INTRODUCTION
The species Dodecaceria concharum Oersted, Reference Oersted1843 was thought by Gibson & Heppell (Reference Gibson and Heppell1995) to be D. fimbriata (Dehorne Reference Dehorne1933). However, the ICZN (2003; Case 2899) arbitrated in favour of the name D. concharum. As a result Oersted's species remained as D. concharum and Dehorne's species, D. fimbriata (D. caulleryi), became D. concharum. The names were reversed which has led to further confusion in the literature. The problem with the original description by Oersted is that it is woefully inadequate. Caullery and Mesnil (Reference Caullery and Mesnil1898) were unable to separate the two species or forms of the species using his description. Dehorne's, Reference Dehorne1933 names D. fimbriata (then D. caulleryi) and D. concharum should, as a result, be retained. Dodecaceria concharum had been described as Form A by Caullery and Mesnil and D. fimbriata as Form B.
The type specimens for D. concharum and D. caulleryi were missing so Gibson & Heppell (Reference Gibson and Heppell1995) attempted to formalize the nomenclature by depositing new types at the National Museums of Scotland. They retained the name used by Dehorne, Reference Dehorne1933.
OERSTED'S TYPE SPECIES
Confusion over the nomenclature of Dodecaceria concharum Oersted, Reference Oersted1843 (Gibson & Heppell, Reference Gibson and Heppell1995) is only partly due to the loss of the type specimen. The original description by Oersted is equivocal: a short paragraph and a sketchy diagram. This confusion is explained by the seminal work on Dodecaceria by Caullery & Mesnil (Reference Caullery and Mesnil1898). They studied specimens from the English Channel at La Hague, Bartleur-Vauville, France (not ’s-Granenhage in the Netherlands). They could not determine whether they were looking at one, two or three species, or three forms (Forms A, B and C) of the same species. They were unable to identify D. concharum, Form A, these three Forms from Oersted's description and one figure. Caullery and Mesnil found Form A to be parthenogenetic (female individuals) and Form C to be a rare parthenogenetic (female) epitoke. They thought that Form A and Form C were the same species and Form B to be sexual.
OERSTED'S TYPE DESCRIPTION
The type description for Dodecaceria concharum by Oersted in 1843 was for a specimen from an unknown oyster bed in the Kattegat. The location of the site from which Dodecaceria was taken was not recorded. It must have been between Skagen (57o45′N 10o35′E) and Frederikshavn (57o28′N 10o31′E) at Hellebaek (56o4′N 12o32′E) a distance of a 125 km. Specimens may have come from galleries in oyster shells produced by a burrowing sponge. However, this was not documented.
Oersted's description of Dodecaceria (1843, p. 44) was translated from colloquial Latin by Geoffrey Thorp MA (Classics teacher at Norwich School, Norfolk). The translation is: It has a body 2′′ long 1–1½′′′ wide around rather smooth dull green, of the 65 segments the middle ones twice as broad as long, head equal in length to the four following segments put together, the front two thread-like branchiae a little longer than the rest, the 7–8 bristles of the superior wings are capillary, the inferior 5–6 of the lower wing stronger and hooked. Obs. A unique specimen differed from the rest in that it had on the first segment two pairs of branchiae, one above other, and the lower ones were short and thicker than the rest. Perhaps it ought to form a species of its own. (′′ = cm; ′′′ = mm; wing = parapodium; around = probably across.)
From this description Oersted appears to have unwittingly described an epitoke (Figure 1). This has not been generally appreciated. The unique specimen in the description in the translation was thought by Oersted to be a separate species. In accordance with normal practice the atoke should have been used for the type description. A problem in describing an epitoke is that epitokes are only found at certain times of the year (Gibson, Reference Gibson1974). Oersted did not record when he took his samples and no type remains.
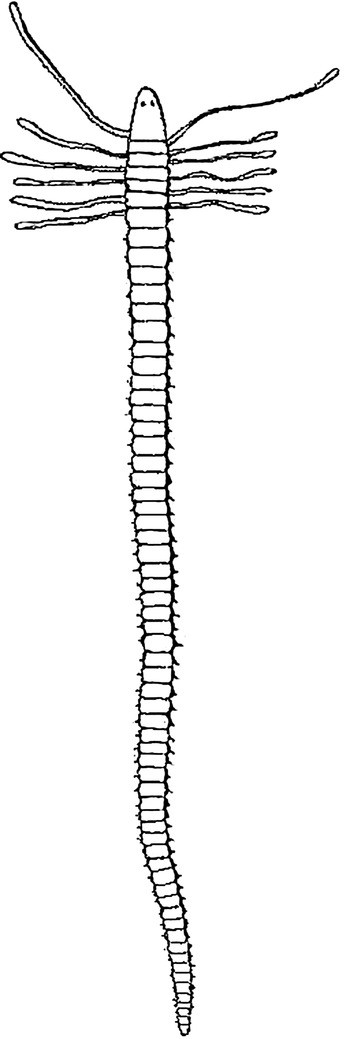
Fig. 1. Plate VI, figure 99 by Oersted (Reference Oersted1843) from his type description for a specimen from the Kattegat. This is likely to be an epitoke since it appears to have a pair of anterior eyes, slender body and apparently atrophied tentacles. If tentacles are still present as in some metamorphosing individuals they should be accompanied by left and right branchiae on the same segment, which they are not in Oersted's figure.
CAULLERY & MESNIL'S DESCRIPTIONS
As noted, Caullery and Mesnil studied specimens Dodecaceria concharum from La Hague in 1898. These authors wrote, categorically, they were uncertain whether they were looking at a single species. Oersted's description was probably for Caullery and Mesnil's Form B 2 (they used the superscript 1 to denote an atoke and superscript 2 for an epitoke) or it could have been for Form A 2 (Form A 2 is the same as Form C). The front two segments of Oersted's specimen had (to quote Oersted) thread-like branchiae (Figure 1). Caullery and Mesnil found the epitoke Form B 2 (Figure 2) had a pair of tentacles of reduced size. Tentacles and the gut of epitokes are absorbed during metamorphosis (Figure 3) (Caullery & Mesnil, Reference Caullery and Mesnil1898; Gibson & Clark, Reference Gibson and Clark1976, Plate II, Figs B & C; Gibson, Reference Gibson1997a , Figure 2). Form B 2 is an epitoke. Oersted under Obs. of his type described a unique specimen with branchiae one above other, and the lower ones were short and thicker than the rest. The lower ones must have been a pair of tentacles (palps) and the specimen was therefore an atoke (Figures 4 & 5). (Caullery and Mesnil's Plate I is missing labelling for figures B 1 & B 2). Therefore the specimen Oersted described was an epitoke. He must have been sampling in late summer and the autumn when most of the atokes, Form B 1 , had metamorphosed.
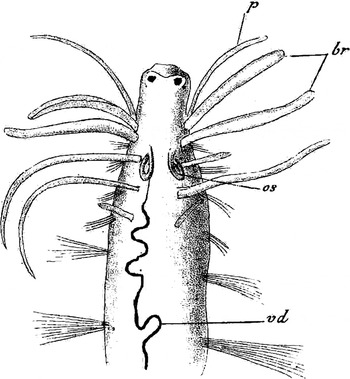
Fig. 2. Plate XXXIX, figure 11, by Caullery & Mesnil (Reference Caullery and Mesnil1898) of the anterior region of Form B 2 , which is in the process of metamorphosing. br = branchiae, p = atrophying palp, os = nephridium, vd = dorsal blood vessel. There is a pair of eyes, and the white area around them is the cerebral ganglion showing through the epidermis (Gibson, Reference Gibson1997a, Figures 1 & 3) (see Figures 2 & 3). The tentacles are slender and in the process of being reabsorbed. The posterior two segments in the figure bear natatory chaetae.
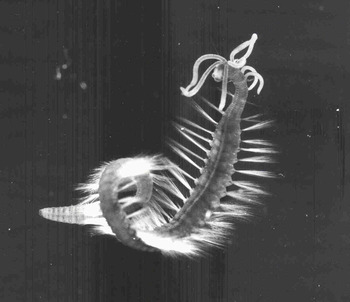
Fig. 3. Photograph of an epitoke of Dodecaceria fimbriata from the east coast of North America (Gibson, Reference Gibson1997b, figure 1D). It has natatory chaetae, a white area surrounding the eyes, as seen in Figure 2 above, and no tentacles.
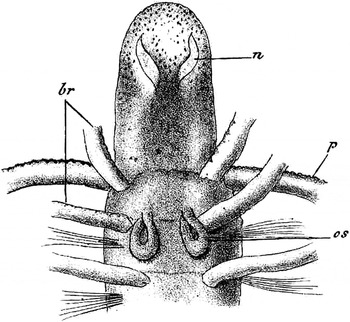
Fig. 4. Plate XXXIX, figure 10 by Caullery & Mesnil (Reference Caullery and Mesnil1898) of the anterior region of Form A. n = nuchal organ, br = branchia, p = palp (tentacle), os = nephridium. This is an atoke, the nuchal organs are prominent and serpentine, and the edges of the prominent tentacles are crenulated.
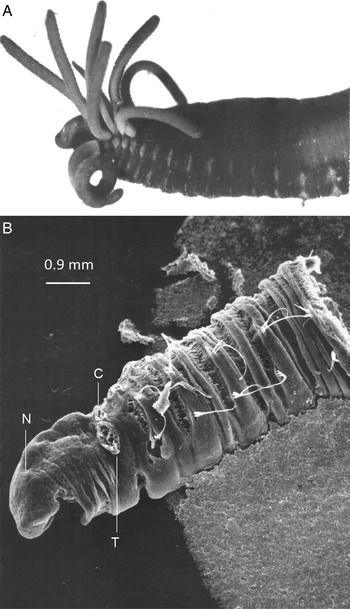
Fig. 5. (A) Photograph of the anterior region of Form A 1 seen from the left side. The slightly coiled tentacle is on first segment below the line of branchiae. The specimen has been preserved and is therefore slightly contracted. In life the tentacles would be longer than the branchiae and are often much coiled. The left serpentine nuchal organ on the head and the region around it can just be made out. Also see figures 1, 2 and 3 in Gibson (Reference Gibson1997a ). (B) Scanning electron micrograph of Form B viewed from the left side. The base of the missing tentacle (T) and branchia (C) are visible. The circular nuchal organ (N) is just visible.
Atokes of Form B 1 were found by Gibson (Reference Gibson1974) to be unable to swim when released from their burrows in the laboratory whereas the epitokes, Form B 2 , could. The epitokes left their burrows at some time in the autumn to spawn (Gibson & Clark, Reference Gibson and Clark1976). They also often left their burrows in the laboratory when disturbed during extraction as specimens and swam vigorously about the collecting dish (Gibson, Reference Gibson1974). They were attracted to the light. They also spontaneously left substratum following the collection of rock samples. The samples were left in sealed plastic bags, which were placed on the lower shore and left for a couple of days. When the bags were opened free epitokes were found. Caullery & Mesnil (Reference Caullery and Mesnil1898) found the same behaviour for epitokes when collecting specimens at La Hague, France.
Oersted appears to have assumed that the predominant species of Dodecaceria in the oyster shells he collected in the Kattegat was B 2 since individuals had probably spontaneously left the shells. He then must have thought the atokes, B 1 , remaining in the shells, were another species, as he suggests. The time of year Oersted sampled oyster shells was not recorded and was probably in late summer or autumn when epitokes are numerous. Sampling in the winter would have been unusual. This would explain why Oersted as well as Caullery and Mesnil never observed asexual reproduction in the species. This occurs after the epitokes have spawned (Dehorne, Reference Dehorne1933; Gibson, Reference Gibson1974).
Caullery & Mesnil (Reference Caullery and Mesnil1898) thought that Form C was derived from the atoke, Form A. Specimens of Form C appeared to be an old epitokes of Form A (Gibson, Reference Gibson1981) and therefore the atoke Form A 1 . At the start of metamorphosis of Form A 1 into Form C (Form A 2 ) the chaetae of both forms were identical. Metamorphosis, as in Form B 1 , occurs only once. It is a terminal spawning stage.
The numbers of epitokes of Form C (A 2 ) are likely to be fewer in number than in the atokes (Form A 1 ) since the atokes have spawned over several years (Gibson, Reference Gibson1981). Because death increases with age Form C would be expected to be rare due to repeated spawning of A 1 . Rare specimens of Forms C (A 1 ) were found by Caullery & Mesnil (Reference Caullery and Mesnil1898) at La Hague and by Gibson (Reference Gibson1981) at Cullercoats. Due to its rarity Caullery and Mesnil suggested that Form C could be a new species.
Both Caullery and Mesnil and Dehorne suggested various names for Form A: Terrebella ostreae Dalyell, Heterocirrus saxicola Grube, H. ater Quatrefages, H. fontifilis Grube, H. multibranchis Grube. However, the descriptions for these are of much the same quality as that of Oersted's (i.e. brief). In fact, Caullery and Mesnil's Form A was not Oersted's species, which was an epitoke of Form B (i.e. Form B 2 ). Dehorne (Reference Dehorne1933) therefore, in effect, defined D. concharum when he separated it from D. caulleryi (now D. fimbriata). Oersted's D. concharum as a result is not a valid species since it cannot be identified from Oersted's description, as noted by Caullery & Mesnil (Reference Caullery and Mesnil1898).
DEHORNE’S SPECIES
Dehorne (Reference Dehorne1933) first described Dodecaceria caulleryi (now D. fimbriata) from Portel, Boulogne-sur-Mer, France in 28 pages with 46 figures. He named the species (the type specimen is missing). This species was Caullery and Mesnil's Form B (Form B 1 ). Dodecaceria caulleryi in Europe was found through its reproduction to be the same as D. fimbriata on the east coast of North America (Gibson, Reference Gibson1997b ).
A key character in Dehorne's description of D. fimbriata (then D. caulleryi) is that it reproduces asexually. Caullery and Mesnil, as noted, did not see asexual reproduction probably because they were sampling at the wrong time of year. As a result of Dehorne's description of the new species D. caulleryi (now D. fimbriata) the name D. concharum described by Oersted for the species found in the Kattegat became the parthenogenetic species Form A (Form A 1 ) of Caullery and Mesnil. Had a type existed this would not have occurred since Dehorne would have realized his new species already existed although inadequately described.
NUCHAL ORGAN, CROTCHETS AND ANUS
The two most useful distinguishing structures of the two species Dodecaceria are the pair of nuchal organs at the front of the head, and the crotchets. In Form B the nuchal organs are small and oval and those of Form A are long and serpentine (Gibson, Reference Gibson1997a , Figure 2) (Figures 4, 5A & B). Each of the two types of crotchets of the two species has a distal depression. In Form B 1 , there is a prominent tooth at the base of depression while Form A lacks this tooth (Caullery & Mesnil, Reference Caullery and Mesnil1898; Gibson, Reference Gibson1978; Gibson & Stoddard, Reference Gibson and Stoddard2005). Caullery and Mesnil show the distribution of the types of chaetae along the body of representative individuals of Form A 1 and Forms B 1 and B 2 . Their method clearly shows the differences between the forms. This was also shown by Martin (1934) for Dodecaceria fimbriata found in Vineyard Sound, USA and expressed graphically by Gibson (Reference Gibson1997b , Figure 4).
Individuals of Dodecaceria have a characteristic dorsal bend halfway down the body. Individuals in the burrow are doubled up with the head and anus projecting in opposite directions from the opening of the burrow. Through contraction of the, presumably, dorsal muscles the body is effectively wedged in the burrow. When released from the burrow individuals appear to continue to contract these muscles, which produce the bend in the body. The individuals initially remain still presumably due to the contraction of these muscles. This bend is not seen in species such as Polydora, which is found in the same habitat, and Cirratulus, which is similar in appearance to Dodecaceria although larger. These species form disorganized writhing spirals.
The shape of the anus of D. fimbriata, Form B, and D. concharum, Form A, differ. Because Form B 1 reproduces asexually the pygidium continues to grow and the four lobes of the anus are therefore small. Form A does not reproduce asexually and therefore the anus is more developed. It has a pair of large rounded ventral lobes and two smaller rounded dorsal lobes. For the same reasons the posterior end of D. fimbriata is generally pointed and has narrower segments. The posterior end continues to grow even after a year. The posterior region of D. concharum, Form A, however, is spatulate (Gibson, Reference Gibson1974). In sexually mature individuals growth ceases since individuals are in the process of producing oocytes (Gibson, Reference Gibson1981). The segments in the posterior region are more defined and broader.
IMPORTANCE OF ASEXUAL REPRODUCTION
Caullery and Mesnil, as noted, never observed asexual reproduction in Form B 1 presumably because fragmentation did not occur at La Hague at the time of year they were sampling. However, the situation appears more complex. The presence of asexual reproduction is retained as a characteristic pattern of pigmentation due to regeneration of new regions, as described by Dehorne (Reference Dehorne1933), Gibson (Reference Gibson1974) and Gibson & Clark (Reference Gibson and Clark1976). If Oersted's species was, as seems likely, Form B he presumably never observed asexual reproduction because he was sampling at the wrong time of the year.
The identification and naming of the two species of Dodecaceria, then, depends, in part upon their methods of reproduction: one is asexual and the other parthenogenetic. Dehorne (Reference Dehorne1933) described the asexual reproduction of D. fimbriata (as D. Caulleryi), Form B 1 , in detail. Although this process occurs in the autumn, the resulting variation in density of pigmentation along the body can be seen throughout the following year and for several years afterwards (Gibson & Clark, Reference Gibson and Clark1976). Specimens ultimately become so deeply pigmented that the regions can only be distinguished with difficulty. The other species, Form A 1 , as noted only has females (Caullery & Mesnil, Reference Caullery and Mesnil1898; Gibson, Reference Gibson1981). This species never reproduces asexually and therefore never shows bands of pigmentation.
IMPORTANCE OF SALINITY
The two species of Dodecaceria can be separated by their tolerance to lowered salinity (Gibson, Reference Gibson1996). Oersted found his specimens, Form B 1 or Dodecaceria fimbriata, in the Kattegat. The species found by Caullery and Mesnil at Portel, Form A or D. concharum, could not have survived in the Kattegat since the salinity was too low. Dodecaceria fimbriata extends into the reduced salinity of the upper reaches of the Firth of Forth (Scotland) (mean 33‰, SD = 2, n = 7, Figure 6A) (Gibson, Reference Gibson1996). Dodecaceria concharum disappears at salinities below 34‰ (SD = 7, N = 7) (Figure 6A). In the Kattegat Dodecaceria, presumably D. fimbriata, survives at salinities lower than 34‰, (Figure 6B) (Jagerskiold, Reference Jagerskiold1971). This difference results in Dodecaceria concharum being found in areas of full salinity in the Channel, Plymouth (Marine Biological Association of the United Kingdom, 1937, 1957). A problem with measurements of salinities is that they vary according to depth, precipitation and temperature (Raymont, Reference Raymont1963; Rodhe & Winsor, Reference Rodhe and Winsor2002).
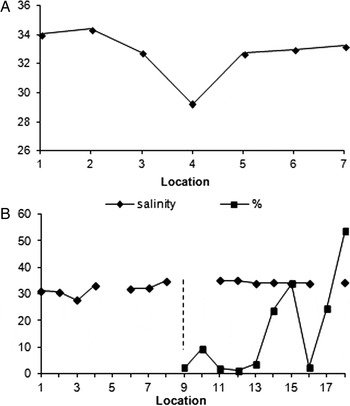
Fig. 6. (A) Changes in salinity along the shoreline of the south side of the Firth of Forth from St Abbs Head (location 18) to South Queensferry (location 1), a distance of some 100 km (Gibson, Reference Gibson1996, tables 5 & 6). Form B, Dodecaceria fimbriata (Dehorne) (see Table 1) was found at all 18 locations and Form A, D. concharum (Gibson & Heppell) (see Table 1) at 10 locations from The Gegan to St Abbs Head (locations 9–18). The mean salinity for locations at low spring tide at which D. concharum was found was 34‰ (SD = 7, N = 7) and the mean for D. fimbriata was 33‰ (SD = 2, N = 18). (B) Changes in salinity along the seabed of the west coast of Sweden plotted from data in Jagerskiold (Reference Jagerskiold1971). From Strömstad (location 1) in north to Halmstad (location 7) in the south, a distance of some 300 km. Dodecaceria was dredged at four locations (Stations 63, 108, 386, 424) and close to three others (Stations 24, 35, 94). Mean recorded surface salinity was 33‰ (SD = 2, N = 6), and the mean depth for the locations was 23 m (SD = 5, N = 7). Identifications were made by A. Eliason and I. Arwidsson but the keys they used were not specified and therefore the species names are unreliable.
Table 1. Summary of differences between Dodecaceria fimbriata, the asexually reproducing species, and D. concharum, the parthenogenetic species
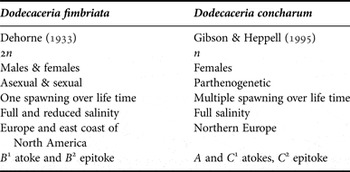
The separation of D. concharum in northern Europe from D. fimbriata in east coast of North America appears to have occurred at the start of the Cretaceous when the north Atlantic was being formed. Letters A, B and C refer to the forms described by Caullery and Mesnil (Reference Caullery and Mesnil1898).
Regenerating asexually produced fragments of Form B 1 and fully regenerated individuals (Gibson & Clark, Reference Gibson and Clark1976) are found in the less saline water of the Firth of Forth (Gibson, Reference Gibson1996, Reference Gibson1999). The atokes must have fragmented there for they could not have migrated from the lower regions of the Firth since the distance is too great. Immature and apparently mature oocytes were also found in atokes of Form B 1 . However, oocytes, shed by the epitokes, B 2 , may not be viable and therefore recruitment would have been through asexual reproduction.
Aberrant gametogenesis and development of the larvae in Form A 1 (Marcel, Reference Marcel1962) were found at lowered salinities. This may restrict the distribution of species to full salinities. The salinity at Portel, Boulogne-sur-Mer, is below 24‰ probably due to the low salinity resulting from the outflow of the Baltic (Gibson, Reference Gibson1996). This drop in salinity at Portel may result in the failure of normal meiosis found in Form A 1 as noted by Marcel (Reference Marcel1962). Some oocytes were found to have more than two asters and others several nuclei. The failure of some coelomic larvae in Form A 1 to develop was noted by Caullery & Mesnil (Reference Caullery and Mesnil1898) who found phagocytosis of oocytes.
There is a drop in salinity along the west coast of Sweden (Jagerskiold, Reference Jagerskiold1971) and the south coast of the Firth of Forth (Figures 6A & B). Along the Firth Form A disappears at salinities below a mean of 34‰ (SD = 7, N = 7). Along the west coast of Sweden Dodecaceria, as defined by Oersted, was found by Jagerskiold at seven sites with a salinity of 33‰ (SD = 2, N = 7). This suggests that the salinities in the Kattegat are too low for the survival of Form A (Gibson, Reference Gibson1996, Reference Gibson1999).
NEW TYPE SPECIES
Since Oersted's description for D. concharum was inadequate Gibson and Heppell deposited a new type (Cat. No. NMSZ 1993063) for D. concharum from Cullercoats, Tyne & Wear, England (Gibson & Heppell, Reference Gibson and Heppell1995) at the National Museums of Scotland (Chambers Street, Edinburgh). A description of the location was given (Gibson, Reference Gibson1974). Specimens were collected from the rock pools outside the harbour wall adjacent to Dove Marine Laboratory, Cullercoats, University of Newcastle upon Tyne, Tyne & Wear (OS NZ 368715). The position of pools was identified to within a few metres from photographs (Gibson, Reference Gibson1974). They were on the lower shore with the laboratory visible in the background. The pools were formed by sandstone strata bedded at about 15° with seams of grey shale running through them. The rock pools were some 25 cm deep and 20–50 m long and contained the encrusting calcareous alga Lithothamnion. Laminaria can be seen in the pools from the photographs. The pools sampled can be found today.
In view of the uncertainty over the identification of D. concharum as described by Oersted the present author suggests that the names D. concharum, Form A, as used by Dehorne, Reference Dehorne1933, should continue to be used. This has the advantage that the species is defined by its method of reproduction. That is, the species is parthenogenetic and shows no signs of asexual reproduction. Dodecaceria fimbriata, Form B, is clearly defined by Dehorne, Reference Dehorne1933 and shows obvious signs of having reproduced asexually at some time in its adult life. Examples of these species, as noted, were deposited as neotype by Gibson and Heppell at the National Museums of Scotland (Cat. No. NMSZ 1993063) in 1995.
ACKNOWLEDGEMENTS
I wish to thank Dr Colin Legg of the University of Edinburgh for helpful criticism of the manuscript. This work is dedicated to David Heppell who died in 2004.









