Introduction
The preimplantation development of mammalian embryos is affected by several nutritional and environmental factors. Soluble growth factors and cytokines produced by preimplantation embryos (Rappolee et al., Reference Rappolee, Brenner, Schultz, Mark and Werb1988; Werb, Reference Werb1990; Sharkey et al., Reference Sharkey, Dellow, Blayney, Macnamee, Charnock-Jones and Smith1995) and the epithelial cells of the fallopian tube (Sakkas and Trounson, Reference Sakkas and Trounson1990; Minami et al., Reference Minami, Utsumi and Iritani1992) and of the uterus (Brigstock et al., Reference Brigstock, Heap and Brown1989; Sakkas and Trounson, Reference Sakkas and Trounson1990; Pampfer et al., Reference Pampfer, Arceci and Pollard1991) have been reported to control early embryonic development. In all species studied, developmental arrest occurred at specific cell-stages in cultured embryos including mouse 2-cell (Goddard & Pratt, Reference Goddard and Pratt1983; Bolton et al., Reference Bolton, Oades and Johnson1984), human 4- to 8-cell (Braude et al., Reference Braude, Bolton and Moore1988), hamster 2-cell (Bavister, Reference Bavister1988), sheep and goat 8- to 16-cell (Sakkas et al., Reference Sakkas, Batt and Cameron1989), cow 4- to 8-cell (Camous et al., Reference Camous, Heyman, Meziou and Menezo1984), and pig 4-cell (Davis, Reference Davis1985) stages. During these stages, the activation of transcription by the embryonic genome occurs (Latham, Reference Latham1999; Minami et al., Reference Minami, Suzuki and Tsukamoto2007). However, this developmental arrest has hampered the generation of in vitro produced preimplantation embryos for animal husbandry, the generation of transgenic animals, and assisted reproductive technology for infertility. In order to analyze the mechanisms underlying these cleavage blocks, substances that act as inducers or inhibitors of the cleavage block are very valuable.
Serum and serum preparations, such as fetal calf serum and bovine serum albumin (BSA), have been widely used as supplements for culture media to support the in vitro development of preimplantation mammalian embryos. The liver is the major organ that synthesizes and secretes serum or serum fractions, some of which are known to affect the early development of mammalian embryos (Saito et al., Reference Saito, Berger, Mishell and Marrs1984; Ogawa et al., Reference Ogawa, Ono and Marrs1987). However, the effects of substances synthesized by hepatocytes on the development of preimplantation embryos are not fully understood. Assuming that hepatocytes, including hepatoma cells, provide an excellent source for finding either positive or negative regulators for the development of mammalian embryos, we have investigated the effects of medium conditioned using rat hepatoma cells, Reuber H-35 (Kobayashi et al., Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1996, Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1997, Reference Kobayashi, Terawaki, Saito, Kasuga and Kojima2009; Kobayashi & Horiuchi, Reference Kobayashi and Horiuchi1998) and BRL (Kobayashi et al., Reference Kobayashi, Terawaki, Saito, Kasuga and Kojima2009), on the development of mouse and bovine embryos cultured in vitro. The cell-conditioned medium (CM) exerted beneficial effects on the development of preimplantation embryos (Kobayashi et al., Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1997, Reference Kobayashi, Terawaki, Saito, Kasuga and Kojima2009; Kobayashi & Horiuchi, Reference Kobayashi and Horiuchi1998). On the other hand, we also found a compound, Fr.B-25, purified from the Mr < 10,000 fraction of Reuber CM that inhibits the development of mouse zygotes at the 2-cell stage (Kobayashi et al., Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1996).
In this study, we identified Fr.B-25 as a purine nucleoside, 5′-deoxy-5′-methylthioadenosine (MTA), using mass spectroscopic analysis and compared the biological activities of Fr.B-25, MTA, and other purines on the development of mouse embryos cultured in vitro.
Materials and Methods
Experimental design
The first experiment was designed to identify the structure of Fr.B-25 by mass spectroscopic analysis. Secondly, the biological activities of Fr. B-25, an authentic compound for Fr.B-25 identified by mass spectroscopic analysis, and its structurally related compounds on the development of mouse zygotes cultured in vitro were compared. Thirdly, we tried to investigate the mode of action of the identified compound as Fr.B-25 underlying the 2-cell blocking effect on mouse embryos.
Media
M2 (Quinn et al., Reference Quinn, Barros and Whittingham1982) was supplemented with 4 mg/ml BSA (A4378, Sigma Chemical Company), and modified Whitten's medium (WM) (Hoppe, Reference Hoppe and Dixon1985) was supplemented with 3 mg/ml BSA (A7030, Sigma Chemical Company). Phenol red was not added to M2 or WM. Dulbecco's modified Eagle's medium (DMEM) was purchased from Sigma.
Embryo collection
Induction of superovulation and subsequent mating of 6- to 12-week-old virgin female CD-1 mice (random bred, Swiss, Charles River Japan) were performed as described previously (Kobayashi et al., Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1996). Zygotes were flushed in M2 from excised oviducts 21–23 h after hCG injection. Cumulus cells were removed from the zygotes by hyaluronidase treatment (150 units/ml in M2 without BSA, Type I-S, Sigma Chemical Company). Two-cell embryos were flushed in M2 from excised oviducts 41–42 h after hCG injection. All animal procedures conformed to the Guidelines for the Care and Use of Laboratory Animals of Akita Prefectural University.
Culture of hepatoma cells and embryos
Rat hepatoma Reuber H-35 cells were routinely maintained in DMEM supplemented with heat-inactivated 2% fetal calf serum (HyClone).
The zygotes were cultured in 0.5 or 1 ml of fresh WM supplemented with MTA (Sigma Chemical Company), adenosine (Wako Pure Chemicals), or hypoxanthine (Wako Pure Chemicals) in the presence of both 3 mg/ml BSA and 50 μM EDTA in 24-well dishes (Asahi Glass) or 4-well dishes (Nunc). EDTA (50 μM) was added because low concentrations (10–50 μM) of this compound have been demonstrated to promote the development of CD-1 zygotes cultured in vitro (Abramczuk et al., Reference Abramczuk, Solter and Koprowski1977). Because the hypoxanthine-induced 2-cell block was reversed by dibutyryl cyclic adenosine monophosphate (dbcAMP), a cAMP analogue, at 100 μM (Fissore et al., Reference Fissore, O'Keefe and Kiessling1992), dbcAMP (Sigma Chemical Company) was used at this concentration. Development to the 2-cell, 4- to 8-cell, and blastocyst stages was observed at 24, 48, and 120 h, respectively, after the start of in vitro culture.
When obtaining 4- to 8-cell embryos, 2-cell embryos developed in vivo were cultured in vitro with WM containing 3 mg/ml BSA without EDTA for ~22 h. Thereafter, 4- to 8-cell embryos were transferred to 0.5 ml of fresh WM supplemented with one of the nucleosides in the presence of 3 mg/ml BSA without EDTA. Development to the morula to blastocyst and blastocyst stages was observed at 24 h and 72 h, respectively, after start of the experiment.
Less than 30 embryos were cultured in each well. The results of each experiment were analyzed by the chi-squared test.
Preparation, ultrafiltration, and column chromatography of medium conditioned using hepatoma cells
Cell-conditioned WM without BSA was obtained by culture with Reuber H-35 cells as described previously (Kobayashi et al., Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1996). In brief, confluent Reuber H-35 cells in 143 cm2 dishes were cultured with 40 ml of WM without BSA, penicillin, or streptomycin for 24 h. Because penicillin was eluted in fractions beside the fraction containing Fr.B-25, it was not added to WM. CM was separated by centrifugal ultrafiltration (Centricon Plus-80, < 10,000 Mr cut off; Millipore) into a subfraction, i.e. the pass-through fraction as the Mr < 10,000. This fraction was stored at −20°C before use.
The Mr < 10,000 fraction was further applied to preparative reversed-phase column chromatography to obtain Fr.B-25 in accordance with the method described previously (Kobayashi et al., Reference Kobayashi, Terawaki, Saito, Kasuga and Kojima2009). The fractions containing Fr.B-25 were lyophilized to remove organic solvents. For embryo culture, the lyophilites were reconstituted with WM to a half of their original volumes.
Analytical reversed-phase column chromatography and high resolution-mass spectroscopic analyses (LC-MS) and tandem mass spectroscopic analysis
Fr.B-25 was further applied to LC-MS analysis. Analytical reversed-phase HPLC (octadecylsilane column, 2 mm × 150 mm, Nomura Chemical, Seto, Japan) was carried out with elution of the column using a linear gradient of acetonitrile with 0.05% perfluorobutanoic acid. High resolutional electrospray ionization mass spectroscopy (HR-ESI-MS) analysis of compounds separated by HPLC was carried out in the positive-ion mode on a JEOL MS-700 (JEOL). Fr.B-25 was also subjected to LC-ESI-MS/MS analysis in the positive-ion mode (API2000 LC-MS/MS system, Applied Biosystems, Japan).
Results
Identification of Fr.B-25 as MTA by mass spectroscopic analysis
Approximately 2 mg of Fr.B-25 was purified from ~4000 ml of the Mr < 10,000 fraction by preparative reversed-phase column chromatography. First, a portion (~3 μg) of purified Fr.B-25 was further applied to analytical reversed-phase HPLC and the molecular weight of Fr.B-25 was determined by high-resolution MS (HR-MS). As shown in Fig. 1a, Fr.B-25 eluted from the column as an apparent peak at about 9.3 min. Positive ion HR-MS of Fr.B-25 showed (M+1)+ at m/z 298.0927 (Fig. 1b) for a possible molecular formula of C11H15O3N5S (calc. 298.0973). Considering that the biological activity of Fr.B-25 resembles that of purine nucleosides in our previous study (Kobayashi et al., Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1996) and its UV spectrum property (λmax at 260 nm, data not shown), Fr.B-25 was suggested to be a purine nucleoside, 5′-deoxy-5′-methylthioadenosine (MTA).

Figure 1 HPLC analysis and high resolutional mass spectrum of Fr.B-25. (a) Chromatogram of Fr.B-25 obtained by the HPLC analysis. Elution was carried out with a linear gradient of acetonitrile containing 0.05% perfluorobutanoic acid from 0% (0 min) to 100% (20 min). (b) High resolutional MS spectrum of Fr.B-25 eluted at 9.3 min in the above chromatogram.
To confirm the structure of Fr.B-25, ESI-MS/MS analysis of Fr.B-25 and MTA was performed (Fig. 2). As shown in Fig. 2a, the ESI-MS spectrum for authentic MTA showed (M+1)+ at m/z 298 as a parent ion together with its sodium complex at m/z 320 (M + Na)+. Product ions found with Fr.B-25 from m/z 298 were found at m/z 136, 119, 92, 75, and 61 (Fig. 2c), and these obtained ions were exactly identical to those of authentic MTA (Fig. 2b). Therefore, it was clearly shown that Fr.B-25 was MTA.

Figure 2 Analysis of authentic MTA and Fr.B-25 by ESI-MS/MS. (a) MS spectrum of authentic MTA at m/z 298 (M+1)+, together with its sodium complex at m/z 320 (M + Na)+. (b) Product ions derived from a parental ion (m/z 298) with authentic MTA. (c) Product ions derived from a parental ion (m/z 298) with Fr.B-25.
Inhibitory activity of authentic MTA and other purines on the development of cleaving 2-cell embryos
In preimplantation mouse embryos, micromolar concentrations of the purines hypoxanthine and adenosine have been shown to block the second or third cleavage of several strains of mouse embryos cultured in vitro (Loutradis et al., Reference Loutradis, John and Kiessling1987; Nureddin et al., Reference Nureddin, Epsaro and Kiesling1990). Thus, the following experiment was conducted to determine and compare the effect of MTA with those of hypoxanthine and adenosine on the development of CD-1 zygotes cultured in vitro.
When a 2-day culture with fresh WM containing 50 μM EDTA was used as a control, 96.7% of the 2-cell embryos cultured from zygotes developed to the 4- to 8-cell stage (Table 1). Fr.B-25 significantly inhibited (p < 0.001) the cleavage of embryos at the 2- or 3-cell stage, i.e. 62.2% of the 2-cell embryos developed to the 4- to 8-cell stage. By means of reversed-phase column chromatographic analysis, the content of MTA in Fr.B-25 was estimated to be about 2 to 3 μM. Under these conditions, the dose-dependent inhibitory effect of authentic MTA on the cleavage of 2-cell embryos was examined. Significant (p < 0.01) inhibitory action was detected at concentrations as low as 0.5 μM; 85.2% of the 2-cell embryos developed to the 4- to 8-cell stage. By culturing with 50 μM MTA, 41.8% (p < 0.001) of the 2-cell embryos developed to the same stage. Inhibitory activities on the development of 2-cell embryos at micromolar levels were also detected using other purines, specifically adenosine and hypoxanthine, whereas these purines at 50 μM potently inhibited the development more strongly than 50 μM MTA. These findings demonstrated that MTA inhibits the development of zygotes at the 2-cell stage as efficiently as Fr.B-25, and the effective concentrations of MTA were similar to those of other purines.
Table 1 The effect of Fr.B-25, MTA, and other purines on the cleavage of 2-cell embryos.

aCD-1 zygotes were cultured with Fr.B-25 or one of the purines in the presence of 3 mg/ml BSA and 50 μM EDTA. Control embryos were cultured in fresh medium containing 3 mg/ml BSA and 50 μM EDTA (Control).
bDevelopment to the 2-cell and 4- to 8-cell stages was observed at 24 h and 48 h, respectively, after the start of in vitro culture.
Significantly different from the control; ap < 0.05, bp < 0.01, cp < 0.001.
Dibutyryl cAMP did not reverse the inhibitory effect of MTA on the development of 2-cell embryos
Fissore et al. (Reference Fissore, O'Keefe and Kiessling1992) reported that hypoxanthine-induced 2-cell block was reversed by adding 100 μM dbcAMP, a cAMP analog, to the culture medium from the start of cultivation of random-bred mouse zygotes. Therefore, to investigate differences in the inhibitory actions among MTA, adenosine, and hypoxanthine, the effect of dbcAMP at 100 μM on the 2-cell blocking effect of these compounds was analysed (Table 2). The addition of dbcAMP from the start of cultivation of zygotes (22–24 h after hCG) did not affect development in the control culture (WM + dbcAMP), as reported previously (Nureddin et al., Reference Nureddin, Epsaro and Kiesling1990; Fissore et al., Reference Fissore, O'Keefe and Kiessling1992). On the contrary, the inhibitory activity of 5 μM hypoxanthine on the development of zygotes (19.7% development to blastocysts) was partially alleviated by supplementation with dbcAMP (32.9% development to blastocysts, p = 0.097), whereas those of Fr.B-25, MTA, and adenosine were not affected even in the presence of dbcAMP.
Table 2 The effect of dbcAMP on the 2-cell blocking effects of Fr.B-25, MTA, adenosine, and hypoxanthine.

aCD-1 zygotes were cultured with or without (WM) Fr.B-25 or one of the purines in the presence of 3 mg/ml BSA and 50 μM EDTA. The concentrations of purines and dbcAMP were 5 μM and 100 μM, respectively.
bDevelopment to the 2-cell, 4- to 8-cell, and blastocyst stages was observed at 24, 48, and 120 h, respectively, after the start of in vitro culture.
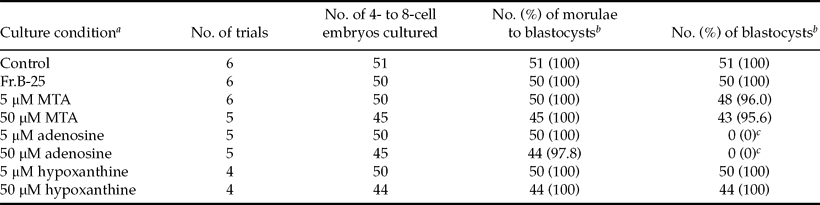
The effect of MTA and other purines on subsequent development of 4- to 8-cell embryos
We reported previously that the subsequent development of 4- to 8-cell embryos (Kobayashi et al., Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1996) and morulae (Kobayashi et al., Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1997) obtained from CD-1 mice was only inhibited slightly by co-culture with Reuber H-35 cells or with its CM, and Fr.B-25 was considered as a 2-cell stage-specific inhibitor of the cleavage of preimplantation mouse embryos. In order to clarify the developmental stage-specific effect of MTA, 4- to 8-cell embryos were cultured with MTA or other purines.
As indicated in Table 3, almost no effect of Fr.B-25 and MTA at 5 and 50 μM on the subsequent development of 4- to 8-cell embryos to blastocysts (a 72-h period) was detected. Similarly, hypoxanthine exerted no effect. However, in the presence of 5 and 50 μM adenosine, degeneration of embryos occurred at the blastocyst stage, and no blastocysts were observed following a 72-h treatment. The rates of morphologically normal blastocysts after a 48-h period of 5 and 50 μM adenosine treatment were 46.0% and 6.7%, respectively, indicating that a rapid degeneration of embryos occurred during the blastocyst stage.
Table 3 The effects of Fr.B-25, MTA, adenosine, and hypoxanthine on the subsequent development of 4- to 8-cell embryos.
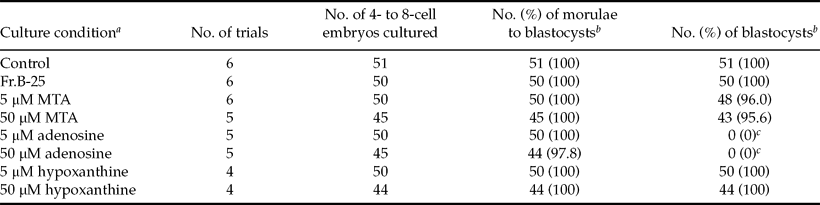
aCD-14- to 8-cell embryos were cultured with Fr.B-25 or one of the purines in the presence of 3 mg/ml BSA without EDTA. Control embryos were cultured in fresh medium containing 3 mg/ml BSA and 50 μM EDTA (Control).
bDevelopment to the morula to blastocyst and blastocyst stages was observed at 24 h and 72 h, respectively, after start of the experiment.
cSignificantly different from the control; p < 0.001.
Discussion
Rat hepatoma Reuber H-35 cells produce a unique compound designated as Fr.B-25, a 2-cell stage-specific inhibitor of the cleavage of mouse embryos cultured in vitro. Here, we identified Fr.B-25 as MTA from the complete identity of their molecular properties and almost identical biological properties.
MTA is a by-product of polyamine biosynthesis in mammalian cells (Pegg and Williams-Ashman, Reference Pegg and Williams-Ashman1969). To date, divergent activities of MTA at micromolar levels have been reported. MTA inhibits the proliferation of various types of mammalian cells, including mouse lymphoma cells (Wolford et al., Reference Wolford, MacDonald, Zehfus, Rogers and Ferro1981; Riscoe et al., Reference Riscoe, Tower and Ferro1984), SV40-transformed mouse fibroblasts (Pegg et al., Reference Pegg, Borchardt and Coward1981), hamster BHK21 cells (Raina et al., Reference Raina, Tuomi and Pajula1982), human glioma cells (Miyaji et al., Reference Miyaji, Tani, Nakano, Ikemoto and Kaba1995), and human hepatoma cells (Yang et al., Reference Yang, Sadda, Li, Zeng, Chen, Bae, Ou, Runnegar, Mato and Lu2004). In addition, MTA inhibited the differentiation of mouse Friend erythroleukemic cells (Di Fiore et al., Reference Di Fiore, Grieco, Pinto, Attadia, Porcelli, Cacciapuoti and Carteni-Farina1984) and stimulated the induction of apoptosis in human leukemia U937 cells (Lee & Cho, Reference Lee and Cho1998), human hepatoma Hep G2 cells (Yang et al., Reference Yang, Sadda, Li, Zeng, Chen, Bae, Ou, Runnegar, Mato and Lu2004), and human colon cancer HT-29 and RKO cells (Li et al., Reference Li, Zhang, Oh, Xia, Chen, Bemanian, Lastra, Circ, Moyer, Mato, Aw and Lu2009b).
To the best of our knowledge, this is a first report that MTA inhibited the in vitro development of preimplantation mouse embryos. Two points should be discussed regarding MTA action. First, how does MTA exert the cleavage blocking effect? The growth arrest produced by MTA in mouse lymphoma S49 cells is primarily due to the inhibition of cAMP phosphodiesterase and the subsequent increase in intracellular cAMP levels (Riscoe et al., Reference Riscoe, Tower and Ferro1984). However, in the present study (Table 2), the addition of dbcAMP to maintain intracellular cAMP as dbcAMP at 100 μM to the control culture of mouse zygotes (WM + dbcAMP) did not result in developmental arrest at the 2-cell stage, suggesting that cAMP elevation is probably unrelated to the blocking effect of MTA, adenosine, and hypoxanthine. On the other hand, Fissore et al. (Reference Fissore, O'Keefe and Kiessling1992) reported that dbcAMP at 100 μM reversed the 2-cell blocking effect of hypoxanthine, which is well-characterized purine nucleoside that acts as an inhibitor of the cleavage of mouse 2-cell embryos (Loutradis et al., Reference Loutradis, John and Kiessling1987; Nureddin et al., Reference Nureddin, Epsaro and Kiesling1990), and they concluded that hypoxanthine suppresses a cAMP-dependent process. Here, the relatively strong inhibitory effect of hypoxanthine was partially alleviated by adding dbcAMP. Conversely, the alleviation by dbcAMP addition was not observed even with relatively weak inhibitory effect of MTA (Table 2). Therefore, we considered that the mechanism of MTA action is different from that of hypoxanthine, i.e., the inhibitory effect of MTA is likely to be exerted without the inhibition of a cAMP-dependent process. MTA is known as a potent inhibitor of the fibroblast growth factor (FGF)-stimulated tyrosine kinase activity of FGF receptors (Maher, Reference Maher1993; Miyaji et al., Reference Miyaji, Tani, Nakano, Ikemoto and Kaba1995), protein carboxymethyltransferase (Pintucci et al., Reference Pintucci, Quarto and Rifkin1996; Lee & Cho, Reference Lee and Cho1998), and DNA methylase (Vandenbark et al., Reference Vandenbark, Ferro and Barney1980). These effects of MTA or other novel regulatory effects seem to induce its inhibitory activity against the development of zygotes.
Secondly, why does MTA specifically inhibit the cleavage of 2-cell embryos? The 2-cell stage-specific inhibition by hypoxanthine indicated here is also a new finding. As reviewed by Minami et al. (Reference Minami, Suzuki and Tsukamoto2007), zygotic genome activation occurs during the 2-cell stage in mouse embryos; therefore, this stage is critical for several nutritional and environmental factors particularly in in vitro culture, including inorganic constituents (Abramczuk et al., Reference Abramczuk, Solter and Koprowski1977; Erbach et al., Reference Erbach, Lawitts, Papaioannou and Biggers1994), energy sources (Chatot et al., Reference Chatot, Ziomek, Bavister, Lewis and Torres1989), growth factors (Lu et al., Reference Lu, Ikeda and Takahashi1994), and reactive oxygen (Lu et al., Reference Lu, Ikeda and Takahashi1994). MTA and hypoxanthine were likely to be involved in zygotic genome activation. As indicated, we broadly defined the mode of MTA action underlying the 2-cell blocking effect on mouse embryos. Further work will be required to determine the exact mechanisms of MTA action.
While the inhibitory effects of hypoxanthine and adenosine on the developing 2-cell mouse embryos have been well characterized (Loutradis et al., Reference Loutradis, John and Kiessling1987; Nureddin et al., Reference Nureddin, Epsaro and Kiesling1990; Downs & Dow, Reference Downs and Dow1991); the effect of these purines on the subsequent development of 4- to 8-cell embryos was not reported. Although exposure to adenosine from the 4- to 8-cell stage induced the degeneration of embryos at the blastocyst stage (Table 3), MTA and hypoxanthine specifically inhibited the cleavage of 2-cell embryos during the preimplantation period. This finding also indicated that the effects of MTA and hypoxanthine are different from that of adenosine on the development of preimplantation mouse embryos. Several studies have shown that extracellular adenosine induces cell death in a variety of cell types, including human hepatoma (Bar-Yehuda et al., Reference Bar-Yehuda, Stemmer, Madi, Castel, Ochaion, Cohen, Barer, Zabutti, Perez-Liz, Del Valle and Fishman2008), human glioma (Grbovic et al., Reference Grbovic, Jovic, Ruzdijic, Pejanovic, Rakic and Kanazir2002), and human arterial smooth muscle cells (Peyot et al., Reference Peyot, Gadeau, Dandre, Belloc, Dupuch and Desgranges2000), through adenosine receptors or adenosine transporters (Eltzschig, Reference Eltzschig2009). Mouse blastocysts also express adenosine receptors, as reported by Kawaguchi et al. (Reference Kawaguchi, Kano and Naito2009). Degeneration of blastocysts induced by adenosine was likely to be involved in a similar manner to that demonstrated in these reports.
Methylthioadenosine phosphorylase (MTAP) plays a major role in polyamine metabolism and the salvage of adenine and methionine by catalyzing the phosphorylation of MTA to methylthioribose-1-phosphate. While MTAP is expressed abundantly in normal cells (Olopade et al., Reference Olopade, Pomykala, Hagos, Sveen, Espinosa, Dreyling, Gursky, Stadler, Le Beau and Bohlander1995), many malignant cells lack MTAP activity (Tang et al., Reference Tang, Li and Kruger2000; Garcia-Castellano et al., Reference Garcia-Castellano, Villanueva, Healey, Sowers, Cordon-Cardo, Huvos, Bertino, Meyers and Gorlick2002). As reported by Savarese et al. (Reference Savarese, Dexter and Parks1983), a lack of MTAP in tumor cells probably leads to the excretion of MTA into the culture medium, owing to their inability to metabolize MTA. In fact, MTA is detectable in the urine of patients with malignant cancer (Li et al., Reference Li, Wang, Liu, Bu, Li, Han, Zhang and Wu2009a). We found previously that the content of Fr.B-25 in Reuber CM is 19-fold higher than that in BRL CM, indicating the productivity of Fr.B-25 is different even in rat hepatoma cell lines (Kobayashi et al., Reference Kobayashi, Terawaki, Saito, Kasuga and Kojima2009). In addition, human hepatoma Hep G2 cells exerted almost no effect to the development of CD-1 zygotes cultured in vitro, suggesting that Hep G2 cells secrete little MTA, as we also reported (Kobayashi et al., Reference Kobayashi, Hirako, Minato, Sasaki, Horiuchi and Domeki1996). However, it is unclear whether hepatoma cells other than Reuber H-35, BRL, or Hep G2, in which the expression of MTAP activity still unknown, secrete a significant amount of MTA. We considered that medium conditioned by established cell lines lacking MTAP activity may also exert the 2-cell blocking effect via MTA. On the other hand, physiological extracellular concentrations of purines, such as adenosine, are at submicromolar levels (Hasko et al., Reference Hasko, Linden, Cronstein and Pacher2008) and correspond well with effective concentrations of purines in 2-cell blocking (Table 1). It is noteworthy that the Mr < 1000 fraction of human cord serum significantly reduced embryonic development in B6C3F1 mice, particularly at the 2-cell stage (Ogawa et al., Reference Ogawa, Ono and Marrs1987). Comparing the levels of MTA, adenosine, and hypoxanthine in serum or secretory fluid of genital tracts, including oviducts or uteri, of between fertile and infertile females could provide valuable information on the effects of these purines in vivo.
Taken together, we identified the active compound in Fr.B-25 as MTA. In view of the present findings, in addition to hypoxanthine and adenosine, MTA is useful as a tool to dissect the mechanism regulating the preimplantation development of mouse embryos. Further studies on MTA or other purines are crucial for understanding the causes of infertility in mammals.
Acknowledgements
Part of this study was supported by a Grant-In-Aid to M.K. for the Promotion of Scientific Research from the President of Akita Prefectural University, Japan.