRD). J2s were collected daily and used immediately.
Two phytohormones (obtained from Sigma) were used in this experiment, auxin (indole-3-acetic acid) and kinetin (6-furfurylamino purine) with cytokinin activity. Both phytohormones were prepared as described by Akhkha et al. (2002). A concentration of 10 μM of both phytohormones was used in all experiments. For the phytohormone treatments, J2s were incubated in distilled water containing auxin or kinetin for 1 h at room temperature, washed and then labelled. For the controls, J2s were incubated in distilled water.
The fluorescent lipid probe 5-N-(octodecanoyl) aminofluorescein (AF18) was obtained from Molecular Probes (Europe BV., Leiden, The Netherlands). Ten μl of stock solution (10 mg/ml) of AF18 in ethanol was layered onto 1 ml of distilled water containing the treated J2s and then rapidly mixed. Following incubation for 15 min at room temperature, J2s were washed in distilled water by 3 cycles of centrifugation and re-suspension.
Fluorescence was quantified using a Leitz fluorescence microscope as described by Akhkha et al. (2002). The fluorescence was determined in the anterior region (see diagram below) of 30 AF18-labelled J2s for each treatment. A Zeiss fluorescence microscope (Carl Zeiss, Light Microscopy, POB 4041, 37030 Göttingen, Germany; www.zeiss.de/micro) mounted with an FITC filter and AxioCam digital camera, and fully controlled by AxioVision software was used to capture and process images.

Since the cuticle of G. rostochiensis J2s is generally impermeable to unmodified mediators, hydrophobic caged compounds were used to introduce signalling molecules into the nematodes. Photolabile caged compounds are inert precursors of biologically active molecules that are modified chemically by the attachment of a photolabile cage. The chemical caging imparts greater membrane permeability to the active molecule (McCray & Trentham, 1989). The active molecule is released from its cage by irradiation with pulses of ultra-violet light (λ=310–360 nm). Caged cAMP [Adenosine 3′,5′-cyclic Monophosphate, P1-(2-Nitrophenyl)ethyl Ester] and NPE-caged Ins 1,4,5-P3 [D-myo-inositol 1,4,5-triphosphate, P4(5)-(1-(2-nitrophenyl) ethyl) ester] were obtained from Molecular Probes (Europe BV., Leiden, The Netherlands). The caged calcium chelator NITR-5/AM [1-(2-Amino-5-(1-hydroxy-1-[2-nitro-4,5-methylenedioxyphenyl] methyl) phenoxy)-2-(2′-amino-5′-methylphenoxy) ethane-N,N,N′,N′-tetraacetic acid, tetraacetoxymethyl ester] was obtained from Calbiochem (CN Bioscience UK, Beeston, Nottingham). All caged compounds were prepared as stock solutions (caged cAMP, 20 mM; IP3, 10 mM and NITR-5/AM, 5 M) in dimethyl sulphoxide (Me2SO) and diluted in distilled water to the required concentrations. The final Me2SO concentration did not exceed 0·1% as it was determined in our laboratory that concentrations up to 1% Me2SO did not affect AF18 uptake, nematode behaviour or infectivity (results not shown). Approximately 1000 J2s were incubated in distilled water containing the caged signalling molecules (10 μM IP3, 500 μM NITR-5/AM or 20 μM cAMP) for 1 h at room temperature. Caged cAMP-treated J2s were also incubated in 1 mM 3-isobutyl,1-methylxantine (IBMX) to prevent cAMP degradation by phosphodiesterase. J2s were washed 3 times by slow centrifugation to remove excess caged compounds, then transferred to a microtitre plate and flashed with UV light (45 mW cm−2 at 10 cm from source) for 30 sec to release the active molecules. The J2s were then labelled. In the controls, J2s flashed with UV in the absence of caged signalling molecules were not significantly different from J2s that were not flashed with UV in the presence or absence of caged signalling molecules. In the results, only UV flashed controls in the absence of caged signalling molecules are shown.
All enzyme stimulators and inhibitors were obtained from Calbiochem (CN Bioscience UK, Beeston, Nottingham).
Forskolin (7β-Acetoxy-8,13-epoxy-1α,6β,9α-trihydroxy-labd-14-en-11-one Colforsin), a cell-permeable diterpene isolated from the Indian plant Coleus forskohlii was used to activate adenylate cyclase in order to increase the intracellular level of cAMP. Forskolin 1,9-dideoxy was used as a negative control as it does not activate adenylate cyclase.
Wortmannin (KY-12420), a fungal (Penicillium wortmanni) metabolite, specifically inhibits PI3-kinase to stop the phosphatidylinositol-(3,4,5)-triphosphate (PIP3) synthesis that is necessary for the activation of many signalling enzymes. LY-294002 [2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one], a specific inhibitor of PI3-kinase. Quercetin (3,3′,4′,5,7-Pentahydroxyflavone), another inhibitor of PI3-kinase.
U-73122 (1-[6-((17b-3-Methoxyestra-1,3,5(10)-trien-17-yl)amino) hexyl]-1H-pyrrole-2,5-dione): acts as an inhibitor of the phosphoinositide-specific phospholipase C that generates two ubiquitous signalling molecules, DAG and IP3 using PIP2. U-73122 inhibits this step and consequently all downstream events in the signalling pathway. U-73343 (1-[6-((17b-3-Methoxyestra-1,3,5(10)-trien-17-yl) amino) hexyl]-2,5-pyrrolidinedione) acts as a very weak inhibitor of PLC. In the present study it was used as a negative control for U-73122. All the above compounds were used at 20 μM concentration.
The means and standard errors (shown in graphs) were calculated using Excel (Microsoft Office 2000). Analysis of variance was performed using Minitab's ANOVA and General Linear Model (version 13).
G. rostochiensis J2s responded similarly to pre-incubation in auxin and kinetin (Fig. 1). The insertion of the lipid probe AF18 into the surface of J2s was significantly (P<0·05) lowered by pre-treatment with auxin or kinetin to about 57±1·4% or 43±3·4% of the controls respectively.

Fig. 1. Effect of the phytohormones auxin and kinetin on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).
In an attempt to investigate signalling pathways that regulate phytohormone-induced surface changes, J2s were treated with phytohormones in the presence of two signalling inhibitors Wortmannin and U-73122 that inhibit the activity of PI3-kinase and PLC respectively. The inhibition of PI3-kinase or PLC reversed the effects of phytohormones on the uptake of AF18 by the nematode surface (Fig. 2), suggesting that phytohormones may induce changes in surface lipophilicity through these two pathways.

Fig. 2. Effects of Wortmannin and U-73122 signalling inhibitors on phytohormone-induced lipophilicity changes in Globodera rostochiensis J2s. Similar results were obtained using auxin or kinetin but only those of the latter were presented. Error bars are standard errors (n=30).
In an attempt to dissect the two pathways, the involvement of signalling molecules in the modification of nematode surface lipophilicity was investigated using the caged compounds (see Materials and Methods section) cAMP, IP3 and NITR-5/AM (caged Ca2+) followed by labelling with AF18 (Fig. 3).

Fig. 3. Effect of signalling molecules on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).
When incubated with caged cAMP and after UV treatment, J2s showed less AF18 insertion, suggesting that auxin and kinetin decrease surface lipophilicity (Fig. 3) through an increase in the level of intracellular cAMP. This could occur through the effects of Ca2+ ions and/or inositol triphosphate (IP3).
Incubation of J2s in NITR-5/AM, a Ca2+ loaded chelator that releases Ca2+ after UV treatment, decreased AF18 insertion into the surface. This suggests that the lipophilicity decrease is associated with a Ca2+ rise; this could occur due to an increase in IP3 that stimulates Ca2+ release.
When the intracellular IP3 was increased in the J2s using the membrane-permeant NPE-caged Ins 1,4,5-P3, AF18 insertion was decreased. This suggests that the rise in Ca2+ could be generated through a rise in IP3. IP3 increases when PLC is activated, leading by cleavage of phosphatidylinositol-4,5-bisphosphate (PIP2) to IP3 and diacylglycerol (DAG).
When J2s were incubated in Forskolin (Fig. 4), an adenylate cyclase stimulator, AF18 insertion was decreased significantly (P<0·05) suggesting that the increase in cAMP was due to adenylate cyclase activation, which is known to happen naturally through increase in cytosolic Ca2+ concentration. The use of inactive form of Forskolin (Forskolin 1,9-dideoxy) had no effect on the insertion of AF18 confirming that activation of adenylate cyclase is necessary for the alteration of nematode surface lipophilicity.

Fig. 4. Effect of enzyme stimulators and inhibitors on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).
When J2s were incubated in a solution containing Wortmannin, AF18 insertion not only came back to the control level but it increased significantly (P<0·01) to about 4 times the level of the control (Figs 4 and 5), suggesting that surface changes due to phytohormones may be induced by PI3-kinase activation which phosphorylates the 3-hydroxyl group of physphatidyl inositol lipids generating PI(3,4)P2 and PI(3,4,5)P3; the latter is a known secondary messenger (Toker & Cantley, 1997).

Fig. 5. Effect of pre-incubation in distilled water (A) or Wortmannin (B) on AF18 insertion. Wortmannin-treated Globodera rostochiensis J2s (B) showed a higher level of AF18 insertion compared to the control (A).
Other inhibitors of PI3-kinase were also used to confirm the results observed using Wortmannin. Pre-incubation of J2s in LY-294002 or Quercetin (Fig. 6) increased the level of AF18 insertion significantly (P<0·05) but not to the extent of that of Wortmannin, confirming that PI3-kinase pathway is necessary for the changes in the surface lipophilicity.

Fig. 6. Effect of PI3-kinase inhibitors on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).
The involvement of IP3 in the surface modification was further examined using U-73122 to inhibit PLC that generates IP3 (Fig. 7). When J2s were incubated in a solution containing the inhibitor, the uptake level of AF18 was increased slightly but significantly (P<0·05), suggesting that the PLC pathway is also necessary for the surface modification. The use of a negative control, U-73343 did not increase the level of AF18 uptake significantly (P>0·05).

Fig. 7. Effect of phospholipase C (PLC) inhibitors on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).
The surfaces of a number of nematode species, including parasites, show changes in structure or composition during entry of the parasite into the host or when coming into contact with nutrients. One very rapid change that has been detected, is a change in the uptake into the surface of a lipophilic fluorescent compound, 5-N-(octodecanoyl) aminofluorescein (AF18). For example, on entry into the mammalian host Trichinella spiralis shows a very large increase in uptake of AF18 (Proudfoot et al. 1993a,b). Caenorhabditis elegans shows an increased uptake of AF18 when progressing from the dauer stage to the feeding stage (Proudfoot et al. 1991) and also when stimulated with bacterial diffusates (unpublished data from our laboratory).
G. rostochiensis also showed an increase in AF18 uptake after treatment with potato or tomato root diffusates (Akhkha et al. 2002). In contrast, Meloidogyne incognita showed no significant changes in uptake when exposed to tomato root diffusates from a susceptible or a resistant variety of tomato. However, AF18 uptake was increased after exposure to exogenous phytohormones. In this paper we have shown that the phytohormones, auxin and kinetin stimulate a decrease in the uptake of AF18, and we have analysed the cell signalling pathways, which may be involved in surface changes leading to AF18 uptake.
G. rostochiensis were incubated with a number of caged signalling molecules, and a number of compounds known to inhibit signalling pathways in mammalian cells. The results suggested two possible signalling pathways (Fig. 8). Activation of phospholipase C (PLC) and the consequently increased level of IP3, or activation of PI3-kinase and consequently increased level of PIP3, appear to induce surface modifications. When PLC is activated, PIP2 is hydrolysed to produce DAG and IP3. The latter is known from mammalian cells to bind to IP3 receptor on the endoplasmic reticulum causing the release of endogenous Ca2+ (Berridge, 1993; Berridge, Lipp & Bootman, 2000). The involvement of the PLC pathway in the surface modification was demonstrated in the present study by inhibiting PLC activity and consequent limitation of IP3 generation. This limitation caused an increased level of AF18 uptake. Increasing the level of IP3 in the nematodes using caged IP3 decreased the AF18 uptake, confirming the role of PLC pathway in regulating surface lipophilicity. Other researchers have also reported an increase in IP3 levels in unhatched G. rostochiensis infective larvae when stimulated by hatching factor(s) from potato root diffusate (Atkinson & Fowler, 1990). The involvement of the PI3-kinase pathway in surface changes was demonstrated using the fungal metabolite Wortmannin, which increased the surface lipophilicity to a surprising extent. This suggests strongly that PI3-kinase and PIP3 are involved in the regulation of surface properties. The fact that the increase was so large may be because Wortmannin is not specific for PI3-kinase; it is also known to inhibit other signalling pathways involving phospholipase D (Bonser et al. 1991) and phospholipase A2 (PLA2) (Cross et al. 1995). However, when we used other inhibitors of PI3-Kinase, such as Quercetin and LY-294002, which are more specific than Wortmannin, there was an increase in AF18 uptake, although not as dramatic as caused by Wortmannin. This supports the view that PI3-kinase, and PIP3 levels are likely to regulate surface lipophilicity in G. rostochiensis. How PIP3 affects downstream events is not known even in well-studied systems (Shepherd, Navé & O'rahilly, 1996). However, PIP3 was suggested to activate protein kinase C (PKC) which is known to be activated also by DAG an end-product of the PLC pathway (Nakanishi, Brewer & Exton, 1993; Toker et al. 1994; Palmer et al. 1995). This suggests that PKC could be the common step between PLC and PI3-kinase pathways in regulating surface lipophilicity.

Fig. 8. Signalling pathways potentially involved in the surface changes in Globodera rostochiensis.
In the scheme depicted in Fig. 8, we envisage that the PLC pathway increases IP3 concentration and consequently releases Ca2+ from intracellular stores; the Ca2+ then activates adenylate cyclase to produce cAMP that may regulate surface lipophilicity through PKA activation. PI3-kinase generates PIP3 that regulates the surface by activating another pathway involving protein kinase C (PKC). Thus, we envisage two pathways that might regulate surface lipophilicity. Surface receptor-activated PI3-kinases were also reported to function in other organisms including C. elegans. Morris, Tissenbaum & Ruvkun (1996) observed that a loss of function in the class IA PI3-kinase homologue encoded by age-1/daf-23 (dauer larvae formation defect-23) causes a non-conditional entry into the arrested dauer stage in C. elegans. This could be related to surface changes observed in our work, as C. elegans dauer stage does not insert the lipid probe AF18 into the surface. However, AF18 insertion occurred after exposure of the dauer stage to a fresh bacterial food source (Proudfoot et al. 1991). Furthermore, the ratio between PIP3 and IP3 may be also crucial to the effect on lipophilicity. This idea of the balance of the activity of regulatory pathways controlling surface modification can be envisaged in other systems such as Brugia pahangi, Nippostrongylus brasiliensis and Acanthocheilonema viteae. The surfaces of these parasites were reported to be stimulated (increased level of AF18 uptake) by Ca2+ and cGMP, and inhibited by cAMP (Proudfoot et al. 1993b). On the other hand, surface modification in Trichinella spiralis was shown to be stimulated (increased level of AF18 uptake) by Ca2+ and cAMP (Modha et al. 1995). The fact that signalling molecules have contrasting effects on the surface of different nematode species could represent key adaptations for the establishment and developmental reactivation in the different hosts, plants or animals.
The phytohormones, auxin and kinetin did not decrease the surface lipophilicity in the presence of the signalling inhibitors, Wortmannin and U-73122; confirming the involvement of the PLC and PI3-kinase signalling pathways in the phytohormone-induced surface changes. If this is true, other host stimuli such as root diffusates may act through the same pathways, as they are known to cause changes to the nematode surface. These may have implications in the hatching and infection processes.
Understanding the mechanism(s) by which the surface of nematodes changes may help in identifying signalling molecules that could be targeted as a control strategy. However, further work should be designed to look at the biological significance of surface changes and the effects of signalling inhibitors and signalling molecules on the infection process.
This work was supported by The Leverhulme Trust. We are also very much grateful to the Scottish Agricultural and Science Agency (82 Craigs Road, East Craigs, Edinburgh, EH12 8NJ) for giving us the licence (No. PH/7/2002) to maintain and work with Globodera. Rothamsted-Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
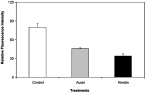
Fig. 1. Effect of the phytohormones auxin and kinetin on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).

Fig. 2. Effects of Wortmannin and U-73122 signalling inhibitors on phytohormone-induced lipophilicity changes in Globodera rostochiensis J2s. Similar results were obtained using auxin or kinetin but only those of the latter were presented. Error bars are standard errors (n=30).

Fig. 3. Effect of signalling molecules on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).

Fig. 4. Effect of enzyme stimulators and inhibitors on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).

Fig. 5. Effect of pre-incubation in distilled water (A) or Wortmannin (B) on AF18 insertion. Wortmannin-treated Globodera rostochiensis J2s (B) showed a higher level of AF18 insertion compared to the control (A).

Fig. 6. Effect of PI3-kinase inhibitors on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).
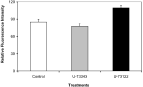
Fig. 7. Effect of phospholipase C (PLC) inhibitors on the uptake of AF18 by Globodera rostochiensis J2s. Error bars are standard errors (n=30).
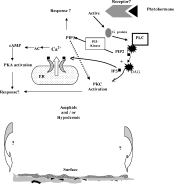
Fig. 8. Signalling pathways potentially involved in the surface changes in Globodera rostochiensis.