Introduction
Insomnia disorder is the most common sleep disorder seen in the primary care setting (National Heart, Lung, and Blood Institute Working Group on Insomnia, 1999), affecting an estimated 10% of the adult population (Morin et al., Reference Morin, LeBlanc, Daley, Gregoire and Merette2006). Cognitive behavioural therapy for insomnia (CBTi) is recommended as the first-line treatment, with several meta-analyses demonstrating benefits in sleep outcomes with fewer side-effects compared with hypnotics (Irwin et al., Reference Irwin, Cole and Nicassio2006; Montgomery and Dennis, Reference Montgomery and Dennis2003). In addition, CBTi has been shown to be effective in improving depression in randomized control trials (RCTs) of patients with both insomnia and co-morbid depression (Blom et al., Reference Blom, Jernelov, Kraepelien, Bergdahl, Jungmarker, Ankartjarn and Kaldo2015). The traditional model of CBTi involves 6–8 weekly sessions delivered in-person alongside the use of sleep diaries. However, lack of local service provision has limited widespread use within the UK (Perils and Smith, Reference Perils and Smith2008).
A variety of digital, psychological therapies are now in use as part of UK’s Improving Access to Psychological Therapies (IAPT) programme, which provides a stepped care approach to the treatment of depression and anxiety. Primary care practitioners have direct access to these psychological therapies, often delivered within a stepped care model where many can access low-intensity support via electronic and telephone communication, with a smaller percentage using higher intensity face-to-face therapy. Use of a similar stepped care model to treat insomnia using digital CBTi (dCBTi) is particularly attractive (Christensen et al., Reference Christensen, Batterham, Gosling, Ritterband, Griffiths, Thorndike and Mackinnon2016; Espie, Reference Espie2009) as access to secondary care sleep services can be variable. There is also a potential for improved cost-effectiveness, although cost-effectiveness remains debated in the primary care setting (Schmitz, Reference Schmitz2016) and has yet to be demonstrated in the UK public health setting (van der Zweerde et al., Reference van der Zweerde, Lancee, Slottje, Bosmans, Van Someren, Reynolds and van Straten2016).
Different dCBTi platforms with varying degrees of support and automatization have been developed (Luik et al., Reference Luik, Kyle and Espie2017). dCBTi has been demonstrated in several RCTs to have comparable efficacy to face-to-face CBTi (Zachariae et al., Reference Zachariae, Lyby, Ritterband and O’Toole2016), but adherence remains a challenge with the perception among users and therapists that dCBTi is less effective (Middlemass et al., Reference Middlemass, Davy, Cavanagh, Linehan, Morgan, Lawson and Siriwardena2012). In addition, many of the RCTs performed to date within clinical practice have relied upon initial screening by health care professionals trained within sleep medicine to ensure correct patient selection (Cape et al., Reference Cape, Leibowitz, Whittington, Espie and Pilling2016). As sleep medicine is not routinely taught within the UK, common insomnia mimics such as obstructive sleep apnoea (OSA), delayed sleep phase syndrome (DSPS) and restless legs syndrome (RLS) may be unrecognized. Likewise, screening for patients with significant psychiatric co-morbidity is required as this population is unsuitable for unsupported dCBTi and this requires secondary care assessment. Hence, although prior screening for other sleep problems and significant psychiatric co-morbidities is necessary to allow patients in primary care to undergo unsupported dCBTi, few researchers have addressed this within real-world clinical practice.
In the Regional Sleep Service in Newcastle upon Tyne, a dCBTi programme was used and evaluated in primary care as part of a stepped care programme. General practitioners (GPs) were able to access dCBTi directly without first referring the patient into tertiary care. Screening and selection of suitable patients for therapy was based upon clinical information provided by the primary practitioners, with another layer of screening in-built into the dCBTi platform using detailed questionnaires to elect suitable patients for dCBTi. There was evaluation of engagement and outcomes alongside those considered unsuitable for therapy as they were more likely to have another sleep disorder.
Method
The Regional Sleep Service in Newcastle has utilized CBTi for insomnia treatment for 10 years either through individualized face-to-face CBTi, or CBTi delivered to small groups using standard published CBTi protocols (Morgenthaler et al., Reference Morgenthaler, Kramer, Alessi, Friedman, Boehlecke and Brown2006). Health professionals including GPs locally have good awareness of the service due to annual educational sessions held since 2013. They also have access to standardized sleep information leaflets and self-help materials aligned to the national IAPT HEE website (National Health Service, 2008).
An automated, online dCBTi programme with email support was made available to the Regional Sleep Service (Sleepstation). The clinical content of videos was developed in 2012 by K.A. and its outcomes have been previously published (Anderson et al., Reference Anderson, Goldsmith and Gardiner2014). The service was available as part of a stepped care programme and was listed within the national e-referral system allowing GPs to directly access dCBTi rather than await a secondary care assessment and face-to-face CBTi. Specific advice that the service was only for insomnia was given.
Patient selection and screening assessments performed as part of dCBTi
Information reviewed by K.A. to select potential dCBTi candidates included the patient’s summary care record which included age, gender, area of residence, current and past medical diagnoses, medications and a brief referral letter. Patients were selected for dCBTi based on typical screening procedures undertaken in clinical trials of dCBTi (Lancee et al., Reference Lancee, van Straten, Morina, Kaldo and Kamphuis2016). Unsuitable patients typically had a significant psychiatric co-morbidity or an undiagnosed primary sleep disorder, and this information was then communicated to the referring clinician to allow appropriate management and referral to secondary care assessment.
Suitable patients were contacted by the dCBTi team by telephone and provided with login details to access the dCBTi platform. There were multiple telephone prompts and further written details posted to those who did not respond initially. Patients completed 1 week of online sleep diaries to confirm the diagnosis of insomnia disorder and obtain baseline sleep measures of sleep efficiency (SE) and total sleep time (TST). Patients with high sleep efficiency of >90% were excluded and given information about sleep misperception. These assessments were reviewed by trained staff at Sleepstation.
Screening assessments performed as part of dCBTi include the Epworth Sleepiness Score (ESS; Johns, Reference Johns1991), Pittsburgh Sleep Quality Index (PSQI; Buysse et al., Reference Buysse, Reynolds, Monk, Berman and Kupfer1989) and the 21-item assessment of the Depression Anxiety Stress Scale (DASS-21; Lovibond and Lovibond, Reference Lovibond and Lovidbond1995). Additionally, the online platform allows for patients to be questioned for the presence of snoring, witnessed apnoeas and restless legs syndrome, after video explanations of these disorders were presented. In addition, the requisite IAPT minimum dataset to assess treatment outcomes, the Patient Health Questionnaire (PHQ-9; Kroenke et al., Reference Kroenke, Spitzer and Williams2001), Generalized Anxiety Depression scale (GAD-7; Spitzer et al., Reference Spitzer, Kroenke, Williams and Lowe2006), Work and Social Adjustment Scale (WSAS; Mundt et al., Reference Mundt, Marks, Shear and Greist2002) and IAPT Employment Status questionnaires (IAPT, March 2011) were administered on the online platform. The defined clinical cut-offs for the PHQ-9 and GAD-7 questionnaires are >9 and >7, respectively. The WSAS scores range from 0 to 40, with scores above 20 suggesting moderately severe to severe impact on work function. Those with significant psychiatric co-morbidity that might be high risk for suicidal ideation, ongoing adjustment of psychotropic medication or variable shift work, were also given information about sleep but advised not to proceed with therapy.
dCBTi and outcome measures
The dCBTi programme was a 6–8 week automated therapy programme based on existing published CBTi treatments manuals with patients accessing and completing an online sleep diary daily. Therapy advice was provided through both videos and printable PDF documents. Treatment was tailored to responses on a weekly basis although all patients received the same components of therapy. The system detected whether sleep diary entries were made daily, and patients were prompted by email if entries were omitted. Post-treatment evaluations (PSQI, PHQ-9, GAD-7 and WSAS) were incorporated into the dCBTi module at the end of the 6-week course of therapy, and post-treatment SE, TST and PSQI were calculated based on the last week of sleep diaries completed. IAPT-defined recovery rates and reliable recovery rates were defined according to IAPT protocols (Gyani et al., Reference Gyani, Shafran, Layard and Clark2013): recovery rate was defined as the percentage of patients who scored below the clinical cut-offs on the PHQ-9 and GAD-7 after therapy over those meeting the clinical cut-offs on the PHQ-9 and GAD-7 before therapy; reliable recovery referred specifically to the percentage of patients who moved to recovery with a >6 point change on the PHQ-9 or a >4 point change on the GAD-7.
Ethical approval was not required for this study as data were anonymized and the study was performed to evaluate an existing and approved clinical service.
Statistical analyses
All statistical analyses were performed using SPSS (version 24; IBM Corporation, Armonk, NY, USA). Data were examined for normality of distribution with visual histograms and Kolmogorov–Smirnov’s test. As assumptions for normality were not met except for sleep efficiency, non-parametric tests were used to compare demographics and clinical characteristics between the different patient groups and outcome measures at baseline and following therapy in patients who had completed therapy. A paired t-test was used to compare sleep efficiency at baseline and 6 weeks following therapy. For all tests, a p-value of <0.05 was considered significant.
Results
Between November 2015 and July 2017, 390 patients were referred from their general practitioners for dCBTi treatment (see Table 1).
Table 1. Demographic information of study participants

Based on clinical information provided by the referral letter and the summary care record available to K.A., 291 (74.6%) patients were screened to be suitable and 99 (25.4%) patients were rejected: 52 had a probable non-insomnia sleep disorder (20 with OSA, 15 with DSPS, and 17 with other primary sleep disorders); 22 patients had significant psychiatric co-morbidity that was felt to increase risk for a non-face-to-face therapy. This is detailed in the flowchart (Fig. 1). Most of the patients (19/20) with probable OSA were above age 40 and most of the patients (9/15) with probable DSPS were below age 30. No statistically significant differences in age (p = 0.064) were observed between groups of patients accepted or rejected for dCBTi. There was a higher proportion of female patients (68.0 vs 55.6%) in the group accepted for dCBTi (p = 0.025).
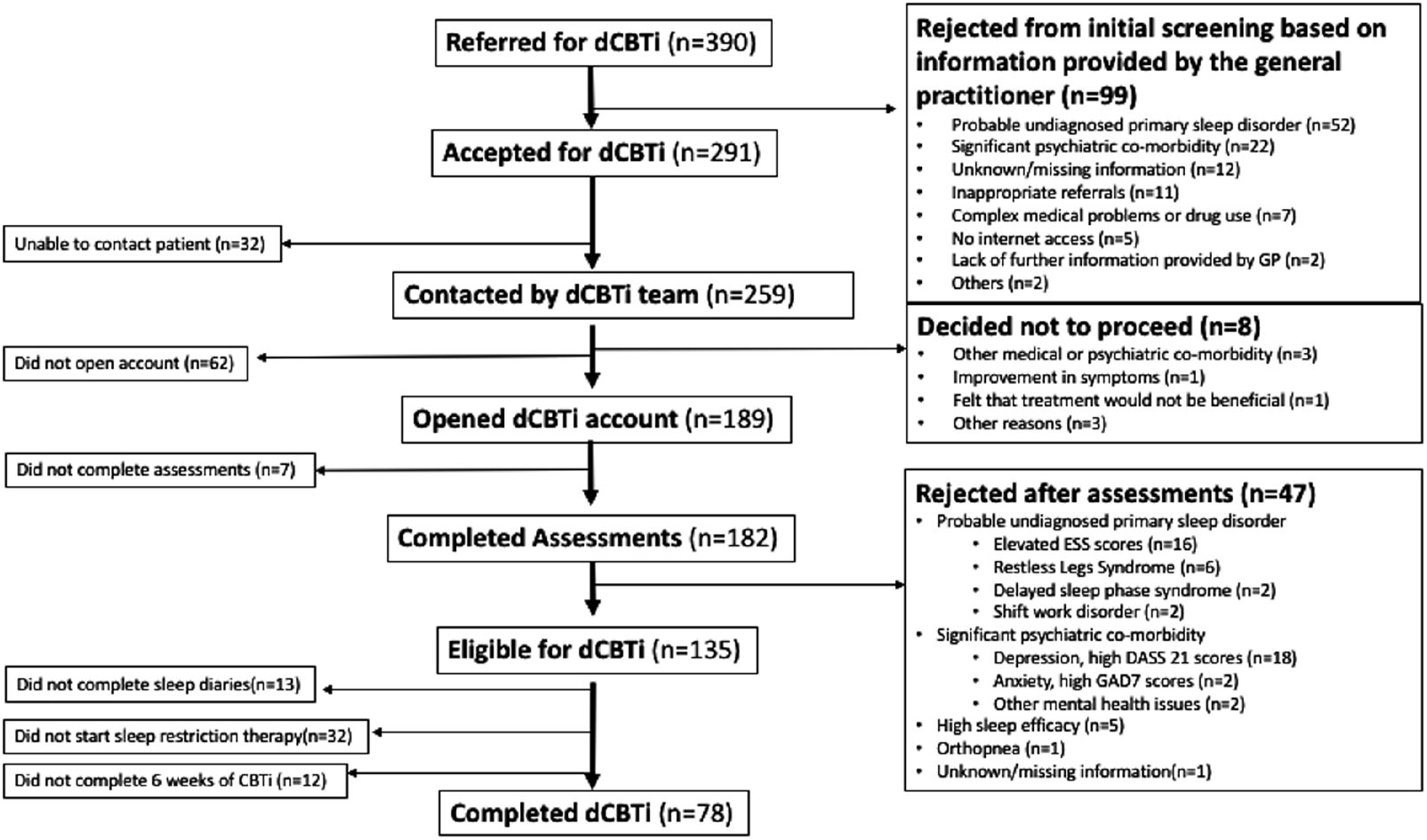
Figure 1. Flowchart detailing outcomes of the 390 patients that were referred for digital cognitive behaviour therapy (dCBTi) from primary care.
Of the 291 patients accepted for dCBTi, 62 who were contacted did not go on to open an account despite prompting by email. Thirty-two (11.0%) patients were uncontactable and eight (2.8%) patients chose not to proceed with dCBTi when contact was made. 189 (73.0%) patients proceeded to open a dCBTi account after contact was made, of which 182 completed the screening questionnaires.
After completing screening and a week of sleep diaries, many did not proceed with therapy either due to recognizing another sleep disorder or psychiatric symptoms. There was no difference in age (p = 0.917) or gender (p = 0.357) when patients were grouped into whether they were accepted or rejected from dCBTi following performance of screening questionnaires.
Following the administration of screening questionnaires, 135 (74.2%) were allowed to progress to the treatment phase of the dCBTi module. Of these 135 patients, 90 (66.7%) initiated dCBTi therapy, 32 (23.7%) only completed sleep diaries but did not start therapy and 13 (9.6%) did not complete sleep diaries.
Of the 90 patients who started therapy, 78 (86.7%) completed all 6 weeks of therapy, which represents 20.0% of the original 390 subject cohort referred by their general practitioners for dCBTi (see Fig. 1).
Demographic, clinical features and outcomes in the 78 patients who completed dCBTi
Of the patients who completed 6 weeks of dCBTi, 55 (70.5%) were female, with an average age of 50.7±13.0 years. Pre- and post-treatment sleep diaries were completed in 73 patients and post-treatment questionnaires were completed in 48 patients. Comparing baseline and 6-week post-treatment outcomes: statistically significant improvements in total sleep time (p = 0.004), sleep efficiency, PSQI, PHQ-9 and WSAS scores (all p < 0.001) were observed. No significant changes in GAD-7 (p = 0.085) scores were observed (see Table 2). Improvements in final sleep efficiency of >20% were observed in 35 (44.9%) patients.
Table 2. Table showing the baseline and post-treatment outcomes in the 78 patients who successfully completed dCBTi therapy

PSQI, Pittsburgh Sleep Quality Index; PHQ-9, Patient Health Questionnaire; GAD-7, Generalized Anxiety Depression scale; WSAS, Work and Social Adjustment Scale. *Seventy-three patients completed post-therapy measures of sleep efficiency and total sleep time, 51 patients completed post-therapy measures of the PSQI and 48 patients completed measure of PHQ-9, GAD-7 and WSAS. aPaired t-test; bWilcoxon signed rank test.
Amongst the 135 patients who were identified to be suitable for dCBTi based on screening questionnaires, dCBTi completers had higher baseline PSQI scores compared with dCBTi non-completers (14.7±2.7 vs 13.5±3.0, p = 0.035) and lower baseline sleep efficiency (58.4±17.8 vs 66.2±12.5%, p = 0.025). Age, gender, sleep, TST, and PHQ-9, GAD-7 and WSAS scores did not differ between dCBTi completers and non-completers (all p > 0.05).
The proportion of patients meeting IAPT definitions for depression and anxiety reduced from 33.3% (26 patients) to 9.0% (7 patients). When only complete cases were analysed, 12 (75.0%) of the 16 patients who scored above the PHQ-9 or GAD-7 threshold at baseline had recovered post-dCBTi. Of these 16 cases, nine (56.3%) had an IAPT defined reliable recovery, which refers to patients with >6 point change on the PHQ-9 or >4 point change on the GAD-7.
Discussion
In this naturalistic study of eligibility, uptake and adherence of dCBTi in the UK primary care setting, only 20.0% of insomnia patients referred from their general practitioners were eligible for dCBTi and completed therapy. However, in this group that completed therapy, 84.6% reported improvements in sleep efficiency and quality, and 44.9% achieved improvements in sleep efficiency of greater than 20%. One-third of the cohort referred from primary care were unsuitable for dCBTi, typically due to the presence of an unrecognized probable primary sleep disorder or complex psychiatric disorder; highlighting both lack of recognition of common insomnia mimics and the need for careful screening in any digital therapy prior to starting therapy. Despite initial patient interest, 35.1% did not engage with the dCBTi to allow screening and treatment to begin.
In this real-world study of dCBTi in primary care, we show that dCBTi can be highly effective in the small subset of patients who both were screened to be suitable for therapy and were sufficiently engaged with the dCBTi platform. This study achieved an IAPT defined recovery rate of 75% and a reliable recovery rate of 56.3%. Our findings are comparable with another study where dCBTi was supplemented by more intensive weekly support telephone calls lasting 20 to 30 minutes (Luik et al., Reference Luik, Bostock, Chisnall, Kyle, Lidbetter, Baldwin and Espie2016), and exceeds the IAPT recovery rate target of >50%, and reliable recovery rate of >43% (Gyani et al., Reference Gyani, Shafran, Layard and Clark2013). The low proportion of patients who are both screened to be suitable for therapy and sufficiently engaged with the dCBTi platform to gain potential benefit was also observed in a previous study recruiting patients from a community health service (Feuerstein et al., Reference Feuerstein, Hodges, Keenaghan, Bessette, Forselius and Morgan2017).
In the patients who commenced therapy, we achieved 86.7% compliance, which is consistent with our previous findings (Anderson et al., Reference Anderson, Goldsmith and Gardiner2014). Age and gender did not determine adherence or rejection from therapy. Our compliance rates compare favourably to the CONSORT clinical trial where participants were given significant financial incentives. Our compliance rates are higher than other studies (Feuerstein et al., Reference Feuerstein, Hodges, Keenaghan, Bessette, Forselius and Morgan2017; Luik et al., Reference Luik, Bostock, Chisnall, Kyle, Lidbetter, Baldwin and Espie2016) which had provided weekly telephone support, an online community (Luik et al., Reference Luik, Bostock, Chisnall, Kyle, Lidbetter, Baldwin and Espie2016) or small financial incentives (Feuerstein et al., Reference Feuerstein, Hodges, Keenaghan, Bessette, Forselius and Morgan2017). The more stringent screening that is incorporated within our dCBTi platform to exclude unsuitable patients may have contributed to the higher adherence rates (Fernandez et al., Reference Fernandez, Salem, Swift and Ramtahal2015; Gearing et al., Reference Gearing, Townsend, Elkins, El-Bassel and Osterberg2014).
One-third failed to engage with the dCBTi platform. Higher engagement levels of 81.7% were achieved in a UK study recruiting patients from a community health service (Luik et al., Reference Luik, Bostock, Chisnall, Kyle, Lidbetter, Baldwin and Espie2016), but the process of subject selection was not defined, patients were existing clients of a community-based provider of psychological services and were already familiar with similar therapies, with dCBTi further supplemented by more intensively weekly support telephone calls lasting 20 to 30 minutes and an online community. Even higher levels of engagement of 100% have been achieved in clinical trials (Ritterband et al., Reference Ritterband, Thorndike, Ingersoll, Lord, Gonder-Frederick, Frederick and Morin2017). The use of delayed cash incentives may well be contributory and some trials had recruited from online advertisements, thus self-selecting for individuals who are more technologically able and are more likely to engage with dCBTi.
Still, patient engagement achieved in this study is considerably higher than the 6–10% reported from the UK public and the primary care setting in the Netherlands (Beaulac et al., Reference Beaulac, Vincent and Walsh2015; Feuerstein et al., Reference Feuerstein, Hodges, Keenaghan, Bessette, Forselius and Morgan2017). Low engagement rates were reported when dCBTi brochures were not actively handed out by the GPs but only provided in the waiting room (Beaulac et al., Reference Beaulac, Vincent and Walsh2015). However, some GPs are not sufficiently familiar with dCBTi to answer questions from their patients (Beaulac et al., Reference Beaulac, Vincent and Walsh2015). Qualitative studies have suggested that in patients who had initiated dCBTi, most had no expectations of the programme and had tried dCBTi due to the failure of other interventions (Chan et al., Reference Chan, West and Glozier2017) or held beliefs in the effectiveness of the programme and their potential for recovery (Barazzone et al., Reference Barazzone, Cavanagh and Richards2012). Hence, GPs play an important role in facilitating dCBTi engagement but may need further training in sleep and insomnia. In our study, referral to dCBTi was via the GPs with oversight by a sleep neurologist, which could afford a greater medical legitimacy, improve acceptance and enhance engagement with dCBTi (Batterham et al., Reference Batterham, Neil, Bennett, Griffiths and Christensen2008). Studies in clinical trial settings have suggested that non-adherence typically occurs during sleep restriction phase (Chan et al., Reference Chan, West and Glozier2017); however, we show that patient engagement in the real-world setting was poor even before therapy could begin.
The most common reasons for non-engagement included the failure to contact the patient and to open an account. About 10% of patients screened to be suitable for dCBTi were repeatedly uncontactable by telephone. Other studies have reported 25% of their patients to be uncontactable (Feuerstein et al., Reference Feuerstein, Hodges, Keenaghan, Bessette, Forselius and Morgan2017). Our study design did not allow us to explore other reasons underpinning poor engagement: possible reasons could include spontaneous remission of insomnia, lack of motivation, technical difficulties, technical issues with the dCBTi platform, or difficulty adhering to sleep diaries or sleep restriction therapy. These reasons for non-engagement would need to be explored in future studies to allow dCBTi to be better designed to be implemented from the primary care setting. However, spontaneous remission of insomnia was uncommon when telephone contact was made.
In the primary care setting, more than one-third of the cohort referred by the GPs were unsuitable for dCBTi. Insomnia mimics such as OSA, DSPS and RLS were common reasons for dCBTi rejection. Our findings are consistent with studies performed on clinical trial subjects recruited from the public where rigorous screening was performed (Lancee et al., Reference Lancee, van Straten, Morina, Kaldo and Kamphuis2016; Ritterband et al., Reference Ritterband, Thorndike, Ingersoll, Lord, Gonder-Frederick, Frederick and Morin2017) and within patients attending a community mental health service (Feuerstein et al., Reference Feuerstein, Hodges, Keenaghan, Bessette, Forselius and Morgan2017). In an earlier study where we had recruited directly from the public, two-thirds were excluded from dCBTi (Anderson et al., Reference Anderson, Goldsmith and Gardiner2014). Demographic factors did not influence subject acceptance to dCBTi. In the UK, GPs generally have insufficient time or knowledge to be able to provide the CBTi themselves (Everitt et al., Reference Everitt, McDermott, Leydon, Yules, Baldwin and Little2014), and tend to treat insomnia pharmacologically. Although using a more dogmatic checklist could have reduced dCBTi rejection rates, we had deliberately designed the referral process to be simple to allow uptake and implementation in busy clinical settings.
Of the patients who had engaged sufficiently with dCBTi to be screened to be suitable for therapy, a further one-third had dropped out before therapy. Average drop-out rates have been estimated at 24.7% in a meta-analysis (Zachariae et al., Reference Zachariae, Lyby, Ritterband and O’Toole2016) and are similar to findings from a large IAPT cohort in the UK (Elison et al., Reference Elison, Ward, Williams, Espie, Davies, Dugdale and Smith2017). dCBTi non-completers had lower baseline PSQI scores and higher sleep efficiency, which reflect better sleep quality when compared with dCBTi completers. Previous studies have suggested that higher sleep efficiency (Espie et al., Reference Espie, Bostock, Paluzzi and Hames2014) and greater depression severity (Luik et al., Reference Luik, Bostock, Chisnall, Kyle, Lidbetter, Baldwin and Espie2016; Yeung et al., Reference Yeung, Chung, Ho and Ho2015) and anxiety (Yeung et al., Reference Yeung, Chung, Ho and Ho2015) were associated with poor adherence; contradictory findings of both longer (Yeung et al., Reference Yeung, Chung, Ho and Ho2015) and shorter sleep time have been associated with poor adherence (Ong et al., Reference Ong, Kuo and Manber2008). It has been suggested that patients with higher levels of depressive symptoms could benefit from increased support as part of dCBTi (Lancee et al., Reference Lancee, Sorbi, Eisma, van Straten and van den Bout2014); however, we were not able to assess differences in depression and anxiety scores to influence adherence given that patients with significant anxiety and depression were excluded from dCBTi using screening questionnaires.
We found improvements in the PHQ-9 and WSAS scores, consistent with another IAPT study which showed that the use of the dCBTi platform resulted in improvements in the PHQ-9, GAD-7 and WSAS scores (Elison et al., Reference Elison, Ward, Williams, Espie, Davies, Dugdale and Smith2017). dCBTi has been shown to be cost-effective in one study of guided dCBTi for insomnia to teachers, with a cost saving of US$418 achieved per patient for the employer (Thiart et al., Reference Thiart, Ebert, Lehr, Nobis, Buntrock, Berking and Riper2016). These findings are in line with studies demonstrating an improvement in depressive symptoms in patients with both insomnia and co-morbid depression (Blom et al., Reference Blom, Jernelov, Kraepelien, Bergdahl, Jungmarker, Ankartjarn and Kaldo2015).
Limitations include a lack of long-term follow-up to see if benefits were maintained. This was intended to be an evaluation of implementation within a care pathway so the study was not designed for long-term follow-up. Patients were rejected based on the presence of a probable primary sleep disorder or psychiatric disorder identified from referral letters or screening questionnaires, but actual confirmation of these diagnoses was not always available. Patients were recruited based on insomnia complaints, rather than DSM-V definitions of insomnia; however, this allows the real-world utility of dCBTi to be examined in the primary care setting. Still, this naturalistic study in the real-world setting provides valuable insight into patient behaviour and engagement with a dCBTi platform in the primary care setting in UK. However, our study design did not allow us to explore the factors underlying non-engagement with the dCBTi module before and during therapy. Future studies in this population group derived from primary practice are necessary to identify reasons for non-engagement with the dCBTi module. Other studies conducted from the primary care setting exploring dCBTi implementation and dissemination from the GP’s perspective are necessary to allow the optimal design of dCBTi to suit the GPs and their patients. We had studied dCBTi evaluated as a stand-alone service within the primary care setting. Future studies evaluating dCBTi as part of a more comprehensive mental health service as part of IAPT are necessary.
A recently published single-blind study of therapist-supported dCBTi utilized a semi-automated algorithm to identify patients at risk of treatment failure during early therapy, and were able to demonstrate that an adaptive treatment strategy with greater contact time for patients at risk of treatment failure improved treatment outcomes (Forsell et al., Reference Forsell, Jernelov, Blom, Kraepelien, Svanborg, Andersson and Kaldo2019). Replication of similar studies in the primary care setting would be needed to confirm the effectiveness of such an adaptive treatment strategy without therapist support.
In conclusion, implementation of dCBTi within the UK NHS public healthcare setting is feasible, achieving sleep-related benefits and outcomes exceeding IAPT targets amongst dCBTi completers. Of those referred, only one in five successfully completed therapy. Those with worse sleep were most likely to engage. There were improvements in sleep outcomes and also other IAPT outcomes. Many were unsuitable for insomnia therapy with evidence of other sleep disorders, highlighting the need for training in sleep disorders across healthcare providers to allow correct recognition of insomnia mimics.
It also highlights the need for safe screening in addition to the IAPT minimum dataset to allow safe treatment and avoid any potential side-effects of, for example, sleep restriction with regard to daytime sleepiness and driving safety. Clinicians or dCBTi providers may need to provide more support to encourage patients to initiate the process of dCBTi, and improvements in the selection of appropriate insomnia patients, specific dCBTi-targeted education in the primary care setting, should be more formally evaluated to allow dCBTi to be better integrated within primary care settings.
Author ORCID
Zheyu Xu, 0000-0002-7404-9240
Acknowledgements
None.
Conflicts of interest
Dr Zheyu Xu has no conflict of interest with respect to this publication. Dr Kirstie Anderson had developed the clinical video content for Sleepstation in 2012, and was responsible for clinical governance between 2012 and 2017. However, she has not received any financial incentive, payment or any kind of honoraria from the Rubrum (the company that developed Sleepstation) throughout the process. She is neither a board member nor a shareholder in Rubrum.
Ethical statement
Ethical approval was not required for this study as data were anonymized and the study was performed to evaluate an existing and approved clinical service. The authors have abided by the Ethical Principles of Psychologists and Code of Conduct as set out by the APA during the conduct of the study.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Key practice points
(1) Only one in five patients referred from the primary care setting for unsupported dCBTi were assessed to be suitable for dCBTi and had completed therapy.
(2) In dCBTi completers, good sleep-related and IAPT outcomes were achieved, suggesting that dCBTI as part of a stepped care approach could be a useful model for insomnia treatment in the primary care setting.
(3) Many patients referred from primary care for dCBTi had unrecognized other primary sleep disturbances or significant psychiatric disturbances rendering them unsuitable for dCBTi therapy.
(4) Careful screening of patient referrals for unsupported dCBTi therapy is necessary to ensure that the use of unsupported dCBTi is safe.






Comments
No Comments have been published for this article.