Introduction
Seed dormancy regulates germination until near optimal conditions for seedling emergence and establishment in species-specific ways. The degree of physiological dormancy is well known to influence germination timing, thereby setting up variation among species in their emergence patterns (Baskin and Baskin, Reference Baskin and Baskin2014). However, another component of dormancy that has received less attention with regard to germination timing is initial embryo size and the amount of embryo growth that must occur within seeds before radicle emergence (Vivrette, Reference Vivrette, Arroyo, Zedler and Fox1995; Verdú, Reference Verdú2006; Vandelook et al., Reference Vandelook, Verdú and Honnay2012). Seed embryos may have distinguishable cotyledons and a radicle, but under-developed embryos must elongate within the seed before radicle emergence, in contrast to fully developed embryos which simply push through the seed coat once key germination conditions are met (Baskin and Baskin, Reference Baskin and Baskin2014). The two types of embryos may also have a physiological block that must be overcome to enable embryo growth and/or germination. Altogether, the degree of physiological dormancy and timing of dormancy loss interacts with embryo growth to time germination and emergence in response to environmental cues.
The flora of Mediterranean ecosystems contains families with species having fully developed embryos in their seeds as well as species with under-developed embryos. Physiological dormancy is prominent in seeds of many species, with germination and seedling recruitment only possible during a relatively narrow window during the winter-wet season that is punctuated by the long summer-dry season (Merritt et al., Reference Merritt, Turner, Clarke and Dixon2007; Carta et al., Reference Carta, Hanson and Müller2016; Bart et al., Reference Bart, Tague and Dennison2017). In these habitats, fire is a major periodic disturbance that provides a primary recruitment opportunity for many plants due to increased resource availability (i.e. moisture, nutrients and light), especially for short-lived fire ephemerals that solely rely on seeds stored in the soil seed bank (Rundel and Parsons, Reference Rundel and Parsons1984; Brown, Reference Brown1993; Miller and Dixon, Reference Miller, Dixon and Lambers2014). Heat and smoke are the main components of fire influencing germination, and within smoke, KAR1 is a key compound that elicits remarkable germination across many taxonomically diverse species (Chiwocha et al., Reference Chiwocha, Dixon, Flematti, Ghisalberti, Merritt, Nelson, Riseborough, Smith and Stevens2009).
During summer months in Mediterranean ecosystems, we speculated that release from physiological dormancy would occur in seeds of our study species regardless of embryo type, but under-developed embryos would not grow as seeds are in a relatively dry state (Turner et al., Reference Turner, Merritt, Ridley, Commander, Baskin, Baskin and Dixon2006). When autumn rains begin in concert with a reduction in temperatures, radicles in seeds with fully developed embryos would readily emerge. In contrast, under-developed embryos would first need time to grow inside the seed before radicle emergence, and thus, germination would be delayed. Concomitantly, under-developed embryos may grow at different rates, leading to non-uniformity in emergence among seeds, and thus spreading emergence over a wider period of time (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012). Germination may also be spread over more than one season, due to variation in dormancy depth amongst individual seeds within a seed population (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012). In addition to germination stimulation, KAR1 and smoke water have been demonstrated to enhance seedling growth and vigour (Daws et al., Reference Daws, Davies, Pritchard, Brown and Van Staden2007; Ghebrehiwot et al., Reference Ghebrehiwot, Kulkarni, Kirkman and Van Staden2009; Nelson et al., Reference Nelson, Flematti, Ghisalberti, Dixon and Smith2012; Zhou et al., Reference Zhou, Kulkarni, Huang, Guo and Van Staden2012). Thus, we reasoned that these smoke products would enhance embryo growth prior to germination and allow earlier emergence for treated seeds compared with non-treated seeds.
In the present study, we investigated the patterns of embryo growth, germination and seedling emergence as influenced by climatic factors and fire-related cues. Four common, sympatric annual species were studied: Podotheca angustifolia (Labill.) Less. (Asteraceae) and Austrostipa macalpinei (Reader) S.W.L. Jacobs and J. Everett (Poaceae) with fully developed embryos, and Trachymene pilosa Sm. (Apiaceae) and Levenhookia pusilla R. Br. (Stylidiaceae) with under-developed embryos. In the winter rainfall region of southwest Western Australia, these species grow and co-occur in fire-prone habitats, such as Banksia woodlands and eucalypt forests (Lamont, Reference Lamont1985; Grant and Loneragan, Reference Grant and Loneragan1999; Barrett and Tay, Reference Barrett and Tay2005), and in less fire-prone areas, such as granite outcrops (Hopper et al., Reference Hopper, Brown and Marchant1997). The highest population densities of these species are usually found in woodlands or forests following fire, although they do also occur and persist in habitats that have not been recently burnt.
The first aspect of our study was to examine the loss of physiological dormancy over time in relation to growth of embryos within seeds prior to radicle emergence and germination under a range of field and laboratory conditions among our study species. To achieve these aims, we tested embryo growth and germination on water, KAR1 and smoke water in light and darkness. Second, we followed the emergence of our study species in a tunnel-house trial, and related the emergence observed to ambient temperatures, soil moisture and the application of smoke. Specifically, we documented the onset, median and time to maximum emergence and the amount of emergence during two winter seasons.
Materials and methods
Plant material
Freshly matured caryopses (Austrostipa), true seeds (Levenhookia), achenes (Podotheca) and mericarps (Trachymene) (hereafter, seeds) were collected during the dispersal season: in November 2007 for Podotheca and Trachymene (hereafter, 2007 cohort) and for all four species in November 2008 (hereafter, 2008 cohort). Due to the ephemeral nature of the populations for each species and the large number of seeds required for experimentation, seeds were obtained from several hundred individuals growing at three sites: recently burnt Banksia woodlands near Perth (31°56′59″S, 115°51′38″E) or near Pinjar (31°39′27″S, 115°49′17″E) or in a historically burnt open Eucalyptus marginata (jarrah) forest near Pinjarra (32°36′59″S, 115°52′38″E), Western Australia. Seeds were kept dry in cloth bags at room conditions (21°C) between collection and initiation of experiments, which began within the first week following collection. Trachymene produces a mix of bristly and tuberculate mericarps, which were combined in approximate equal proportions for experiments as embryo growth and germination did not differ between them (S.N. Hidayati and J.L. Walck, unpublished data).
General embryo growth and germination test procedures
Every time embryo growth was recorded prior to radicle emergence, embryos from 25 seeds were excised using a razor blade and measured under a dissecting microscope with a micrometer. The length of the seeds from which the embryos were excised was also measured and embryo lengths were converted to a ratio (percentage) of seed length. If a seed had germinated, embryo length was estimated by determining its fully elongated length inside the seed (i.e. the point of emergence from the seed); thus, the ratio for a germinated seed was 100%. Seeds in a dry condition (i.e. freshly collected or stored in the field or laboratory) were imbibed at room temperature for 24 h before embryos were excised.
To test germination, seeds were placed on two glass microfibre 1.2 µm filter papers in plastic Petri dishes [9.0 cm (diameter) × 1.5 cm (depth)] moistened with one of three solutions: distilled water, 0.67 µM KAR1 (3-methyl-2H-Furo[2,3-c]pyran-2-one) synthesized from pyromeconic acid as described in Flematti et al. (Reference Flematti, Ghisalberti, Dixon and Trengove2005), or 1:10 (v/v) smoke water produced following Dixon et al. (Reference Dixon, Roche and Pate1995). The purpose of using the two smoke solutions was to simulate some of the effects of fire under laboratory conditions; we have found consistently that smoke-sensitive species respond readily to the concentrations used (e.g. Merritt et al., Reference Merritt, Kristiansen, Flematti, Turner, Ghisalberti, Trengove and Dixon2006; Stevens et al., Reference Stevens, Merritt, Flematti, Ghisalberti and Dixon2007; Commander et al., Reference Commander, Merritt, Rokich and Dixon2009; Turner et al., Reference Turner, Merritt, Renton and Dixon2009; Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012).
Three replications of 25 seeds per dish were used in each treatment. Dishes were wrapped with plastic film to reduce water loss, and those in dark treatments were additionally wrapped in aluminium foil to exclude all light. Protrusion of the radicle was the criterion for germination. Incubation lasted for 4 weeks in light or in darkness to ensure adequate time for embryo growth and radicle emergence; seeds were not examined while they were in darkness. At the end of 4 weeks, germinated seeds were counted and embryos in non-germinated seeds were examined and determined to be viable if they were firm and white (confirmed by tetrazolium tests; Grabe, Reference Grabe1970), or non-viable if they were soft and grey. Germination percentages were determined based on the number of viable seeds.
A temperature- and light-controlled incubator was used to study embryo growth as well as to test germination. It was set at daily alternating (12 h/12 h) 18/7°C that approximated the mean daily maximum and minimum air temperatures for Perth from May to September (Bureau of Meteorology, 2009). This temperature is optimum for germination of the study species (S.N. Hidayati and J.L. Walck, unpublished data) and simulates the cooler wet season when seedling emergence occurs for most species in southwest Western Australia (Merritt et al., Reference Merritt, Turner, Clarke and Dixon2007). The 12-h photoperiod corresponded to the high-temperature period with the light source being 30 W cool white (400–700 nm) fluorescent tubes with photon flux density at seed level of 50 μmol m–2 s–1.
Patterns of embryo growth and germination
To understand the timing and amount of embryo growth and germination in relation to physiological dormancy loss, if any, over summer and into autumn, we studied seeds placed on/into the soil in the field and seeds stored under laboratory after-ripening conditions. At the start of the experiment, 2008-cohort seeds were separated into batches and the following occurred: (1) the lengths of embryos and seeds were measured for Levenhookia and Trachymene, (2) germination tests were performed for all four species in light and in darkness at 18/7°C, and (3) about 500 seeds for the four species were each placed into fine-mesh nylon bags [5 cm (length) × 5 cm (width)]. The bags of seeds were stored in the field for Podotheca, Levenhookia and Trachymene or in the laboratory for all four species, starting on 9 December 2008.
For field storage, we established a site in natural Banksia woodland at Kings Park and Botanic Garden; the soil at this site was classified as Karrakatta sands of the Spearwood Dunes (Bennett, Reference Bennett1988). We used 24 bags per species allowing for extra bags in case some were destroyed or lost and placed one bag into a plastic tray [7 cm (depth) × 15 cm (length) × 7 cm (width), with drainage holes] filled with soil from the site. In the tray, the bag was either (1) buried at 5-cm depth or (2) placed on top of the soil surface with ca 1 mm of soil sprinkled on top. Four trays (two with a buried bag and two with a surface bag) each per species were placed into each of six plots [60 cm (length) × 60 cm (width)]. The plots were placed at 5 m intervals along an unused sand track in the woodland. In each plot, the trays were randomly positioned (with respect to species and bag placement) alongside each other (in a 2 × 6 design) and sunk into the ground so that the top was even with the surrounding soil surface. All the trays in a plot were covered with a metal screen secured to the ground with metal pins to protect against predators. Part of this woodland was burnt in January 2009, and while the immediate site remained non-burnt, two plots were destroyed by vehicles extinguishing the fire. In January, March and May 2009, a randomly selected buried and surface bag was removed from a plot. After removal, the bag was opened and the number of germinated seeds counted. For non-germinated seeds, (1) lengths of embryos and seeds were measured for Levenhookia and Trachymene, and (2) germination was tested in light on each solution at 18/7°C for all three species.
For laboratory storage, we placed 20 bags above a non-saturated solution of LiCl (370 g l–1) in an air-tight polycarbonate box [14 cm (depth) × 28 cm (length) × 28 cm (width); NHP Fibox, Australia]. The atmosphere inside the box was maintained at ca 50% relative humidity (Gold and Hay, Reference Gold and Hay2014). The box was kept in a 30°C incubator, simulating summer temperature, with a 12-h photoperiod. One randomly selected bag for each species was obtained from the box each month between January 2009 and May 2009 (except for Austrostipa, which was removed every other month). Every time a bag was removed, (1) lengths of embryos and seeds were measured for Levenhookia and Trachymene, and (2) germination was tested in light and in darkness on each solution at 18/7°C for all four species.
To determine the start of embryo growth and germination on a fine-interval scale, we measured embryos and recorded germination in seeds of Levenhookia and Trachymene that had been after-ripened in the laboratory. We placed 50 seeds each of these two species into three Petri dishes on each solution at 18/7°C when they were removed after 5 months of dry storage at laboratory conditions (in May 2009). At 4-day intervals up to 28 days (or until all seeds germinated), we randomly selected 8–9 germinated or non-germinated seeds from each dish (to total 25) and: (1) recorded if a seed had germinated, and (2) measured the length of the embryo and seed. We determined the relative growth rate of the embryos on each solution over the total measurement period: [(logeE 2 – logeE 1)/(t 2 – t 1)], where E is the embryo length and t is time between two intervals, 1 (in fresh seeds) and 2 (seeds incubated 28 days for Levenhookia or 20 days for Trachymene) (Walck et al., Reference Walck, Baskin and Baskin1999). Calculations were made for paired seeds on the basis of ranking by embryo length in each of two sequential measurements.
Patterns of seedling emergence
To document the timing and amount of emergence, we used a tunnel-house, located in Kings Park and Botanic Garden (Perth, Western Australia). This tunnel-house had no heating or air conditioning and had only the roof and the top-half of its sides covered with fibreglass sheeting forming a rain-out shelter. For each species, 200 seeds each were sown on 23 November 2007 (2007 cohort) or on 9 December 2008 (2008 cohort) into eight 10-cm diameter plastic pots. Seeds were sown on the surface and then lightly covered with about 1 mm of soil. The pots were filled with a soil mixture (4:2:1 composted jarrah sawdust:fine river sand:coarse river sand, pH adjusted to 6.0 with addition of 1 kg of lime and 800 g of dolomite per 1 m3 of soil; 2007 cohort) or with fine river sand (2008 cohort) and placed on benches. Before use, the soil mix, sand and pots were steam-pasteurized for 1 h at 60–70°C.
The soil mix and sand were watered to maximum capacity weekly during summer and early autumn (dry season, September to March), twice a week in mid-autumn (first 2 weeks in April), then daily from late autumn into winter (wet season, last 2 weeks in April to August) to simulate natural precipitation (Turner et al., Reference Turner, Merritt, Ridley, Commander, Baskin, Baskin and Dixon2006; Merritt et al., Reference Merritt, Turner, Clarke and Dixon2007). One week after the start of daily watering, four randomly selected pots were exposed to 1 h of aerosol smoke in late April of 2007 and 2008 (following Dixon et al., Reference Dixon, Roche and Pate1995), while the other four served as control pots. Following smoking, the pots were returned to the tunnel-house and daily watering of all pots resumed the next day. Experiments in the tunnel-house ended on 26 June 2009, as no new seedlings had emerged for at least 2 weeks prior to this date.
Scoring was done weekly using a magnifying glass to examine the soil surface for signs of emergence. If seedlings (with an emerged radicle and/or cotyledon) were present, they were counted and gently removed using small forceps. We defined emergence seasons as the period between the commencement of twice weekly watering in April and the point where no additional seedling emergence was observed during the wet season. Emergence percentages were calculated based on the number of seeds sown. Parameters estimated from the cumulative emergence curves during each season using GERMINATOR software (Joosen et al., Reference Joosen, Kodde, Willems, Ligterink, van der Plas and Hilhorst2010) were: maximum emergence (defined as Gmax in the software), onset of emergence (i.e. time to 1% of Gmax), median emergence (i.e. time to 50% of Gmax), and the spread of emergence (i.e. time between 10 and 90% of Gmax).
Temperature and precipitation records
Maximum and minimum daily air temperatures throughout the study (23 November 2007 to 26 June 2009) were determined from regional records at the Perth weather station (#009225; Bureau of Meteorology, 2009), approximately 5 km from Kings Park and Botanic Garden. Data loggers (Tinytag Plus 2, Gemini Data Loggers, Chichester, West Sussex, UK) were placed on top of the soil surface in a pot in the tunnel-house and on the soil surface in the woodland in Kings Park and Botanic Garden and recorded soil temperatures on an hourly basis. Data loggers were located approximately 10 and 50 m from our study pots or plots, respectively. Daily precipitation during the study was obtained from the Perth weather station, as previously described.
Statistical analyses
Generalized linear models (GLMs; binomial error structure, logit link function) or analyses of variances (ANOVAs) followed by protected least significance difference tests (PLSD, P = 0.05) were used to examine the data (SPSS, 2012; R Core Team, 2014). For GLMs, the main factor terms and their two-way interactions were added sequentially followed by a Wald test to validate the final model (Fox, Reference Fox2015). All factors in the GLMs were analysed as discrete terms. Germination from seeds was examined using GLM with species, time for storage, location (buried, surface), and solution as factors in the field storage experiment and with species, time for storage, light regime (light, dark), and solution as factors in the laboratory experiment. Relative growth rates for embryos were examined using a Welch's ANOVA with species and solution (water, KAR1, smoke water) as factors. To analyse patterns of emergence, the effects of species and smoke treatment on onset, median and spread of emergence were examined by a two-way ANOVA. Emergence was analysed using GLMs with species and smoke treatment as factors.
Results
Patterns of embryo growth and germination
Embryo lengths in fresh Levenhookia seeds were 10% the length of the seed and those in Trachymene seeds were 11%. Between January 2009 and May 2009, Levenhookia embryos remained 10–14% the length of the seed, with no consistent growth, regardless of whether they were stored in the field (buried in soil or placed on the soil surface) or in laboratory after-ripening conditions. Trachymene embryos in field-stored seeds increased linearly up to 32% (while buried) or up to 17% (on soil surface) by May 2009 but were 11–13% under laboratory conditions.
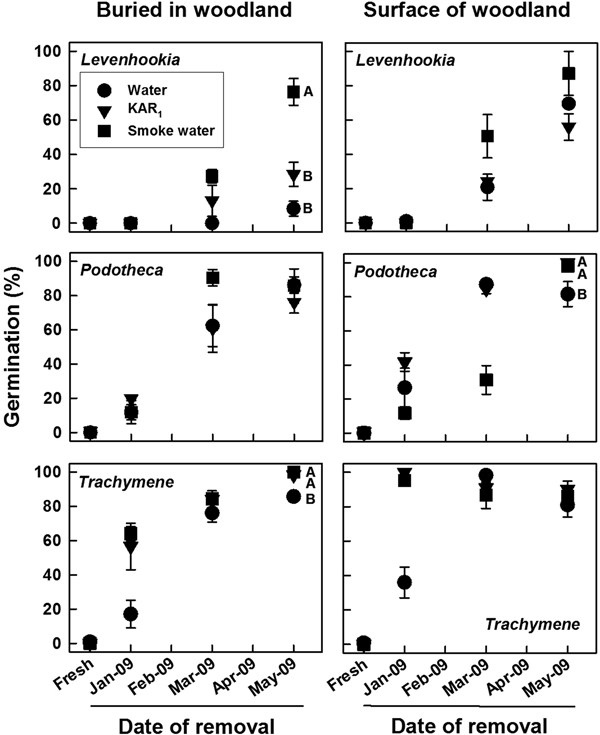
For seeds that were removed from storage in the field, germination was highly species specific and dependent on storage location in the field, time when exhumed and tested, and test solution (all factors and interactions, Wald χ2 ≥ 11.879, P ≤ 0.018). No freshly matured seeds of these species germinated during 4 weeks of incubation at 18/7°C in light on any solution (Fig. 1), and no germinated seeds were observed within bags exhumed in January, March and May 2009. When removed from buried or surface bags from January to May 2009 and incubated at 18/7°C in light, germination of Podotheca and Trachymene increased to 73–100% on all solutions. Levenhookia germination increased to 56–87% on all solutions when removed from surface bags but to 8, 28 and 76% on water, KAR1 and smoke water, respectively, from buried bags. Germination of all three species increased progressively with time, but at different rates; for seeds of Trachymene >90% germination was recorded as early as January (2 months old), for seeds of Podotheca >80% germination was recorded from March (4 months old), and for seeds of Levenhookia >80% germination was not recorded until May (6 months old).
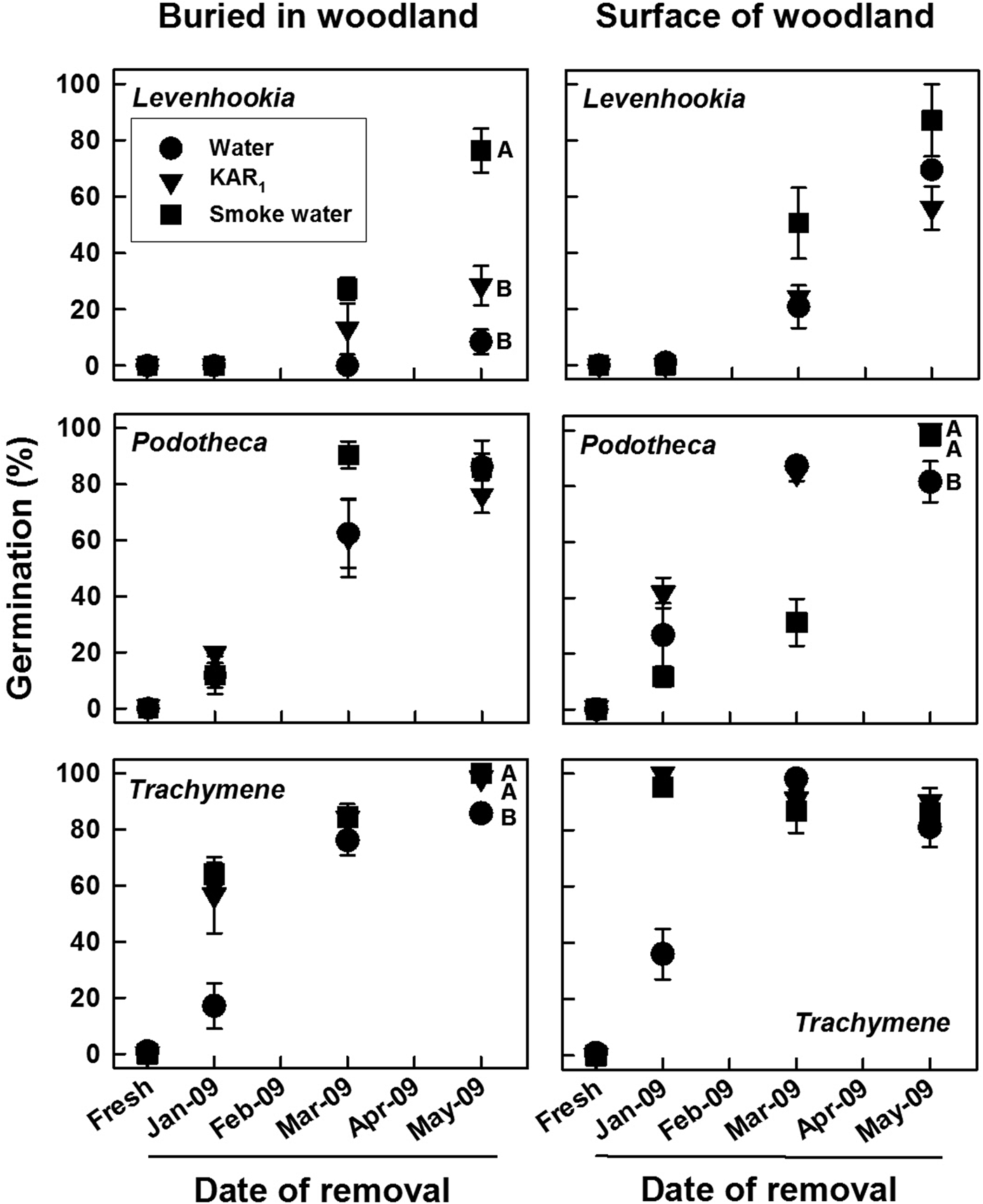
Fig. 1. Mean (±SE, SE shown if ≥5%) germination of three fire ephemerals on water, KAR1 and smoke water during 4 weeks of incubation in light at 18/7°C following 0 (fresh) to 5 months in situ soil storage under Banksia woodland conditions (either buried in soil or placed on the soil surface). Seeds were buried on 9 December 2008. Means with different letters within each panel are significantly different among solutions for the May 2009 test (PLSD, P ≤ 0.05); those without letters are not. Data are based upon three replicates of 25 seeds each.
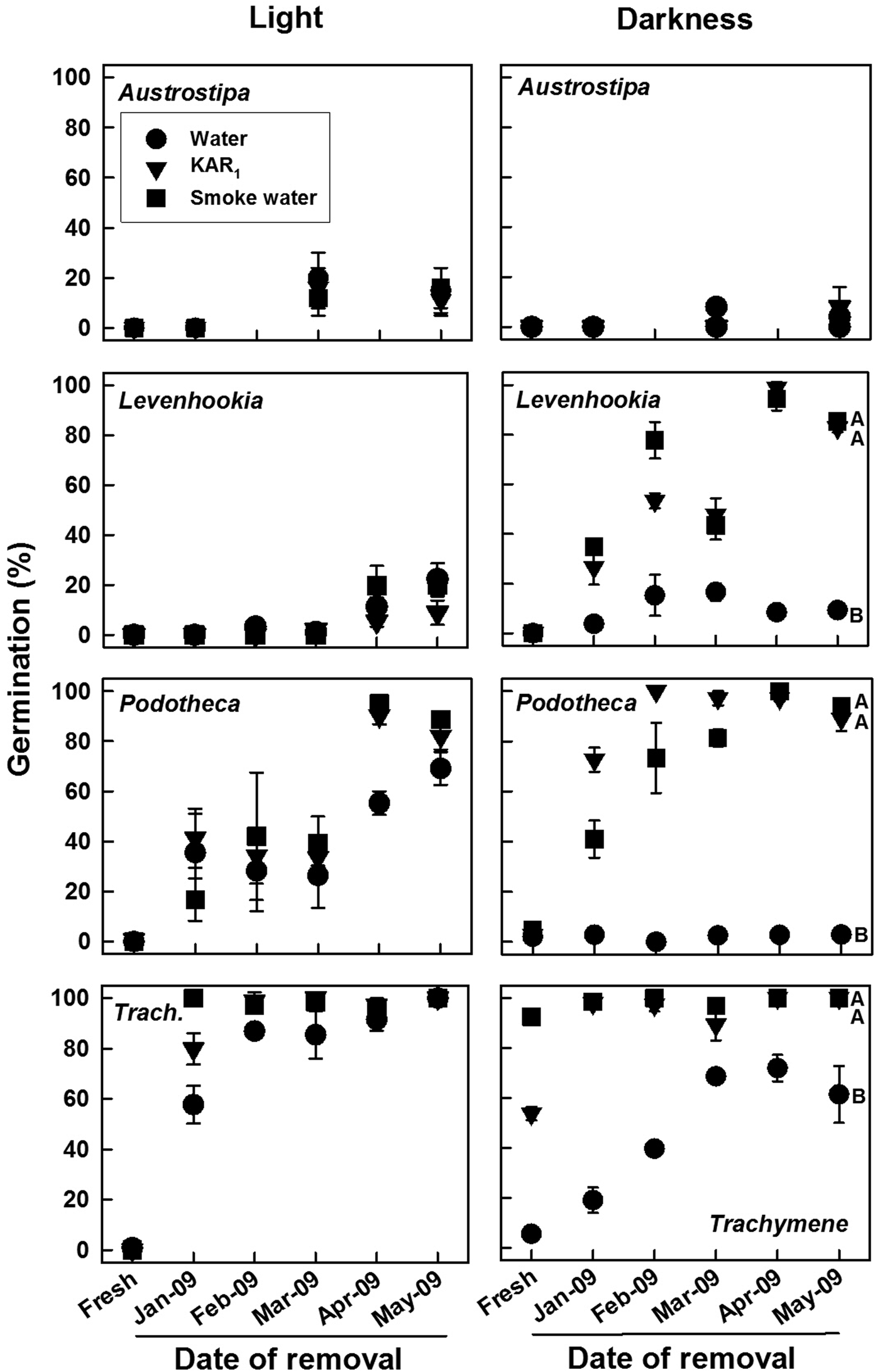
Germination of laboratory-stored seeds was highly species specific and dependent on time when tested, test solution and test light regime (all factors and interactions, Wald χ2 ≥ 21.358, P ≤ 0.006). Fresh seeds of Austrostipa, Podotheca and Levenhookia germinated ≤5% during incubation at 18/7°C in light and darkness, regardless of solution (Fig. 2). In contrast, Trachymene fresh seeds germinated to ≤1% in light on all solutions but to 92, 54 and 5% in darkness on smoke water, KAR1 and water, respectively. Germination for Podotheca and Trachymene increased over time to 69–100% on all solutions in light, but for Levenhookia to only 9–22%. In darkness, germination for these three species increased over time to 100% on KAR1 and on smoke water but up to only 9% (Podotheca, Levenhookia) or 72% (Trachymene) on water. Seeds of Austrostipa only germinated up to 20% regardless of light regime or solution.
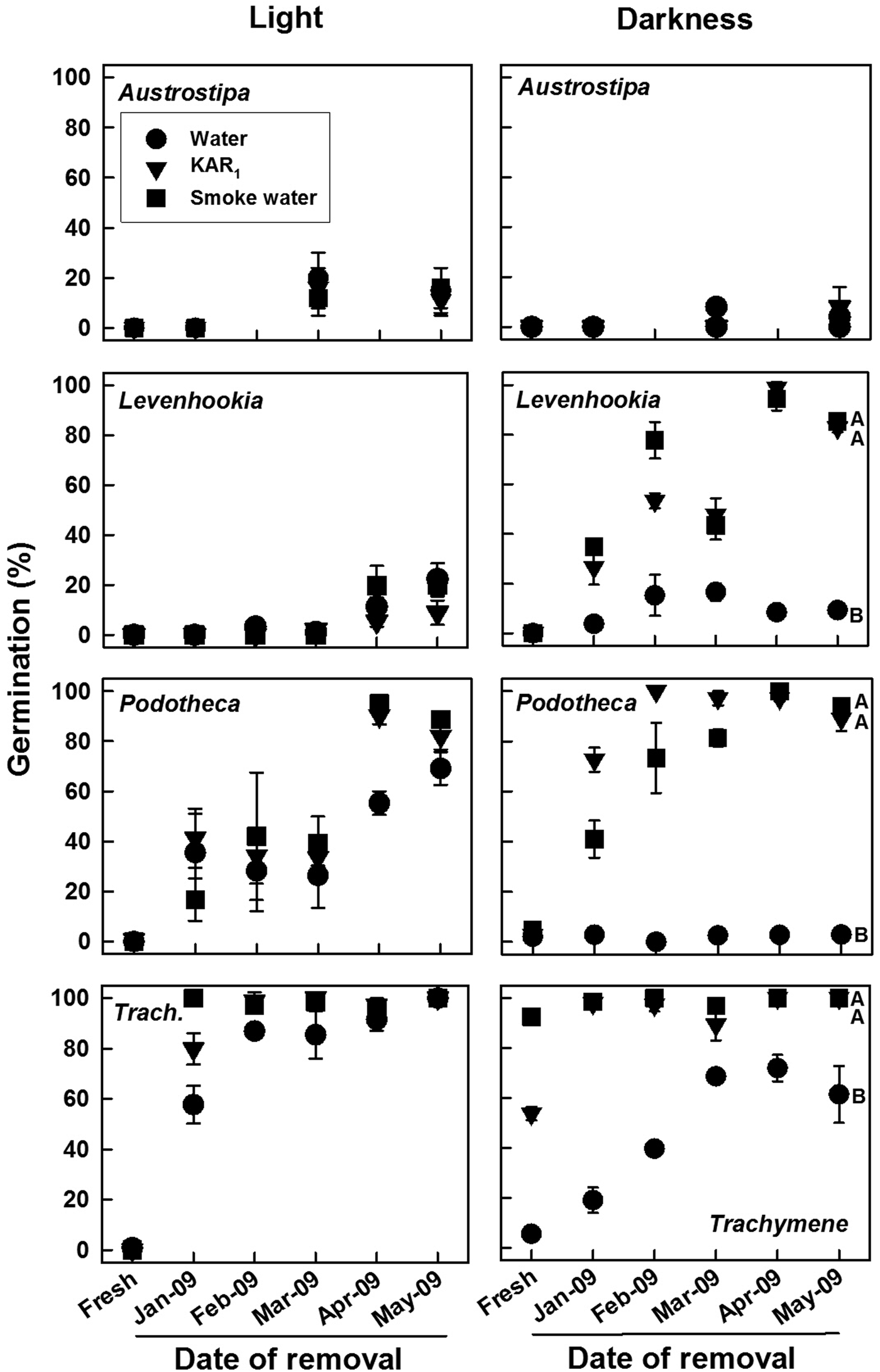
Fig. 2. Mean (±SE, SE shown if ≥5%) germination of four fire ephemerals on water, KAR1 and smoke water during 4 weeks in two light regimes at 18/7°C following 0 (fresh) to 5 months storage under standardized laboratory conditions (polyethylene box with 50% relative humidity in an incubator at 30°C). Seeds were placed into the after-ripening box on 9 December 2008. Means with different letters within each panel are significantly different among solutions for the May 2009 test (PLSD, P ≤ 0.05); those without letters are not. Data are based on three replicates of 25 seeds each.
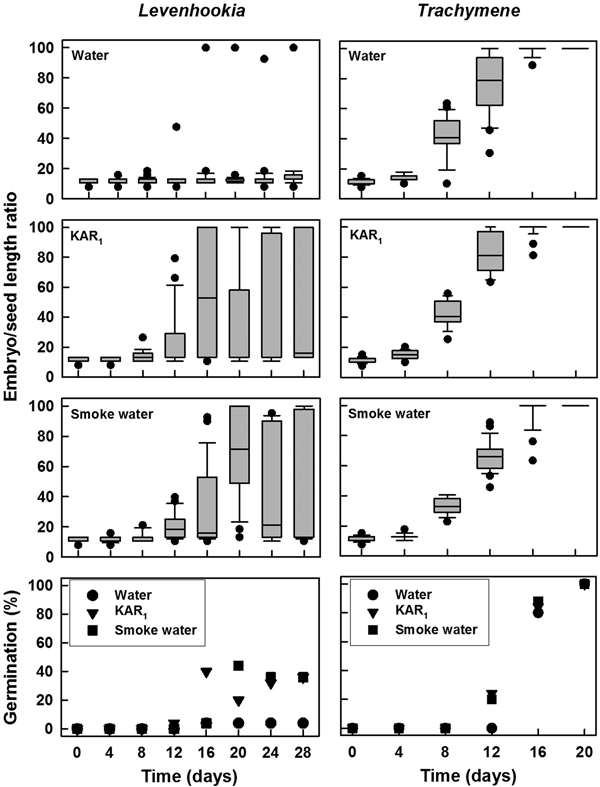
Once incubated for germination, relative growth rates for embryos in laboratory-stored, after-ripened seeds differed among solutions (F = 7.887, P = 0.001) and between species (F = 766.446, P < 0.0001), and the response to solutions was species specific (interaction, F = 8.284, P < 0.0001). Growth rates of Levenhookia embryos differed among solutions as follows: smoke water (0.0415 ± 0.0063 mm mm–1 day–1) = KAR1 (0.0390 ± 0.0061 mm mm–1 day–1) > water (0.0138 ± 0.0024 mm mm–1 day–1). For Levenhookia seeds, embryo growth was most evident by day 12 on all solutions (Fig. 3). Germination of Levenhookia seeds was evident on day 16 for KAR1 and on day 20 for smoke water; no seeds germinated on water. Growth rates for Trachymene embryos did not differ among solutions: water (0.1181 ± 0.0012 mm mm–1 day–1), KAR1 (0.1165 ± 0.0011 mm mm–1 day–1) and smoke water (0.1186 ± 0.0013 mm mm–1 day–1). Embryo growth of Trachymene seeds started noticeably on day 8 regardless of solution, and germination was obvious on day 12 for seeds treated with both KAR1 and smoke water, but on day 16 for water (Fig. 3).

Fig. 3. Box plots and mean (±SE, SE shown if ≥5%) germination for after-ripened seeds of two fire ephemerals with small under-developed embryos incubated for up to 28 days on water, KAR1 and smoke water. Embryos in 25 seeds were measured for each treatment at each time, and germination is based upon three replicates of 25 seeds each.
Patterns of seedling emergence
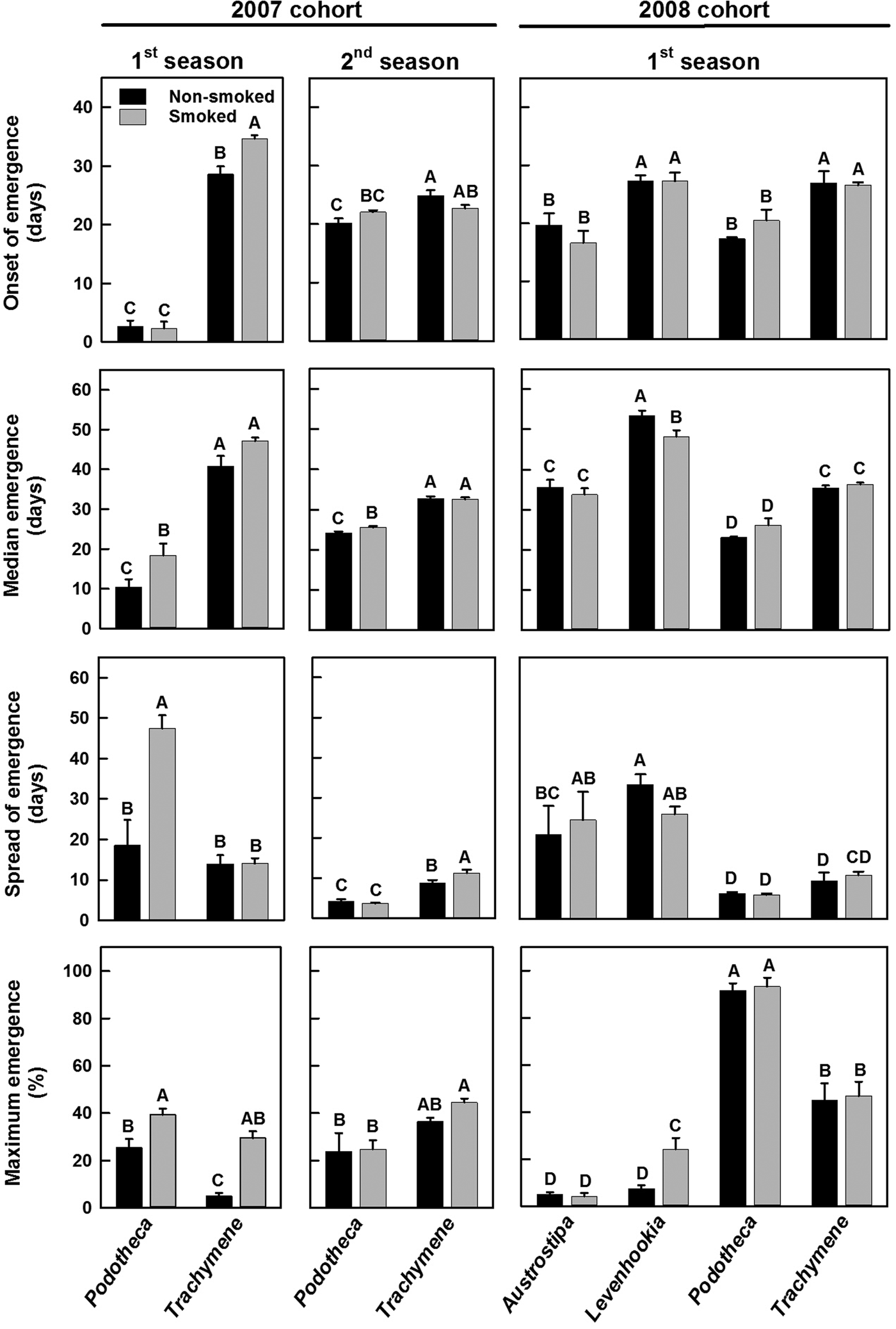
For the 2007 cohort during the first germination season, onset, median and spread of emergence (F ≥ 7.511, P ≤ 0.018) and maximum emergence (Wald χ2 = 72.522, P < 0.0001) varied significantly between species and between treatments. While the responses to smoke for the onset and spread of emergence (interaction, F ≥ 9.460, P ≤ 0.010) and maximum emergence (Wald χ2 = 36.059, P < 0.0001) varied between species, the response for median of emergence was similar between species (F = 0.106, P = 0.751). During the second germination season, no treatment effect occurred for any of the four emergence parameters (F ≤ 2.940, P ≥ 0.112; Wald χ2 = 3.427, P = 0.064). All four parameters varied between species (F ≥ 14.463, P ≤ 0.002; Wald χ2 = 40.895, P < 0.0001), but for only onset and spread of emergence (interaction, F ≥ 6.670, P ≤ 0.024) did species have dissimilar responses between treatments (F = 3.543, P = 0.084 for median emergence, Wald χ2 = 0.822, P = 0.365 for maximum emergence). Onset and median emergence was significantly earlier for Podotheca than for Trachymene during both emergence seasons (Fig. 4; Supplementary Material Fig. S1); seeds of Podotheca emerged almost immediately (within 3 days), whilst seeds of Trachymene required ca 30 days to emerge. This discrepancy in onset and median emergence times between species was reduced in the second season, but for the most part, seeds of Podotheca still emerged significantly earlier than seeds of Trachymene. Smoking delayed the onset of emergence for Trachymene seeds only during the first season, whereas it slightly delayed median emergence for Podotheca in both seasons. There was a smaller difference in the spread of emergence between the two species in both emergence seasons. However, smoke greatly spread emergence of Podotheca seeds during the first season and did so to a lesser extent for Trachymene in the second season. Smoke treatment induced significantly greater maximum emergence of both species during the first emergence season but not the second season.

Fig. 4. Mean (± SE) time to onset (1%) of emergence, to median (50%) emergence, and spread (time interval) between 10 and 90% emergence of fire ephemerals exposed to aerosol smoke during the first and second emergence seasons for the 2007 cohort and during the first season for the 2008 cohort (for additional details, see Supplementary Material Figs S1 and S2). Means with different letters within each graph are significantly different (PLSD, P ≤ 0.05); those with similar letters are not.
For the 2008 cohort, no treatment effect occurred for any of the four emergence parameters (F ≤ 0.480, P ≥ 0.495; Wald χ2 = 2.029, P = 0.154). All four parameters varied among species (F ≥ 17.237, P < 0.0001; Wald χ2 = 176.615, P < 0.0001), but for only median emergence did species have dissimilar responses between treatments (interaction, F = 3.601, P = 0.028; F ≤ 1.537, P ≥ 0.230 and Wald χ2 = 5.281, P = 0.152 for the other three parameters). Onset of emergence was significantly earlier for Austrostipa and Podotheca compared with Levenhookia and Trachymene (Fig. 4, Supplementary Material Fig. S2). Median emergence varied among the species as (earliest to latest): Podotheca < Austrostipa = Trachymene < Levenhookia. Onset of emergence, median emergence and total emergence were unaffected by smoke for all species except Levenhookia; for Levenhookia seeds smoke significantly reduced the median emergence time and significantly increased maximum emergence.
When comparing the first season emergence characteristics of Podotheca and Trachymene seeds collected in 2007 with seeds collected in 2008, seeds of Podotheca in the 2008 cohort had a greatly delayed onset of emergence (ca 18 days versus ca 3 days), and a much greater maximum emergence (ca 92% versus ca 32%), than those in the 2007 cohort. Such differences between seed batches collected in 2007 and 2008 were not evident in Trachymene seeds.
Temperature and precipitation records
Mean daily maximum and minimum temperatures during summer (December to March) 2007–2008 and 2008–2009 were 30.7 and 17.6°C, respectively, at the regional level and 40.2 and 17.7°C, respectively, in the tunnel-house (Supplementary Data Fig. S1). Seeds of all species emerged in 2008 and 2009 during April to May when mean daily maximum and minimum temperatures were 24.1 and 10.8°C, respectively, at the regional level and 31.8 and 12.2°C, respectively, in the tunnel-house (Supplementary Material Figs S1 and S2). Our daily watering in the tunnel-house commenced at about the same time as the regional wet season during 2008, but it was earlier compared with regional precipitation in 2009 (Supplementary Material Fig. S1).
Discussion
Fresh seeds of all four study species were dormant upon release in November and after-ripening to overcome physiological dormancy subsequently occurred at summer conditions in all species, during which no embryo growth was observed for the two species (Levenhookia and Trachymene) with under-developed embryos (Fig. 5). As dormancy loss proceeded (albeit very little in Austrostipa), germination increased in light and darkness on all three solutions at a simulated winter temperature of 18/7°C. When seeds were tested for germination at simulated summer temperatures (33/18°C), very low germination occurred, indicating that a rain event during summer would not trigger germination (S.N. Hidayati and J.L. Walck, unpublished data). In addition to this thermal suppression of germination, intermittent summer rain would not maintain soil moisture long enough to initiate embryo growth in Levenhookia and Trachymene seeds – which required at least 8–12 days of high moisture conditions (at winter temperatures). Thus, both of these processes ensure that sporadic summer rainfall does not lead to a false germination event.
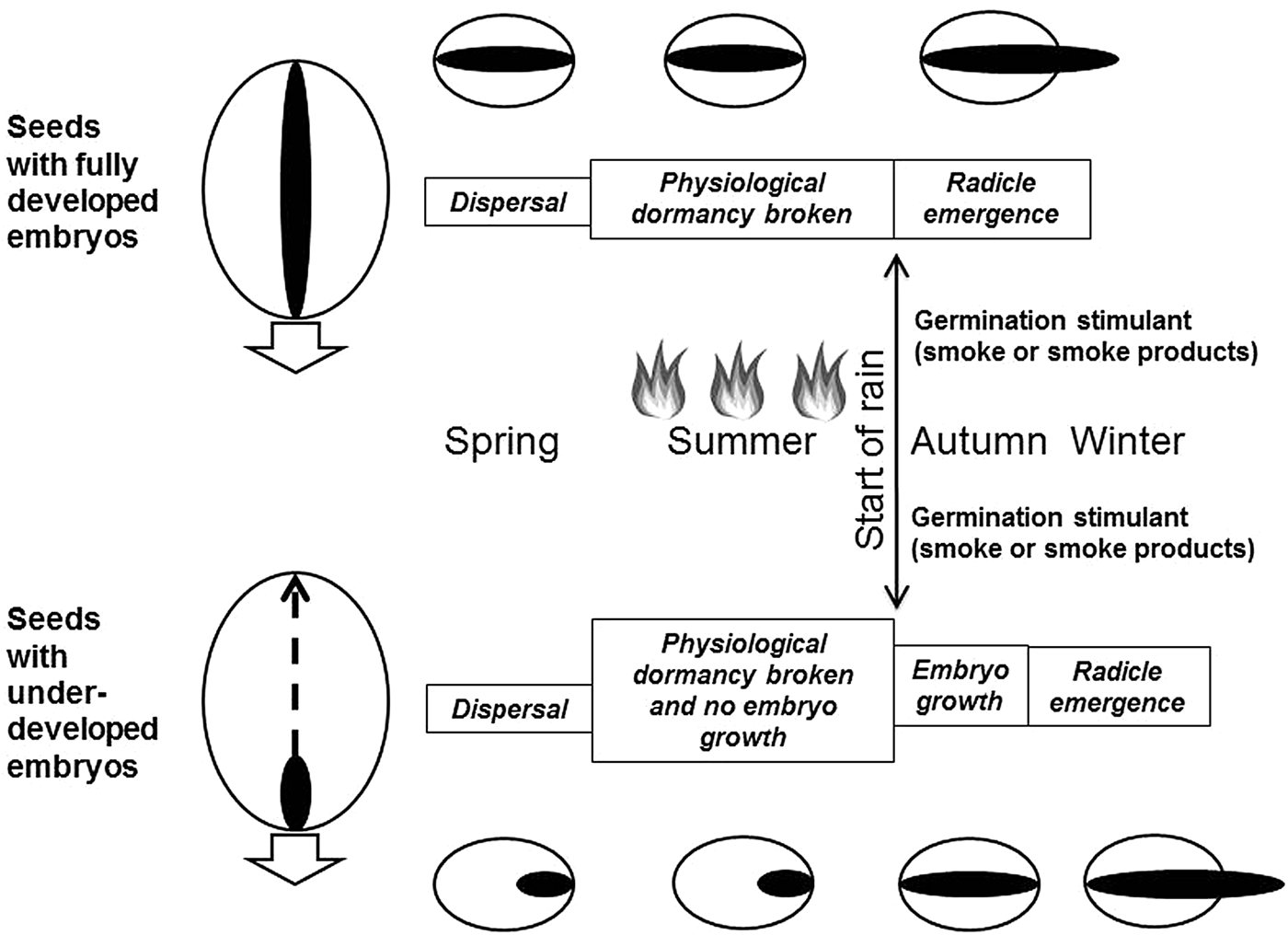
Fig. 5. Conceptual diagram showing seeds with different types of embryos (fully developed and under-developed) in relation to key life stage transitions from seed dispersal to germination according to seasons. Fire (indicated by flames) could occur at any time primarily in summer. Seeds with under-developed embryos germinate later than those with fully developed embryos due to a period of embryo growth. Under-developed embryos must develop and elongate (dashed arrow) inside the seed before radicle emergence (white arrow).
An after-ripening requirement for loss of physiological dormancy is reported for a large number of species (Baskin and Baskin, Reference Baskin and Baskin2014), including many belonging to families well represented in the Australian flora (Merritt et al., Reference Merritt, Turner, Clarke and Dixon2007). In our study, the degree of physiological dormancy loss was highly dependent on the location of after-ripening. The difference in after-ripening between seeds buried versus placed on the soil surface in the woodland was not as dramatic as that between seeds after-ripened in the woodland versus laboratory. This difference was most evident in Levenhookia. For this species, at the end of the after-ripening period, seeds placed on the soil surface in the woodland germinated to high percentages on water and smoke water and moderate percentages on KAR1, seeds buried in the woodland germinated to high percentages on smoke water and low percentages on water and KAR1, and seeds kept in an incubator germinated to low percentages on all three solutions. Thus, after-ripening was most effective on the soil surface, perhaps due to the warmer and/or dryer conditions compared with burial or the laboratory. Dormancy loss via after-ripening occurs more rapidly at increased temperature (Steadman et al., Reference Steadman, Crawford and Gallagher2003) and also with alternating wet/dry conditions for some species (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012), as would be experienced for seeds on the soil surface. In contrast, seeds of Austrostipa germinated to low percentages, regardless of after-ripening condition in our study. This result is surprising since Baker et al. (Reference Baker, Steadman, Plummer and Dixon2005) reported high germination at constant temperatures of 5, 10 and 15°C in the same species following dry storage at 15°C. However, no seeds germinated at an alternating temperature of 25/15°C in their study. We also used an alternating temperature regime and perhaps this was the reason for our very low germination.
At the start of the autumn–winter wet season when seeds were least dormant, seedlings emerged earlier from seeds with fully developed embryos than from seeds with under-developed embryos (Fig. 5). This pattern was particularly evident for the onset of germination in the tunnel-house: by 30 days for Podotheca versus Trachymene in the 2007 cohort during the first emergence season and by ca 10 days for Austrostipa and Podotheca versus Levenhookia and Trachymene in the 2008 cohort. A similar pattern was observed in the field. In remnant seed bags kept on the soil surface (following the completion of our experiments), high percentages of Podotheca seedlings were observed in late May–early June 2009, 2 weeks before seedlings of Levenhookia and Trachymene were observed (S.N. Hidayati and J.L. Walck, unpublished data). The delay in emergence observed in the tunnel-house and field was due to embryo growth that had to occur inside seeds with under-developed embryos before radicles emerged versus no prior growth for seeds with fully developed embryos (Fig. 5).
We thought that the timing of induction of embryo growth, and the rate of embryo growth, would differ amongst seeds of species with under-developed embryos, leading to seedling emergence being more spread out during the wet season compared with fully developed embryos. Barring differences between smoked and non-smoked treatments, the spread of emergence for the 2007 cohort was not different between embryo types during the first emergence season but was during the second emergence season. If we consider the germination of seeds in light following after-ripening in the laboratory as a degree of physiological dormancy, then the hierarchy of species (from shallowest to deepest dormancy) would be: Trachymene > Podotheca > Levenhookia ≈ Austrostipa. Thus, for the 2008 cohort, spread of emergence was related more to degree of (physiological) dormancy than to embryo growth. The two deep-dormant species (Austrostipa, Levenhookia) had a wider spread than the two shallow-dormant species (Podotheca, Trachymene). In addition to physiological dormancy break during the summer, additional dormancy release may have occurred during the autumn–winter period to allow continued emergence of seedlings in Austrostipa and Levenhookia.
We suggested that seedlings from seeds treated with smoke would emerge earlier than those from untreated seeds regardless of embryo type. In our study where differences occurred, smoked seeds emerged later in three instances and earlier in only one case. This is in contrast to a number of studies where the rate of emergence was enhanced in seeds treated with smoke (Dixon et al., Reference Dixon, Roche and Pate1995; Roche et al., Reference Roche, Dixon and Pate1997a, Reference Roche, Koch and Dixonb). The effects of smoke were more noticeable in terms of number of emergents rather than timing of emergence: with some smoked treatments having more emergents than non-smoked ones. Our study demonstrated the loss of smoke reactiveness after a previous smoke application. In the second emergence season for the 2007 cohort, smoke did not enhance emergence, strongly contrasting with the first season. Apparently, seeds then respond to regular seasonal cues outside of the previous ‘fire’ event. We observed a similarly reduced requirement for a smoke cue over time in Hibbertia species, speculating that it might be a bet-hedging strategy in the event a fire does not occur for some time (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012).
Unlike other studies that have shown enhanced seedling growth (Daws et al., Reference Daws, Davies, Pritchard, Brown and Van Staden2007; Nelson et al., Reference Nelson, Flematti, Ghisalberti, Dixon and Smith2012; Zhou et al., Reference Zhou, Kulkarni, Huang, Guo and Van Staden2012), KAR1 and smoke water did not increase embryo growth consistently between our two species with under-developed embryos. While under-developed embryos grew faster in seeds placed on KAR1 or smoke water than on water for Levenhookia, embryos grew at the same rate on all solutions for Trachymene. The faster embryo growth for Levenhookia resulted in faster germination on KAR1 and smoke water than on water. These differences again appear to be driven by degrees of physiological dormancy, with Levenhookia being more deeply dormant than Trachymene.
Conclusions
Physiological dormancy was overcome in seeds of four fire ephemerals during summer, enabling them to be non-dormant at the start of the autumn–winter wet season. However, no embryo growth was observed in seeds with under-developed embryos during summer. Once rains started, seedling emergence from seeds of two species with fully developed embryos occurred earlier than from seeds of two other species with under-developed embryos. In a non-consistent manner among our study species, smoke and smoke compounds influenced the rate of embryo growth and amount of germination, suggesting a strong interaction between seed dormancy status and sensitivity to smoke. Seeds of these species use thermal suppression and/or embryo growth as a means to avoid summer false breaks, which would be lethal as follow-up rains rarely occur in Mediterranean systems. A shift to reliance of regular seasonal cues for emergence and a reduced requirement for smoke occurred in the second germination season after a fire.
Author ORCIDs
Siti N. Hidayati, 0000-0001-6979-8477, David J. Merritt, 0000-0002-3250-6861, Shane R. Turner, 0000-0002-9146-2977, Jeffrey L. Walck, 0000-0002-8518-9900
Acknowledgements
The authors thank Wolfgang Lewandrowski for his assistance with statistical analyses.
Financial support
S.N.H. was supported by the Australian Research Council's Linkage Projects funding scheme LP0455415, and D.J.M. was supported by the Botanic Gardens and Parks Authority – Alcoa of Australia Limited Seed Conservation Partnership. The research was conducted under the auspices of the Millennium Seed Bank Project, Kew, which is supported by the UK Millennium Commission, the Wellcome Trust and Orange plc.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0960258519000084.