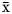Introduction
Animals develop different strategies and modify their behaviour throughout their life history to adapt in response to environmental stimuli (Tinbergen, Reference Tinbergen1963). These various strategies are deployed and tested during their ecological interactions (Medel et al., Reference Medel, Aizen and Zamora2009); however, with the accelerated expansion of occupied territory for the development of human activities, these processes are constantly being modified and organisms are forced by anthropic factors to quickly change their behaviour in order to survive (Faeth et al., Reference Faeth, Warren, Shochat and Marussich2005). For instance, agriculture and livestock demand space, and thus continuously alter and modify the landscape, limiting habitat availability as well as food for various species of both herbivores (Lee & Graham, Reference Lee and Graham2006) and carnivores (Amador-Alcalá et al., Reference Amador-Alcalá, Naranjo and Jiménez-Ferret2013; Peña-Mondragón & Castillo, Reference Peña-Mondragón and Castillo2013). This situation has created long lasting conflicts between humans and wildlife, which have been widely documented, and generate considerable financial losses as well as violent responses towards the species involved, resulting in serious injuries and probably death for many individuals in populations of these species (Goldstein, Reference Goldstein2013).
Fishing is another common and worldwide distributed activity with a similar scenario; for instance, non-target species (i.e. accompanying and by-catch fauna such as marine mammals and turtles) are frequently reported as accidentally entangled in nets or even killed by fishers' violent responses; this occurs in both industrial and artisanal fisheries (Bearzi, Reference Bearzi and Di Sciara2002; Jiménez & Domínguez, Reference Jiménez and Domínguez2007; Adimey et al., Reference Adimey, Hudak, Powell, Bassos-Hull, Foley, Farmer, White and Minch2014; Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2017). Particularly in the latter, in which various rustically constructed gear are used (Jiménez & Castro, Reference Jiménez, Castro, Granados, Arenas and Vargas2007), marine mammals such as dolphins, especially of the species Tursiops truncatus (Montagu, 1821), are the ones with the highest frequency of interaction reports (Reeves et al., Reference Reeves, Read and Notarbartolo di Sciara2001; Lauriano et al., Reference Lauriano, Fortuna, Moltedo and Notarbartolo di Sciara2004; Waples et al., Reference Waples, Thorne, Hodge, Burke, Urian and Read2013; Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014). The opportunistic feeding habits of bottlenose dolphins (Hill et al., Reference Hill, Burrows and Hughes2003; Rocklin et al. Reference Rocklin, Santoni, Culioli, Tomasini, Pelletier and Mouillot2009) may play an important role in their frequent interactions with fisheries, as a result of competition for common prey species (Reeves et al., Reference Reeves, Read and Notarbartolo di Sciara2001; Lauriano et al., Reference Lauriano, Fortuna, Moltedo and Notarbartolo di Sciara2004; Waples et al., Reference Waples, Thorne, Hodge, Burke, Urian and Read2013; Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018).
These interactions are commonly reported and widely distributed across the world (Rocklin et al., Reference Rocklin, Santoni, Culioli, Tomasini, Pelletier and Mouillot2009; Powell & Wells, Reference Powell and Wells2011; Jaiteh et al., Reference Jaiteh, Allen, Meeuwig and Loneragan2013; Adimey et al., Reference Adimey, Hudak, Powell, Bassos-Hull, Foley, Farmer, White and Minch2014; Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014); however, there are no studies in Mexico that describe in detail the strategies used by these cetaceans, as well as their behavioural changes in relation to the presence of artisanal fishing activities. Therefore, we studied a bottlenose dolphin population in the coastal waters of the western Gulf of Mexico, where the dolphin–fisheries interactions are well known to cause conflict due to depredation on fishing gear (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012, Reference Morteo, Rocha-Olivares and Abarca-Arenas2014, Reference Morteo, Rocha-Olivares and Abarca-Arenas2017; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018). Our main goal was to document behavioural variations in relation to dolphin group size and age structure, using their proximity towards the artisanal fishing as an indicator of the types of interactions.
Materials and methods
Study area
The municipality of Alvarado is located within the central coast of the state of Veracruz in the Gulf of Mexico (18°47′47.22″N95°44′43.77″W; Figure 1). The coastal waters are influenced by one of the largest rivers in the country (Papaloapan River), and the Alvarado Lagoon System (Jiménez et al., Reference Jiménez, Granados, Ortiz, Granados, Arenas and Vargas2007); this ecosystem is highly productive, thus this area is of nationwide importance for the abundance of shrimp and coastal fish (López et al., Reference López-Portillo, Martínez, Hespe, Hernández, Méndez, Vásquez-Reyes, Gámez-Aguilar, Jiménez-Orocio and Gachuz-Delgado2011).

Fig. 1. Study area in Alvarado, located on the central coast of Veracruz, Mexico.
Artisanal fisheries support many families in communities along the coastal waters of the Gulf of Mexico, and Alvarado is no exception. Much of its economy depends on artisanal fisheries, mainly for products of commercial importance such as sea bass (Centropomus parallelus), mackerels (Gerres cinereus, Scomberomurus regalis), snappers (Lutjanus sp.), mullets (Mugil curema), red drums (Sciaenops ocellatus) and jacks (Caranx hipos) (Morteo, Reference Morteo2011; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018). Fishers generally use 7 m long fibreglass outboard motorboats (40 hp) in fish captures; their implements are diverse and depend on the target species, such as hand lines, long lines and different types of gillnets (Jiménez & Castro, Reference Jiménez, Castro, Granados, Arenas and Vargas2007; Morteo & Hernández-Candelario, Reference Morteo, Hernández-Candelario, Granados, Arenas and Vargas2007; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018). The latter are the most frequently deployed in this study area (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012, Reference Morteo, Rocha-Olivares and Abarca-Arenas2014; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018), and are over ~700 m long and 3.5 cm mesh size in average. These nets are not species selective and capture all kinds of pelagic fish (Bjordal, Reference Bjordal2002).
Observations from fixed point
From October 2015 to July 2016 we made monthly field observations between 07:00 and 16:00 h from a vantage land point at the top of a 22 m lighthouse (18°46′56.69″N 95°44′44.59″W, Figure 1) located over a lowland tropical dry forest known as ‘Monte Simón' at the eastern breakwater of the Alvarado lagoon entrance (Figure 1). Behavioural observations of dolphins were accomplished by means of the continuous recording scanning method (Altmann, Reference Altmann1974).
The most common behaviours with the longest durations were categorized into general states (Villamizar, Reference Villamizar2001); these were represented by (1) locomotion, (2) feeding, (3) social, (4) socio-sexual, (5) play and (6) rubbing with objects (Table 1). Most of these observations were carried out in the absence of fisheries, and only the latter were used as control. Behavioural records were also filmed with a high definition digital camera (Panasonic SDR – H80 or Nikon Coolpix p500), in order to reproduce and analyse behavioural displays with higher detail.
Table 1. Behavioural description for bottlenose dolphins in the coastal waters off the Alvarado Lagoon according to Morales-Rincon (Reference Morales-Rincon2016)

Distances between dolphins and human activities (either fishing gear or boats) were calculated by means of a theodolite (Sokkia Mod. FOIF-DT 205), recording the horizontal and vertical angles relative to the height of the lighthouse. Such distances (m) were estimated at the centre of each group and these were categorized using the limits proposed by Morales-Rincon (Reference Morales-Rincon2016); therefore all dolphin sightings were divided following Nadeau (Reference Nadeau2013) in: (1) operational interactions (<30 m from nets), (2) non-operational interactions (31–150 m from the net) and (3) no interactions (>150 m or no fishing gear).
Surveys
During the same months, we also carried out monthly boat-based surveys between 07:00 and 16:00 h during the normal operations of the artisanal fishers of Alvarado. Behavioural recordings used the same sampling method as on land, and through the information provided by the control observations, all states were then partitioned into different events related to instantaneous and sporadic movements according to their proximity to fishing activities (sensu Nadeau, Reference Nadeau2013).
The activities were based on the ethogram developed by Morales-Rincon (Reference Morales-Rincon2016) specifically for the dolphin population inhabiting the study area (available at: http://eduardo.morteo.mx/WebPage/PDF/Morales_2016.pdf).
Due to the proximity of target objects (i.e. gear, boats and dolphin groups), estimates from the survey boat usually had sufficient precision, as these were made by experienced crew and based on well-established references (e.g. GPS distance to the coast, dimensions of known fishing gear, nearby boats and coastal landmarks) (Morteo & Hernández-Candelario, Reference Morteo, Hernández-Candelario, Granados, Arenas and Vargas2007; Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012, Reference Morteo, Rocha-Olivares and Abarca-Arenas2014; Morales-Rincon, Reference Morales-Rincon2016; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018). However, these were also calibrated regularly during the fixed point observations by means of the theodolite as described earlier (Cox et al., Reference Cox, Read, Swanner, Urian and Waples2003).
Also, all behavioural records were divided by platform (i.e. fixed point or surveys) and compared to determine the extent of observer bias.
Behaviour in the presence and absence of fisheries interaction
Behavioural observations categorized as events were used to construct discovery curves (Fisher et al., Reference Fisher, Corbet and Williams1943); these were used to measure the rate of appearance for new behavioural events throughout the sampling effort. The variety of these events over time was classified according to the presence and the distance towards fishing activities by means of linear regressions.
Likewise, the frequency of occurrence for the behavioural state of each sighting was calculated, as well as their local rate; the latter defined as the intensity, measured as the frequency per unit of time of each behaviour (Lopéz-Rull, Reference López-Rull, Martínez-Gómez, Lucio and Rodríguez-Antolín2013). Since the behaviour and location of dolphin groups was variable across the observation period, the frequency with which individuals approached the gillnet was calculated, using only one session per group; the session was randomly selected from the records in order to reduce any bias due to pseudo-replication (Hurlbert, Reference Hurlbert1984). A session consisted of every sighting period registered per group with and without fishing interaction, which had an average duration of 1.8 min. Consequently, differences in frequencies and local rates were also assessed according to the presence and distance towards fishing activities using the three categories (sensu Nadeau, Reference Nadeau2013) by means of contingency tables (χ2).
Variations in group size
Our definition of a group followed the chain rule, in which dolphins were considered associated if they remained within two body lengths from each other, usually but not always moving in the same direction and engaged in similar behaviour (Shane, Reference Shane, Leatherwood and Reeves1990; García-Vital, Reference García-Vital2012). As mentioned earlier, the distance between these dolphin groups and fishing activities were estimated from the boat, and were calibrated regularly.
Again, only one observation session per group was randomly selected to reduce the bias from pseudo-replication (Hurlbert, Reference Hurlbert1984). Likewise, the number of individuals involved in each of the three categories of interaction (i.e. operational, non-operational and without interaction) was estimated and graphed according to the distance from the fisheries activity.
Differences by age class
All sighted individual dolphins were categorized by age (Bearzi, Reference Bearzi and Di Sciara2002); taking into account the knowledge of the crew and the experience of fishers, and considering that all boats that are commonly used in the artisanal fisheries are about the same size (~ 7 m length), we used them as scale for the estimation of age classes (Read et al., Reference Read, Kraus, Bisak and Palka1993; García-Vital, Reference García-Vital2012; La Fauci, Reference La Fauci2017), such that: (1) adults were individuals over 2.5 m; (2) juvenile dolphins had an approximate length of two-thirds of an adult, and (3) calves were one-third the size of an adult.
Frequencies of behavioural events were computed for each age class combination (i.e. adults, juveniles and calves), during both interaction categories and in the absence of interaction with fisheries. Once again, only one observation session per group was randomly selected (Hurlbert, Reference Hurlbert1984). The computed frequencies were divided by the number of individuals involved in the sighting, in order to determine the divergence of the group towards any behavioural state and then averaged this index for each condition (interaction and non-interaction). This divergence index was compared for each age class, as well as in the absence and the presence of fishing interactions, both operational and non-operational, using an ANOVA (P < 0.05).
Results
Sampling effort and group size
We conducted 20 field trips in both the mobile platform and the fixed point, and all occurred during the normal activities of the fishers. In total 64 dolphin groups were sighted from which 30 h of recording were obtained for behaviour classification. From these sightings, 28 groups were recorded around fishing activities and the remaining 36 were not. Average dolphin group size was 3.2 ± 2.2 individuals, where trios were the most frequent (41%). Group size was variable and fewer individuals were present when interaction with fisheries occurred (1–7 individuals), compared with the absence of interactions (1 to 16 individuals), but this was not significant (P > 0.05).
Behaviour in the presence and absence of fisheries interactions
A total of 110 different behavioural events were recorded during the observations (74 from fixed point and 72 from surveys; where 32 and 36 were exclusive for each platform, respectively). In total, 53 occurred in the absence of fishing interaction, 36 during operational interaction and 21 in the non-operational condition. The discovery curves showed a linear trend throughout the sampling period in all cases; however, the slope was different for each case and also for each platform. A marked difference in the appearance of new behaviours was observed during the absence of fishing activity when these were observed from land. In contrast, new behaviours were constant for the three conditions when recorded from the boat, and the rate of appearance was higher for the operational interaction (Figure 2). Locomotion was the most common behaviour during all the sightings in the three conditions (Figure 3A–C). Feeding was also recorded regardless of the interaction with fisheries, but it was more frequently observed during the operational interaction when measured from the boat (Figure 3A). Conversely, in the non-operational interaction this behaviour was displayed only a few times; but, from fixed point observations, its intensity (i.e. local rate) was higher (Figure 3D). The behaviours associated with socio-sexual, play and rubbing also showed low occurrences in the different conditions and platforms (Figure 3A, C); however, they presented the highest intensity rates (Figure 3B, D). Social behaviour was only present in the absence of fishing interactions with the second highest local rate (Figure 3A–D). Significant differences were found in the frequency of the behavioural states, in relation to the platforms during the three conditions (operational χ2 = 50.33, df = 7; P < 0.001; non-operational χ2 = 21.73, df = 7; P < 0.002; No interaction χ2 = 39.88, df = 7; P < 0.001). Local rates also showed differences in frequency of behaviours in the operational and non-operational distances (χ2 = 41.80, df = 5; P < 0.001; χ2 = 29.28, df = 4; P < 0.001) but not in the absence of interaction with fisheries (χ2 = 12.85, df = 7; P < 0.075).

Fig. 2. Discovery curves for behavioural events in bottlenose dolphin sightings recorded by survey sample. Black lines show the linear regression with their respective equations and determination coefficient according to the type of interactions with fisheries.

Fig. 3. Histograms of behavioural records for bottlenose dolphins in the study area in the absence and presence of fisheries interactions (operational and not operational) in two platforms. (A) Behavioural frequencies in surveys; (B) Intensity rates in surveys; (C) Behavioural frequencies from the lighthouse; (D) Intensity rates from the lighthouse (N = 64 sightings). Asterisks show statistical differences (P < 0.05).
Interaction distance vs group size
Most of the recorded interactions were classified as operational since the cumulative curve showed that over half of the randomly selected records (54% in land based and 82% in surveys) occurred between 0 and 30 m of the gear (Figure 4A, B, right axis), and were carried out mainly individually (Figure 4A, B, left axis). Non-operational interactions (>30 m) involved the participation of more group members, although in a smaller proportion (Σ = 26% and Σ = 7%).

Fig. 4. Proportion of bottlenose dolphin records during interactions with fisheries (left scale) according to the number of individuals involved and the distance to the fishing gear (N = 28 sightings, 44% of the total). The grey dashed line at the top denotes the cumulative proportion for all records (right scale). (A) Distance of interaction in the surveys; (B) Distance of interaction in the land-based platform.
Differences by age class
Groups composed exclusively of adult animals were the most common during and in the absence of fishing interactions (64% and 75%, respectively). The number of behavioural events in relation to group size and age structure (i.e. divergence index) showed that adult groups were more variable independently of their interaction with fisheries (Figure 5), followed by groups with juveniles and calves; however, these differences were not significant (χ2 = 2.96, df = 4, P < 0.56).

Fig. 5. Divergence index (i.e. frequencies of behavioural records by age class divided by the number of individuals in each sighting) for bottlenose dolphins according to the categories of their interaction with fisheries in the study area (N = 64 sightings) in the two platforms. (A) Divergence index in surveys platform; (B) Divergence index in land-based platform.
Discussion
Sampling effort
Observation time (N = 30 h) was lower compared with similar studies (![]() ${\bar{\rm x}}$ = 80 h; Chilvers & Corkeron, Reference Chilvers and Corkeron2001; Neumann & Orams, Reference Neumann and Orams2005; Miller et al., Reference Miller, Solangi and Kuczaj2010); the latter was due to the persistence of cold northern fronts that limited our sampling efforts in the study area. However, the number of sightings was sufficient to detect significant differences in some cases. This may be attributed to the fact that, unlike most of the studies focused on behaviour that use descriptions from other populations (e.g. Shane, Reference Shane, Leatherwood and Reeves1990), we used an ethogram developed specifically for the dolphins present in our study area (Morales-Rincon, Reference Morales-Rincon2016). Therefore, we deem likely that our samples are representative for the study period and the individuals involved. Also, the use of two platforms (fixed and mobile) allowed behaviour classification from two perspectives, with direct (surveys) and indirect (land based) presence of the researchers. This led to significant differences between the platforms in terms of the frequency of behavioural states and their local rates for dolphin sightings, recording and quantifying both the behavioural responses of dolphins in situ, and also the observer bias (Simultea & Lomac-MacNair, Reference Simultea and Lomac-MacNair2016). It has been argued that from elevated platforms, the larger field of vision provides a better context and accurate readings; whereas during navigation, the field of vision is more limited, but the superficial observations may be more detailed (Würsig et al., Reference Würsig, Cipriano, Würsig, Pryor and Norris1998; Yin, Reference Yin1999). However, we believe that, as shown by our data, the differences found in dolphin behaviour are more related to the effect of the presence and distance of the survey boat (i.e. a local fishing skiff), possibly due to the anticipation of antagonistic responses by fishers posing risk for the dolphin population, as have been described for the study area (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012, Reference Morteo, Rocha-Olivares and Abarca-Arenas2017; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018).
${\bar{\rm x}}$ = 80 h; Chilvers & Corkeron, Reference Chilvers and Corkeron2001; Neumann & Orams, Reference Neumann and Orams2005; Miller et al., Reference Miller, Solangi and Kuczaj2010); the latter was due to the persistence of cold northern fronts that limited our sampling efforts in the study area. However, the number of sightings was sufficient to detect significant differences in some cases. This may be attributed to the fact that, unlike most of the studies focused on behaviour that use descriptions from other populations (e.g. Shane, Reference Shane, Leatherwood and Reeves1990), we used an ethogram developed specifically for the dolphins present in our study area (Morales-Rincon, Reference Morales-Rincon2016). Therefore, we deem likely that our samples are representative for the study period and the individuals involved. Also, the use of two platforms (fixed and mobile) allowed behaviour classification from two perspectives, with direct (surveys) and indirect (land based) presence of the researchers. This led to significant differences between the platforms in terms of the frequency of behavioural states and their local rates for dolphin sightings, recording and quantifying both the behavioural responses of dolphins in situ, and also the observer bias (Simultea & Lomac-MacNair, Reference Simultea and Lomac-MacNair2016). It has been argued that from elevated platforms, the larger field of vision provides a better context and accurate readings; whereas during navigation, the field of vision is more limited, but the superficial observations may be more detailed (Würsig et al., Reference Würsig, Cipriano, Würsig, Pryor and Norris1998; Yin, Reference Yin1999). However, we believe that, as shown by our data, the differences found in dolphin behaviour are more related to the effect of the presence and distance of the survey boat (i.e. a local fishing skiff), possibly due to the anticipation of antagonistic responses by fishers posing risk for the dolphin population, as have been described for the study area (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012, Reference Morteo, Rocha-Olivares and Abarca-Arenas2017; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018).
Behaviour in the presence and absence of fishery interaction
The discovery curves for recorded behavioural events showed a linear trend for our three categories of distances towards the fisheries; the latter indicates that not all the behavioural variants of the studied population were documented throughout the study. However, there were differences in the rate of appearance of all these new events (see Figure 3); therefore, the distance towards the fisheries seems to affect the variety of activities that dolphins may perform. This has been widely documented, and has different impacts on the health of dolphin populations, according to the type, frequency and level of interactions, causing stress, feeding deficiencies and even incidental deaths (Shane et al., Reference Shane, Wells and Wursig1986; Owen et al., Reference Owen, Wells and Hofmann2002; Brotons et al., Reference Brotons, Grau and Rendell2008; Rocklin et al., Reference Rocklin, Santoni, Culioli, Tomasini, Pelletier and Mouillot2009; Adimey et al., Reference Adimey, Hudak, Powell, Bassos-Hull, Foley, Farmer, White and Minch2014).
Locomotion and feeding were the most frequent behaviours regardless of the distance to the fisheries, and their intrinsic relation is explained by the daily and seasonal shifts in the abundance and distribution of prey (Neumann & Orams, Reference Neumann and Orams2005), causing the dolphins to move continuously between the locations with available food. The latter also evidences the opportunistic nature of the bottlenose dolphin, since both these activities were especially recorded around fishing gear, which captures fish that are part of their diet (Jaiteh et al., Reference Jaiteh, Allen, Meeuwig and Loneragan2013; Chávez-Martínez, Reference Chávez-Martínez2017), and also the importance of this site as a feeding ground (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012, Reference Morteo, Rocha-Olivares and Abarca-Arenas2014, Reference Morteo, Rocha-Olivares and Abarca-Arenas2017; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018).
The most frequent interactions were operational (within the first 30 m from the fishing gear), where the extraction of fish was evidenced; actually, the crew witnessed what was interpreted as creaking noises produced by the dolphin's teeth while releasing the netted prey. Conversely, within the non-operational distance (~31–150 m), dolphins spent their time moving and patrolling around the fishing spot. Our findings are consistent with Brotons et al. (Reference Brotons, Grau and Rendell2008) in the Balearic Islands during artisanal fishing; however, unlike their study, where the gear damage was focused only at the top, our nets were damaged in the upper half, which could be related to differences in the fishing techniques or in the distribution of the prey across the water column (Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018). Our data also suggest that dolphins may change their behaviour, but also the frequency and intensity of their activities while using the space around the area where fisheries take place, thus bottlenose dolphins may reorganize their activities as an energy-efficient strategy around the fisheries (Powell & Wells, Reference Powell and Wells2011).
It is noteworthy that frequencies for socio-sexual and play were low, but they showed significantly higher local rates during the operational interaction (Figure 4A and B, respectively). This may be explained considering that dolphins that are interacting with the fisheries are not categorized as residents, as shown by the occurrence records from the long-term photo-identification programme for this dolphin population since 2002 (Morteo, Reference Morteo2011; Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012, Reference Morteo, Rocha-Olivares and Abarca-Arenas2014, Reference Morteo, Rocha-Olivares and Abarca-Arenas2017, Garcia-Vital et al., Reference García-Vital, Morteo, Martínez-Serrano, Delgado-Estrella and Bazúa-Durán2015). Thus intense social and playful contact between dolphins may be indicative of recognition among the individuals involved (Dudzinski, Reference Dudzinski1998).
Interaction distance vs group size
One of the most important attributes for the study of population ecology in dolphins is their social structure (Whitehead & Dufault, Reference Whitehead and Dufault1999; Dinis et al., Reference Dinis, Alves, Nicolau, Ribeiro, Kaufmann, Cañadas and Freitas2018); elements such as group size and individual identification are fundamental to understanding complex social relationships between these animals (Whitehead & Dufault, Reference Whitehead and Dufault1999). In this sense, bottlenose dolphin group formations are often determined by age, sex, hierarchical patterns and environmental cues (e.g. predators and food availability; Connor et al., Reference Connor, Heithaus and Barre2001, Bouveroux & Mallefet, Reference Bouveroux and Mallefet2010). Group size in coastal bottlenose dolphins usually varies between 5 and 15 individuals (Morteo, Reference Morteo2011); however, smaller groups (<5 individuals) were found in the coastal waters of Alvarado. This seems typical for the studied population (Morteo, Reference Morteo2011), thus it has been hypothesized to constitute a strategy that reduces detectability by fishers while also decreasing the chances of negative outcomes for the dolphins (García-Vital et al., Reference García-Vital, Morteo, Martínez-Serrano, Delgado-Estrella and Bazúa-Durán2015); the latter as a response to the pressure by fishers that this dolphin population has suffered for decades, which was evidenced during this study, including violent retaliation to individuals that interacted with their gear (Del Castillo-Olvera, Reference Del Castillo-Olvera2010; Morteo, Reference Morteo2011; Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2017; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018). Conversely, in other study areas, where human-dolphin competition for fish is not as noticeable, dolphin group sizes observed around fishing activities are mostly related to the biomass of prey captured in the gear (Lauriano et al., Reference Lauriano, Fortuna, Moltedo and Notarbartolo di Sciara2004; Rocklin et al., Reference Rocklin, Santoni, Culioli, Tomasini, Pelletier and Mouillot2009).
Although differences in group sizes were not significant in our study, it is noteworthy that most individual dolphins and pairs occurred within the first 30 m (see Figure 2), which seems to argue in favour of the ‘lower detectability' hypothesis. We feel inclined towards this explanation, given the long-term exposition to antagonistic responses by fishers, and the decrease in behaviours that imply greater body exposure as dolphins approach the fishing spots. Admittedly, it is a common assumption that dolphins may be attracted to gear settings since fish are congregated and/or weak when entangled, and therefore are easier to catch (Lauriano et al., Reference Lauriano, Fortuna, Moltedo and Notarbartolo di Sciara2004; Rocklin et al., Reference Rocklin, Santoni, Culioli, Tomasini, Pelletier and Mouillot2009; Powell & Wells, Reference Powell and Wells2011); in that sense, the participation of multiple dolphins would be unnecessary. Other studies have classified the interaction radius between dolphins and fishing activities within a much broader range (e.g. 400 m by Lauriano et al., Reference Lauriano, Fortuna, Moltedo and Notarbartolo di Sciara2004, and 200 m by Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012), such that these may have referred mostly to non-operational interactions, and thus preventing feasible comparisons.
Interactions may depend on sex and age class
The coastal waters of Alvarado are highly productive such that artisanal fishing is frequent and intense (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018); also, over 125 distinct dolphins are present every day, and many of them are resident (Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2017). Therefore, in light of the antagonistic nature of their interactions, the behavioural changes reported here, in addition to the reported trends in temporal and spatial distributions for the bottlenose dolphins (Morteo, Reference Morteo2011; Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014; La Fauci, Reference La Fauci2017), there is an apparent ‘tolerance’ or at least a certain degree of ‘habituation’ to artisanal fisheries in the study area. This has been documented in other bottlenose dolphin populations, with different human activities, and with other dolphin species as well (i.e. Delphinus delphis) (Neumann & Orams, Reference Neumann and Orams2005; Waples et al., Reference Waples, Thorne, Hodge, Burke, Urian and Read2013).
For instance, the overall annual distribution of bottlenose dolphins and artisanal fishing in the area show reciprocal evasion, thus a high proportion of their encounters are deemed fortuitous (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012). Moreover, dolphins display different core distribution areas according to sex (Medellín-Ortiz, Reference Medellín-Ortiz2012) and age (La Fauci, Reference La Fauci2017), showing sexual segregation (Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014), where nursing groups tend to be further away from the areas with greater human activities. However, intentional interactions, measured as higher than expected interaction rates with fisheries, have actually been documented for some individuals within the study area by means of dorsal fin photo-identification, which were first assumed to be males (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012), and then confirmed through direct and indirect sexing methods (Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014).
Bottlenose dolphins group into fluid and dynamic associations by sex and age classes (Nowacek & Wells, Reference Nowacek and Wells2001; Owen et al., Reference Owen, Wells and Hofmann2002; Bouveroux & Mallefet, Reference Bouveroux and Mallefet2010; Dinis et al., Reference Dinis, Alves, Nicolau, Ribeiro, Kaufmann, Cañadas and Freitas2018). The social structure of the females in the study area resembles ‘bands’ of multiple individuals, including offspring and juveniles; whereas males form smaller and more durable unions similar to the so-called ‘first order alliances’ (see Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014 after Connor et al., Reference Connor, Heithaus and Barre2001). Although the sex of individuals that participated in interactions with fisheries recorded during this research was initially unknown, comparisons of their dorsal fins to the photo-id catalogue of bottlenose dolphins in the area found no matches with any of the 84 known females, and that at least five of the 15 known males were involved in these encounters (Morteo, unpublished data). Consequently, given the strong social affiliation and low number of affiliates in male alliances, it is highly likely that a large part of these sightings involved only males.
Conclusion
The adaptation of strategies for food acquisition (Sargeant & Mann, Reference Sargeant and Mann2009), where adult male dolphins moving alone or in small groups are more frequently associated with fishing gear depredation (Adimey et al., Reference Adimey, Hudak, Powell, Bassos-Hull, Foley, Farmer, White and Minch2014; Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014; Labadie et al., Reference Labadie, Tixier, Barbraud, Fay, Gasco, Duhamel and Guinet2018; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018) has been linked to their curious and predatory behaviour, as well as their easy habituation to the varying conditions in their habitat (Adimey et al., Reference Adimey, Hudak, Powell, Bassos-Hull, Foley, Farmer, White and Minch2014; Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014, Reference Morteo, Rocha-Olivares and Abarca-Arenas2017; La Fauci, Reference La Fauci2017). Many of these interactions have been attributed to adult males and point to the development of a sex-biased strategy to take advantage of captured fish (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012, Reference Morteo, Rocha-Olivares and Abarca-Arenas2017; Garcia-Vital et al., Reference García-Vital, Morteo, Martínez-Serrano, Delgado-Estrella and Bazúa-Durán2015; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018). Furthermore, female individuals within the population are more resident and thus experienced (Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2014), and especially nursing or lactating individuals are known to avoid fishing areas (La Fauci, Reference La Fauci2017), since these present a higher risk of injury (Srinivasan et al., Reference Srinivasan, Swannack, Grant, Rajan and Würsig2017), due to fishers' antagonistic responses to the encounters with bottlenose dolphins (Morteo et al., Reference Morteo, Rocha-Olivares, Arceo-Briseño and Abarca-Arenas2012; Rechimont et al., Reference Rechimont, Lara-Domínguez, Morteo, Martínez-Serrano and Equihua2018). Therefore, the risks involved in this activity would also potentially represent a sex-biased pressure for this population (Morales-Rincon, Reference Morales-Rincon2016; Morteo et al., Reference Morteo, Rocha-Olivares and Abarca-Arenas2017). Behavioural variations in the dolphins' repertoire are likely a strategy to reduce the risk of injuries or death when interacting with human activities; thus these dolphins seem to have habituated or at least tolerate fishing activities within the study area.
Acknowledgements
The authors are thankful to M.E. Rechimont, K. Chávez, M. Quiroga, E. Bolaños, J. Bolaños, M. Tenorio, E. Tiburcio and J. Tiburcio for their support in the field during data collection. We also appreciate the insights of I. Martínez-Serrano and. P. Dias at Universidad Veracruzana for their contributions during the writing of the thesis from which this paper derived. We thank the authorities of the Mexican Federal Secretariat of Communications and Transport (SCT) and the Mexican Navy (SEMAR) for the permission to use the facilities of the sea lighthouse at Monte Simón to collect data.
Financial support
This work was part of the MSc thesis of the lead author who benefited from a National Council of Science and Technology Grant (CONACyT grant No. 635385, 2014). This work was part of the project ‘Trophic ecology of bottlenose dolphins (Tursiops truncatus) and artisanal fisheries interactions in coastal waters off Veracruz state’ funded by the CONACyT project number 221750. All boat-based behavioural observations were non-invasive and were also carried out without intervention during the normal fishing operations of the local artisanal fleet, thus no official permit for fieldwork was required.