Introduction
Pyrochlore-group minerals are common rare-metal accessories of carbonatite intrusions, and are typically present in niobium ores in Indian carbonatite occurrences. More than 25 carbonatite complexes have been reported in India (Fig. 1a). Some of them are characterised by different mineral resources, such as REE, Sr, Ba, Nb and F (Krishnamurthy, Reference Krishnamurthy2019). Considerable economic concentrations of pyrochlore are found in the Newania, Sevathur, Sung Valley, and Samchampi carbonatite complexes (Viladkar, Reference Viladkar1998; Viladkar and Ghose, Reference Viladkar and Ghose2002; Melluso et al., Reference Melluso, Srivastava, Guarino, Zanetti and Sinha2010; Sadiq et al., Reference Sadiq, Ranjith and Umrao2014; Viladkar and Bismayer, Reference Viladkar and Bismayer2014; Viladkar et al., Reference Viladkar, Bismayer and Zietlow2017; Hoda and Krishnamurthy, Reference Hoda and Krishnamurthy2020; Randive and Meshram, Reference Randive and Meshram2020). The Amba Dongar, Sevathur [also known as Sevattur], Koratti, Jogipatti, Samalpatti and Pakkanadu carbonatites are potential targets for locating REE, Nb, U and Th resources (Viladkar, Reference Viladkar1981; Viladkar and Bismayer, Reference Viladkar and Bismayer2010; Viladkar and Bismayer, Reference Viladkar and Bismayer2014; Nagabhushanam et al., Reference Nagabhushanam, Durai Raju, Mundra, Rai, Purohit, Verma and Nanda2018). The Amba Dongar carbonatites host significant resources of fluorite, REE, P, Ba, Sr and Nb (Palmer and Williams-Jones, Reference Palmer and Williams-Jones1996; Nanda et al., Reference Nanda, Verma, Purohit, Khandelwal, Rai, Mundra, Viladkar, Duraiswamy and Krishnamurthy2017; Nagabhushanam et al., Reference Nagabhushanam, Durai Raju, Mundra, Rai, Purohit, Verma and Nanda2018). Exploration of the Amba Dongar carbonatites has indicated homogeneous mineralisation down to the explored vertical depth of 120–200 m in calcite and ankerite carbonatites, and also the carbonatite breccia (Krishnamurthy, Reference Krishnamurthy2019).
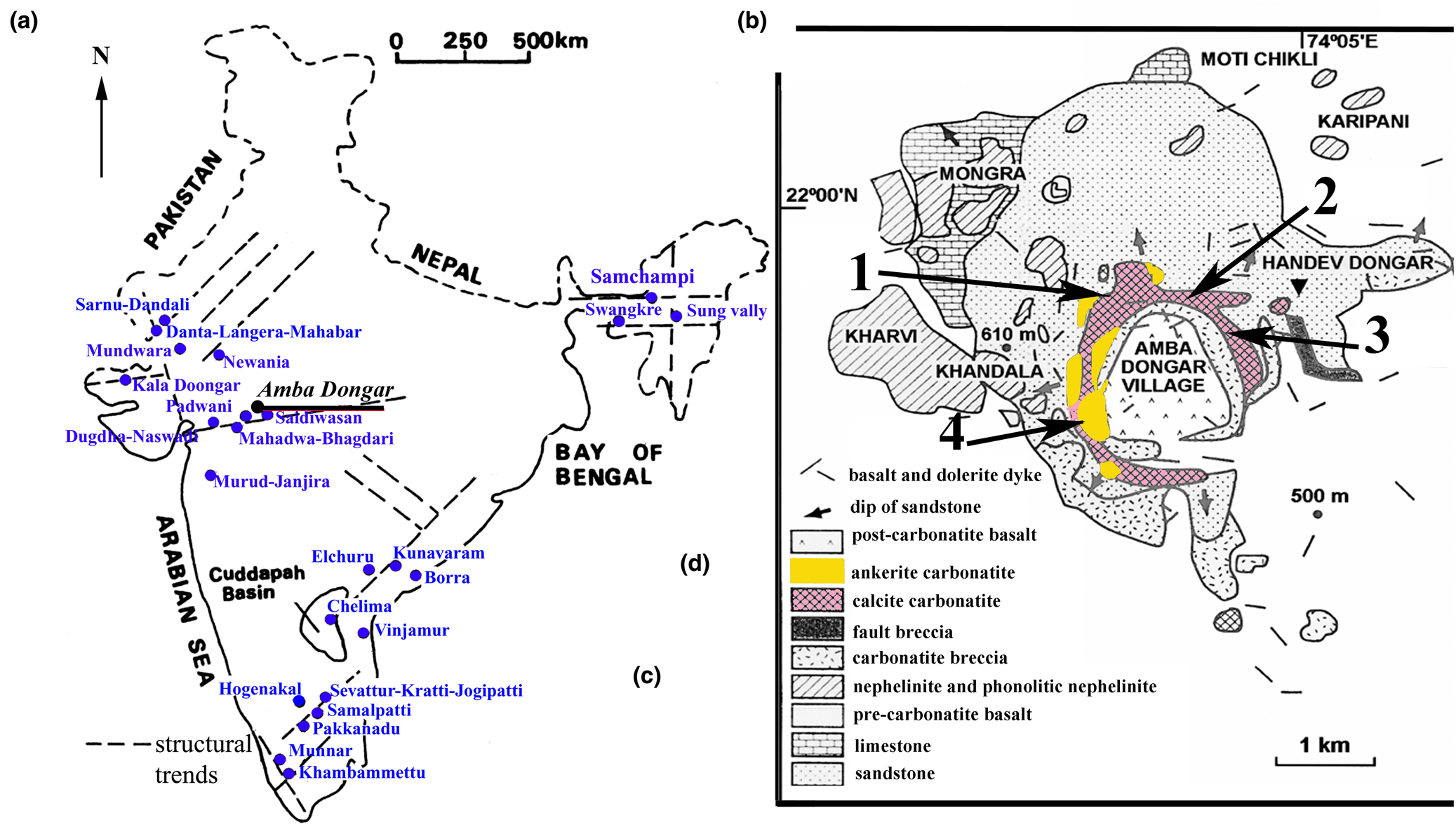
Fig. 1. (a) Schematic location map of Indian carbonatite complexes (Krishnamurthy, Reference Krishnamurthy2019, reprinted by permission from Springer Nature, COPYRIGHT 2019). (b) Geological map of the Amba Dongar carbonatite complex (Viladkar, Reference Viladkar1996, reprinted by permission of the author) with locations of samples investigated: 1–1203; 2–1012; 3–1223; 4–1114.
Pyrochlore is a widespread accessory mineral in the calcite and ankerite carbonatites of the Amba Dongar complex (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Ghose et al., Reference Ghose, Fialin, Kienast and Viladkar1997; Viladkar and Bismayer, Reference Viladkar and Bismayer2010; Nanda et al., Reference Nanda, Verma, Purohit, Khandelwal, Rai, Mundra, Viladkar, Duraiswamy and Krishnamurthy2017; Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020). Pyrochlore is a primary magmatic mineral that occurs as an early crystallising phase in the carbonatites (Viladkar and Bismayer, Reference Viladkar and Bismayer2010). The composition of pyrochlore is known to be modified by transition from a carbonatite magma to a fluid-rich system at the latest stages of formation of pyrochlore and shows a variety of ionic substitutions involving Nb, Ta, Ti, Zr, Ca, Sr, Ba, Si, U, REE, F, OH and other species (Hogarth, Reference Hogarth and Bell1989; Lumpkin and Ewing, Reference Lumpkin and Ewing1995; Hogarth et al., Reference Hogarth, Williams and Jones2000; Lumpkin, Reference Lumpkin2001; Bonazzi et al., Reference Bonazzi, Bindi, Zoppi, Capitani and Olmi2006).
Previous studies of pyrochlore from the Amba-Dongar carbonatites were focused on individual pyrochlore crystals from one type of carbonatite (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Viladkar and Bismayer, Reference Viladkar and Bismayer2010), or on pyrochlores with a specific composition (Ghose et al., Reference Ghose, Fialin, Kienast and Viladkar1997; Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020). The objective of the present study is to summarise the new and exisiting literature data on pyrochlore occurrence and composition in the various carbonatites of the Amba Dongar complex and to compare these data with the available information on pyrochlore-group minerals from other Indian carbonatite complexes. This study also discusses the evolution of pyrochlore composition from the early to late stages of carbonatite formation.
Geological setting and origin of the carbonatites
Amba Dongar is the largest subvolcanic complex located within the Narmada rift zone ~140 km east of Vadodara, Chhota Udaipur district, Gujarat, India (Fig. 1a). The complex intrudes the Late Cretaceous Bagh sandstone and limestone, and is overlain by the Late Eocene Deccan basalts. The Amba Dongar complex, together with the overlying basalts, fenites and country rocks occupy an area of ~30 km2 (Gwalani et al., Reference Gwalani, Rock, Chang, Fernandez, Allégre and Prinzhofer1993; Srivastava, Reference Srivastava1997; Viladkar, Reference Viladkar1981; Reference Viladkar1996; Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992). The alkaline and carbonatite magmatism represents a late-stage magmatic event of the Deccan volcanic episode: the U–Pb apatite radiometric ages are 62 ± 22 and 63 ± 19 Ma for carbonatites and 62.3 ± 1.6 Ma for nephelinites (Fosu et al., Reference Fosu, Ghosh, Chew and Viladkar2019).
The Amba Dongar complex consists of different generations of calcite and ankerite carbonatites, carbonatite breccia, nephelinite, phonolite and fenitised sandstone (Fig.1b). The carbonatite intrusion hosts a large fluorite hydrothermal deposit. The carbonatite activity commenced with the emplacement of carbonatite breccia and calcite carbonatite. Calcite carbonatite makes up the major part of the intrusion and occurs as a ring dyke collaring an inner rim of carbonatite breccia composed of fragments of calcite carbonatite, gneissic country rock, sandstone, basalt and nephelinite. Calcite carbonatites of the ring dyke were emplaced in several phases. The earlier phase is a coarse-grained calcite carbonatite. These were folllowed by fine-grained calcite carbonatites at a later stage (Viladkar, Reference Viladkar1981; Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992). Alkaline silicate rocks are represented by nephelinite plugs and a few dykes of phonolite exposed in the peripheral parts of the complex, in low-lying regions around the carbonatite dome. Nephelinite xenoliths are abundant in the calcite carbonatite in the northern part of the ring dyke (Viladkar, Reference Viladkar1981, Reference Viladkar1996, Reference Viladkar2015). The late-stage ankerite carbonatites occur as thin dykes of the first phase and as large plugs of the late phase in the south-western part of the calcite carbonatite ring dyke. The end of carbonatite activity is marked by the formation of hydrothermal fluorite (Deans et al., Reference Deans, Sukheswala, Sethna and Viladkar1972, Reference Deans, Sukheswala, Sethna and Viladkar1973; Palmer and Williams-Jones, Reference Palmer and Williams-Jones1996).
The detailed geology, petrography and mineralogy of the Amba Dongar carbonatites are described in numerous publications (Gwalani et al., Reference Gwalani, Rock, Chang, Fernandez, Allégre and Prinzhofer1993; Srivastava, Reference Srivastava1997; Viladkar, Reference Viladkar1981, Reference Viladkar1984, Reference Viladkar1996, Reference Viladkar2000, Reference Viladkar and Panagiotaras2012, Reference Viladkar2015, Reference Viladkar2017, Reference Viladkar2018; Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Palmer and Williams-Jones, Reference Palmer and Williams-Jones1996; Viladkar and Schidlowski, Reference Viladkar and Schidlowski2000; Doroshkevich et al., Reference Doroshkevich, Viladkar, Rip and Burtseva2009). According to these studies, the intrusion was formed in the following sequence: (1) nephelinites and phonolites; (2) carbonatite breccia; (3) coarse-grained calcite carbonatite; (4) fine-grained calcite carbonatite dykes in sandstone, basalts and carbonatite breccia; (5) ankerite carbonatite dykes and plugs within the calcite carbonatites; (6) thin veins of sideritic carbonatite in the ankerite carbonatite; (7) fenitised sandstones; and (8) the final hydrothermal phase of fluorite mineralisation.
Calcite carbonatites are the predominant rock type; and exhibit a wide variation in texture, grain size and colour, which varies from white to brown. The coarse-grained calcite carbonatites occur in different varieties and can be banded, pyroxene-, pyrochlore- or magnetite-rich (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Viladkar, Reference Viladkar2017; Chandra et al., Reference Chandra, Paul, Viladkar and Sensarma2018). The calcite carbonatites are formed at magmatic and postmagmatic-hydrothermal stages and consist of primary and secondary minerals. The main primary mineral is calcite, making up to 80 vol.%. Fluorapatite, aegirine–augite, aegirine, phlogopite, magnetite, perovskite, titanite, pyrochlore, zirconolite, columbite and zircon are common accessories, in decreasing order of abundance. Quartz, fluorite, strontianite, Sr-rich baryte, Ba-rich celestine, florencite-(Ce), bastnäsite-(Ce), parisite-(Ce), synchysite-(Ce), monazite-(Ce), ankerite, chalcopyrite, dickite, galena, pyrite, Fe and Mn oxides, ilmenite, vanadinite, wakefieldite-(Ce) and wakefieldite-(La) represent secondary minerals (Viladkar, Reference Viladkar1981; Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Palmer and Williams-Jones, Reference Palmer and Williams-Jones1996; Srivastava, Reference Srivastava1997; Doroshkevich et al., Reference Doroshkevich, Viladkar, Rip and Burtseva2009; Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020). According to Doroshkevich et al. (Reference Doroshkevich, Viladkar, Rip and Burtseva2009), the hydrothermal minerals at Amba Dongar were formed by re-equilibration and recrystallisation of the primary carbonatite minerals with a hydrothermal solution. There is evidence that (OH)–, (SO4)2–, F–, REE, Al and Si were important components of the hydrothermal fluid (Palmer and Williams-Jones, Reference Palmer and Williams-Jones1996; Williams-Jones and Palmer, Reference Williams-Jones and Palmer2002; Doroshkevich et al., Reference Doroshkevich, Viladkar, Rip and Burtseva2009).
The ankerite carbonatite is a late differentiate of a primary carbonatite magma (Viladkar, Reference Viladkar1996; Reference Viladkar2018). These carbonatites are dark red in colour and strongly oxidised. Medium- to fine-grained rocks occur in distinct phases. The first phase of ankerite carbonatite intrudes calcite carbonatite, fenite, carbonatite breccia and sandstone. Xenoliths of the calcite carbonatites were digested and metasomatised and contain significant amounts of Fe, Mg, Mn, Sr, Ba and REE (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992). The second-phase ankerite carbonatites form large plugs within the calcite carbonatite ring dyke. All ankerite carbonatites consist predominantly of ankerite with minor proportions of calcite and fluorite, and have a high abundance of REE, in comparison to the calcite carbonatites and carbonatite breccia. The abundance of Ba, Sr, Nb and REE increases from the early to late carbonatite phases. Accessory minerals of the ankerite carbonatites vary in abundance and comprise baryte, hematitised magnetite, apatite, bastnäsite-(Ce), parisite-(Ce), synchysite-(Ce), monazite-(Ce), pyrochlore, quartz, pyrite, galena, chalcopyrite and dickite. Thorite and cerite are not uncommon (Viladkar, Reference Viladkar1996; Reference Viladkar2018). Phlogopite is observed only in the earlier phase of the ankerite carbonatite. Some samples show intense silicification (Viladkar, Reference Deans, Sukheswala, Sethna and Viladkar1972). The end of carbonatite activity is marked by the formation of a hydrothermal quartz–fluorite rock (Palmer and Williams-Jones, Reference Palmer and Williams-Jones1996).
According to isotopic data, the calcite carbonatites are of magmatic origin as their Pb, Rb/Sr, Nd/Sr isotopic ratios are considered to approach mantle values (Simonetti and Bell; Simonetti et al., Reference Simonetti and Bell1995; Ray et al., Reference Ray, Trivedi and Dayal2000). The C and O isotope characteristic of the calcite carbonatites are also consistent with their primary magmatic nature and their trace-element distribution patterns indicate the evolution of these rocks by differentiation of a primary carbonatite melt into initial calcite carbonatites and late-stage calcite carbonatites. The carbonatite magma was initially more magnesian, and this is evident from the presence of phlogopite and periclase in the calcite carbonatite (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Viladkar and Schidlowski, Reference Viladkar and Schidlowski2000; Viladkar, Reference Viladkar and Panagiotaras2012). According to trace-element modelling and petrological constraints, the parental melt that formed the Amba Dongar carbonatite was derived from a metasomatised carbonatised mantle source of garnet lherzolite composition at a depth of >100 km (>3 GPa) aided by CO2-rich fluids and plume-derived heat. The evolutionary transformation of carbonatised silicate magma, concurrent liquid immiscibility, fractional crystallisation, and assimilation, took place at crustal depths (Chandra et al., Reference Chandra, Paul, Viladkar and Sensarma2018; Fosu et al., Reference Fosu, Ghosh, Chew and Viladkar2019). The ankerite carbonatites are characterised by variation in Pb, C and O isotopic ratios, which are related to fluid activity caused by low-temperature hydrothermal F-rich fluids emanating from the carbonatite magma (Simonetti et al., Reference Simonetti, Bell and Viladkar1995). Variations in the C–O isotope data and petrographic observations reveal the coupling of fractional crystallisation and post-magmatic fluid–rock interactions to bulk-rock composition. After emplacement, the resetting of clumped isotope signatures in carbonatites is facilitated by post-magmatic processes in both open and closed systems (Fosu et al., Reference Fosu, Ghosh and Viladkar2020). Banerjee and Chakrabarti (Reference Banerjee and Chakrabarti2019) found that the calcite and ankerite carbonatites are characterised by geochemical and Ca, Nd and Sr isotopic variations. These are interpreted in terms of crustal contamination, hydrothermal alteration, recycling of ancient subducted carbonates, mantle mineralogy and the depth of magma origin. Mixing calculations suggest that the δ44/40Ca compositions of the Amba Dongar carbonatites reflect up to 20% recycled carbonates in the mantle source.
The temperature regime of hydrothermal fluids for the Amba Dongar carbonatites and fenitised rocks has been studied using primary and pseudo-secondary fluid inclusions hosted by apatite, quartz and fluorite (Deans et al., Reference Deans, Sukheswala, Sethna and Viladkar1972, Reference Deans, Sukheswala, Sethna and Viladkar1973; Palmer and Williams-Jones, Reference Palmer and Williams-Jones1996; Williams-Jones and Palmer, Reference Williams-Jones and Palmer2002). The results of modelling showed that the intrusion of Ca-rich carbonatite magma and subsequent K–Na metasomatism of the surrounding sandstone were accompanied by separation of aqueous–carbonic fluids containing significant concentrations of Ca, Al and Si. The exsolution of fluids from the carbonatite took place at a temperature of 700°C and a depth of >10 km. The surrounding sandstone was affected by the fluids at temperatures from 400 to 150°C.
Pyrochlore associations in the carbonatites
Pyrochlore in the Amba Dongar carbonatites has been described by Viladkar (Reference Viladkar1981) and Viladkar and Wimmenauer (Reference Viladkar and Wimmenauer1992). Pyrochlore of early generations are a magmatic accessory phase and occur in variable amounts in all types of carbonatite. In general, pyrochlore is more abundant in the calcite than in ankerite carbonatites (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Ghose et al., Reference Ghose, Fialin, Kienast and Viladkar1997; Viladkar and Bismayer, Reference Viladkar and Bismayer2010; Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020). Recently, it was discovered that the content of this mineral in the calcite carbonatite from the eastern part of the complex can locally reach 50 vol.% and such rocks are composed mainly of pyrochlore, calcite and magnetite (Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020).
Calcite and ankerite carbonatites with pyrochlore mineralisation from different areas of the complex show varied accessory mineralogy. The morphological features of pyrochlore from Amba Dongar are typical for this mineral from intrusive carbonatites (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Viladkar, Reference Viladkar1996; Reference Viladkar2000; Nanda et al., Reference Nanda, Verma, Purohit, Khandelwal, Rai, Mundra, Viladkar, Duraiswamy and Krishnamurthy2017; Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020). Pyrochlore forms individual euhedral grains, octahedral or cubic crystals, and intergrowths of crystals in a carbonate matriх (Fig. 2a–e). The grain sizes vary from 0.02 to 0.4 mm. The colour of pyrochlore ranges from translucent pale golden-yellow to dark brown, and almost opaque (Fig. 2a–c). Pyrochlore from the calcite carbonatites is characterised by a homogeneous texture, with locally a weak blocky and concentric zonation (Fig. 2d–f). The primary magmatic pyrochlore crystallised simultaneously with fluorapatite, and earlier than calcite (Fig. 2d,e). Pyrochlore from the late-stage ankerite carbonatites has distinct repetitive concentric zoning, indicating mechanical disturbance and alteration (Fig. 2g–i). The zoning is very common in large crystals of pyrochlore, which consists of a dark core and a lighter margin. Sometimes pyrochlore in the calcite carbonatite overgrows magnetite (Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020), or occurs as inclusions in large crystals of phlogopite, together with apatite and zircon (Viladkar, Reference Viladkar2000). Rarely, phlogopite forms thin bands in association with abundant pyrochlore and Nb-bearing zirconolite, which show a parallel orientation (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992). In some cases, pyrochlore can form discontinuous rims on discrete calcite grains. Large grains of pyrochlore show replacement by calcite together with wakefieldite, baryte and vanadinite (Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020).
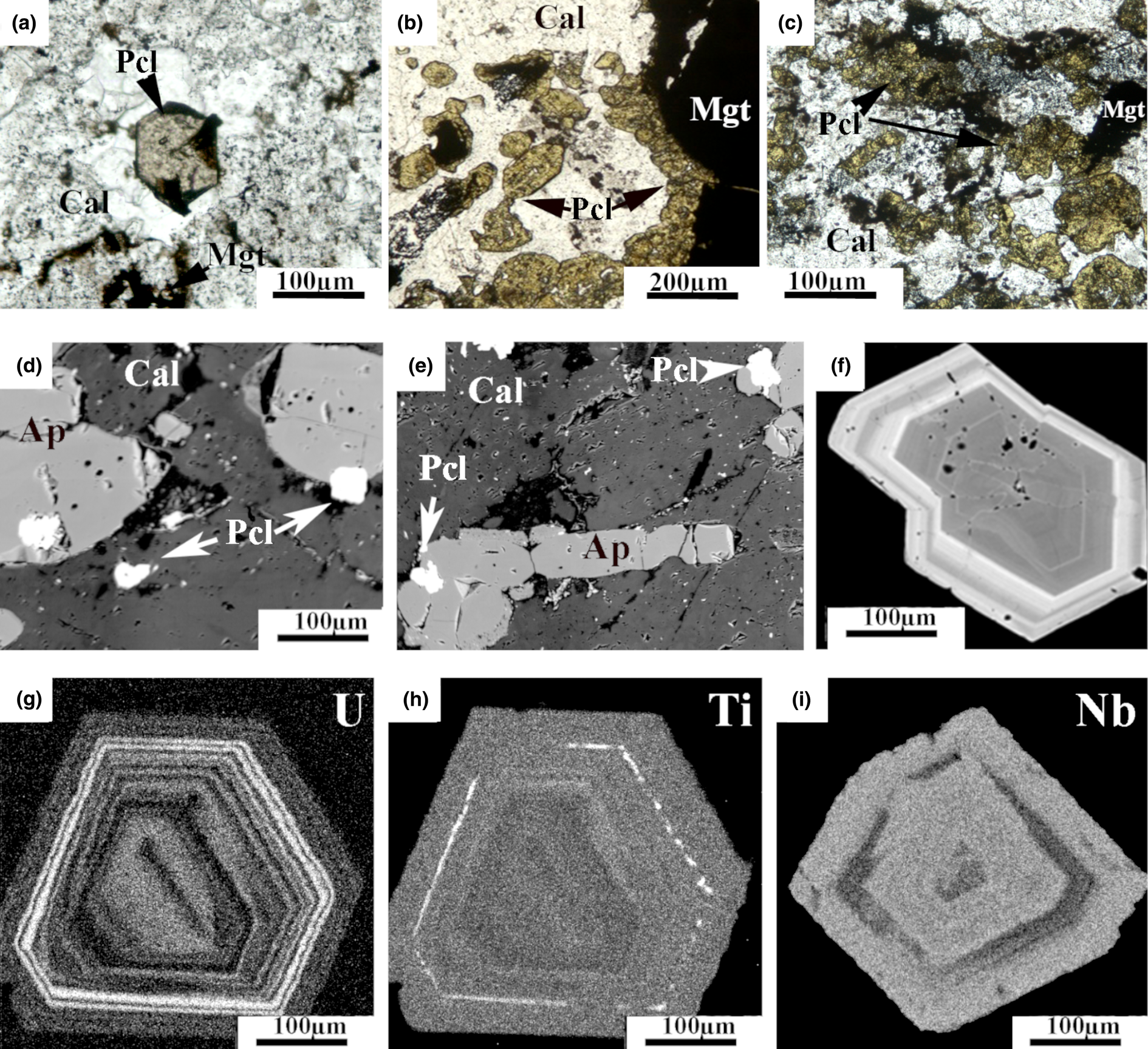
Fig. 2. (a–c) Transmitted-light images of calcite (Cal), pyrochlore (Pcl) and magnetite (Mgt) in calcite carbonatites. (d–f) Back-scattered electron images: (d, e) primary fluorapatite (Ap) associated with homogeneous primary pyrochlore from coarse-grained calcite carbonatites; (f) zoned pyrochlore from coarse-grained calcite carbonatite. (g–i) Element maps showing the distribution of U, Ti and Nb in rhythmically zoned pyrochlore from ankerite carbonatites.
Methods
Pyrochlore in calcite carbonatite thin sections was analysed using a CAMECA SX-100 microprobe at the Mineralogisch-Petrographisches Institute, Hamburg University, Hamburg, Germany. The microprobe was operated at 15 kV accelerating voltage and a beam current of 20 nA. Pyrochlore from ankerite carbonatite (sample 1114) were analysed by electron microprobe analysis (EMPA) using a JEOL JXA 8600 MX Superprobe at the University of Muenster, Germany. This instrument was operated at 20 kV acceleration voltage and a beam current of 15 nA.
The PAP correction procedure was applied to correct for matrix effects. The following standards were used: Nb – Nb metal; F – apatite; Na – jadeite; Si – andradite; Mg – synthetic MgO; Ba – Ba glass; La, Ce and Nd – monazite-Ce; Ta – Ta metal; Ca and Fe – andradite; Sr – synthetic SrTiO3; Zr – synthetic ZrO2; Pb – vanadite; Th – Th glass; U – synthetic UO2; Ti – synthetic MnTiO3. The detection limits do not exceed 0.03 wt.% for most analysed elements and do not exceed 0.05 wt.% for Ta, and 0.1 wt.% for REE, Th, U and F. All the standards were provided by the Smithsonian Institution (National Museum of Natural History), Washington, USA. More detailed information about the standards and applied corrections is available from the analytical laboratories or the authors on request.
Terminology of the pyrochlore supergroup
The pyrochlore supergroup is a group of complex cubic oxides, with the general formula A 2–mB 2X 6–wY 1–n (Hogarth, Reference Hogarth1977; Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010). The A site is typically occupied by eightfold-coordinated large cations: Na+, Ca2+, Sr2+, Pb2+, Sn2+, Sb3+, REE 3+, U4+, Mn2+, Ba2+, Th4+, however it can also contain vacancies and H2O. The B site is represented by octahedrally coordinated cations: Nb5+, Ta5+, Ti4+, Sb5+, Sn4+, Zr4+, Hf4+, Fe3+, Al3+ and Si4+. The X site typically is O2– and can include subordinate (OH)– and F–. The Y site hosts (OH)–, F–, O2–, vacancies, H2O, or a very large cation (K+, Cs+, Rb+). The symbols m, w and n indicate incomplete occupancy of the A, X and Y sites, respectively. The names of each member of the pyrochlore supergroup are composed of the group name plus two prefixes. The first prefix refers to the dominant cation or anion of the dominant valance or H2O or a vacancy at the Y site: OH – hydroxyl; F – fluor; O – oxy; H2O – hydro and vacancy – □. The second prefix refers to the dominant cation of the dominant valance or H2O or a vacancy at the A site, e.g. strontio- or natro-. When the first and second prefixes are equal, only one prefix is applied (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010).
The pyrochlore supergroup is divided into seven groups on the basis of the atomic proportions of the B site cations: pyrochlore group, if Nb is the dominant cation; microlite group (Ta dominant); roméite group (Sb dominant); betafite group (Ti dominant cation); elsmoreite group (W dominant); ralstonite group (Al dominant); and coulsellite group (Mg dominant) (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010, Reference Atencio, Andrade, Bastos Neto and Pereira2017; Christy and Atencio, Reference Christy and Atencio2013).
Unfortunately, it is impossible to identify pyrochlore-group minerals in a petrological context using this classification as there are no precise electron microprobe analytical methods for the determination of the occupancy of the X and Y sites or the structural role of the (OH)-group and H2O (Hogarth, Reference Hogarth2013). The correct identification of individual members is possible only if a combination of several advanced analytical techniques is used. Because of these ambiguities, we will use the terminology proposed by Hogarth (Reference Hogarth1977). Within the pyrochlore subgroups, individual species are named according to the dominant the A site cations (Na, Ca, Ba, REE, Pb, U, Th, etc.). A mineral will be referred to simply as pyrochlore, if the contents of Na and/or Ca are dominant and no other large cation exceeds 20% of their total. If the content of any other large cation exceeds 20% of sum of Na and Ca, the species are named on the basis of a dominant large cation in the A site (e.g. REE- or Pb-rich pyrochlore).
Composition of pyrochlore
New data on pyrochlore composition from different types of the Amba Dongar calcite carbonatites (38 analyses for pyrochlore from coarse-grained, banded and pyrochlore-rich carbonatites from the ring dyke) and ankerite carbonatites (132 analyses for different samples from plugs) were obtained in this study (Tables 1, 2). Additionally, pyrochlore compositional data (112 analyses) from previous investigations of the complex were used (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Ghose et al., Reference Ghose, Fialin, Kienast and Viladkar1997; Viladkar and Bismayer, Reference Viladkar and Bismayer2010; Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020). Data from other Indian carbonatite complexes were used for the discussion of pyrochlore compositional evolution: Newania dolomite and dolomite–ankerite carbonatites (Viladkar, Reference Viladkar1998; Viladkar and Ghose, Reference Viladkar and Ghose2002; Viladkar et al., Reference Viladkar, Bismayer and Zietlow2017); Samchampi calcite carbonatites (Hoda and Krishnamurthy, Reference Hoda and Krishnamurthy2020); Sung Valley (Melluso et al., Reference Melluso, Srivastava, Guarino, Zanetti and Sinha2010; Sadiq et al., Reference Sadiq, Ranjith and Umrao2014) and Khamambettu (Burtseva et al., Reference Burtseva, Ripp, Doroshkevich, Viladkar and Varadan2013); and Sevathur dolomite carbonatite and its weathered zones (Viladkar and Bismayer, Reference Viladkar and Bismayer2014). The evolution of pyrochlore compositions from these Indian carbonatite complexes is presented as binary and ternary plots (Figs 3, 4).

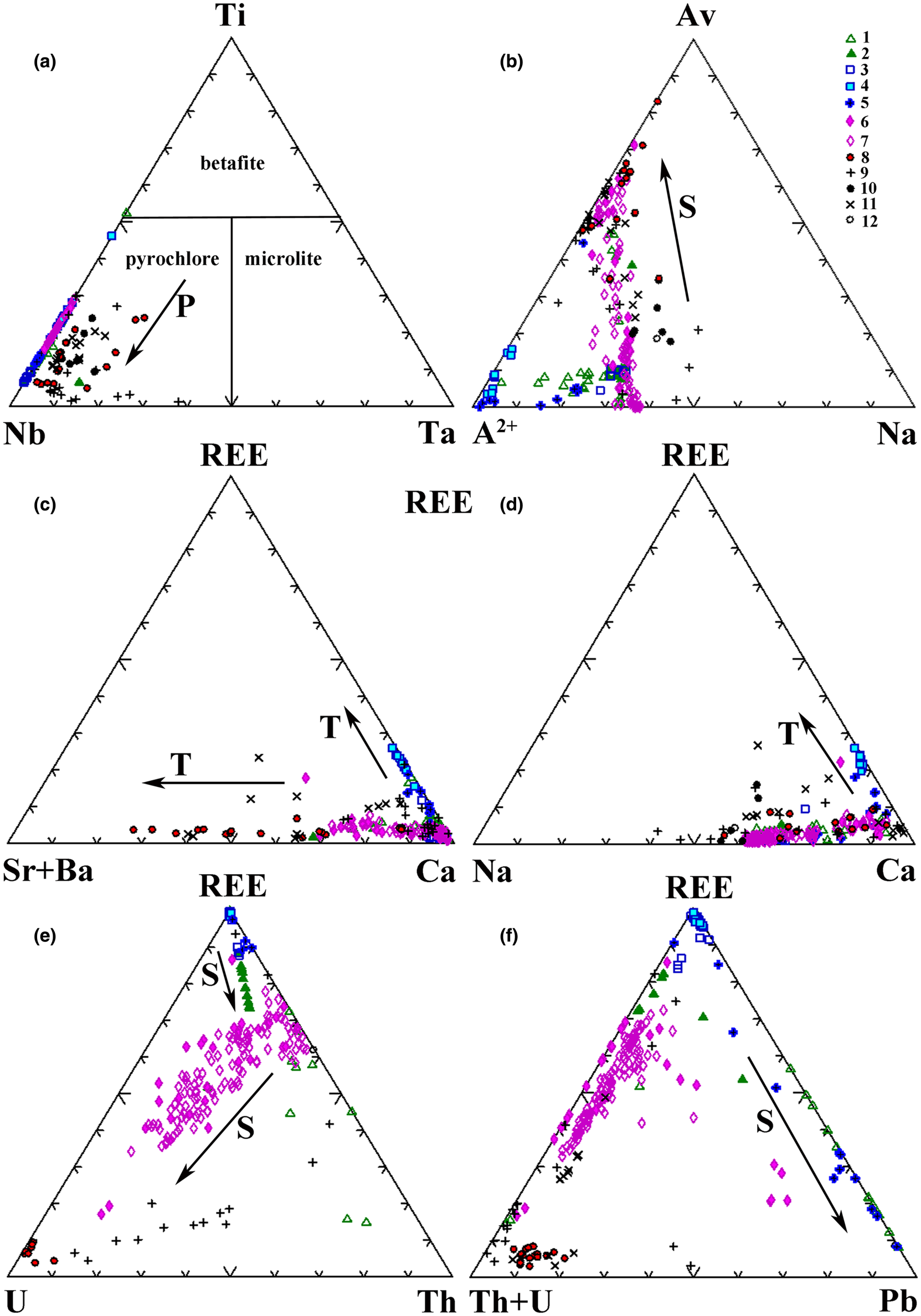
Fig. 3. Composition (apfu) of pyrochlore from Indian carbonatite complexes in terms of cation occupancy in (a) the B site and (b–f) the A site (Hogarth, Reference Hogarth1977; Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010; Lumpkin and Ewing, Reference Lumpkin and Ewing1995). (a) Fields of different pyrochlore-group mineral species are shown. (b) The triangle corners are Av – A-site vacancy, A2+ – sum of divalent cations in the A site and Na. Compositional variation of pyrochlore from the Amba Dongar carbonatites is shown as arrows pointing towards progressively more altered compositions: P – primary, T – transitional and S – secondary. The symbols for samples from the Amba Dongar (1–7) and other Indian carbonatite complexes (8–12) are: 1 – coarse-grained calcite carbonatites in samples 1272, 195, 1231, 1223 (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Ghose et al., Reference Ghose, Fialin, Kienast and Viladkar1997; Viladkar and Bismayer, Reference Viladkar and Bismayer2010; Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020); 2 – coarse-grained calcite carbonatite in sample 1012; 3 – coarse-grained calcite carbonatite in sample 1203 (our data and Viladkar and Bismayer, Reference Viladkar and Bismayer2010); 4 – banded and 5 – pyrochlore-rich variety of coarse-grained calcite carbonatites from the ring dyke in samples AD-1 and V-1, respectively (our data); 6 – ankerite carbonatite in sample 1114 (our data and Viladkar and Bismayer, Reference Viladkar and Bismayer2010); 7 – ankerite carbonatites from different locations in the large plug (our data); 8 – Newania dolomite–ankerite carbonatites (Viladkar, Reference Viladkar1998; Viladkar and Ghose, Reference Viladkar and Ghose2002; Viladkar et al., Reference Viladkar, Bismayer and Zietlow2017); 9 – Sung Valley calcite carbonatites (Melluso et al., Reference Melluso, Srivastava, Guarino, Zanetti and Sinha2010; Sadiq et al., Reference Sadiq, Ranjith and Umrao2014); 10 – Khamambettu calcite carbonatites (Burtseva et al., Reference Burtseva, Ripp, Doroshkevich, Viladkar and Varadan2013); 11 – Sevathur dolomite carbonatite (Viladkar and Bismayer, Reference Viladkar and Bismayer2014); 12 – Samchampi calcite carbonatites (Hoda and Krishnamurthy, Reference Hoda and Krishnamurthy2020).
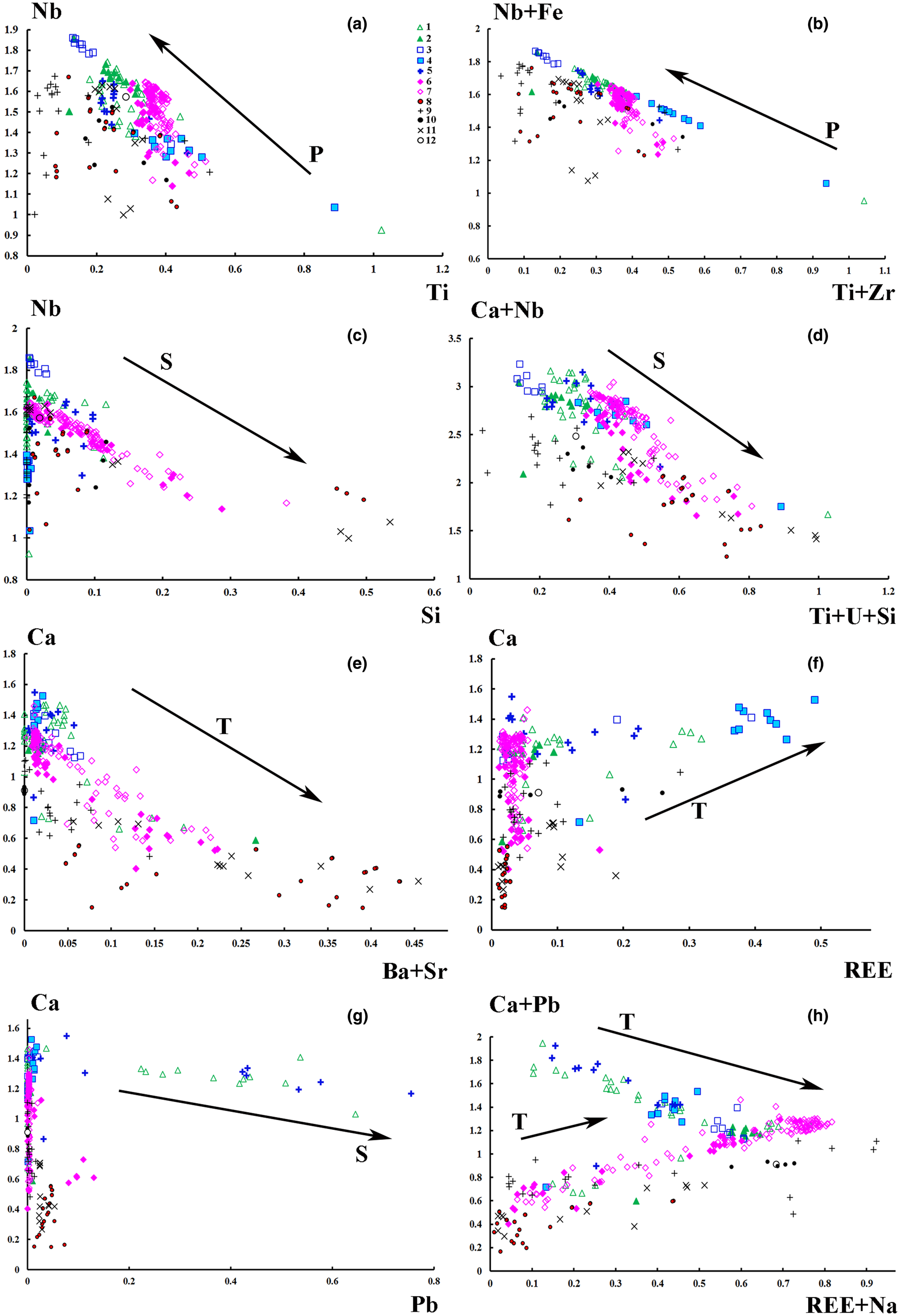
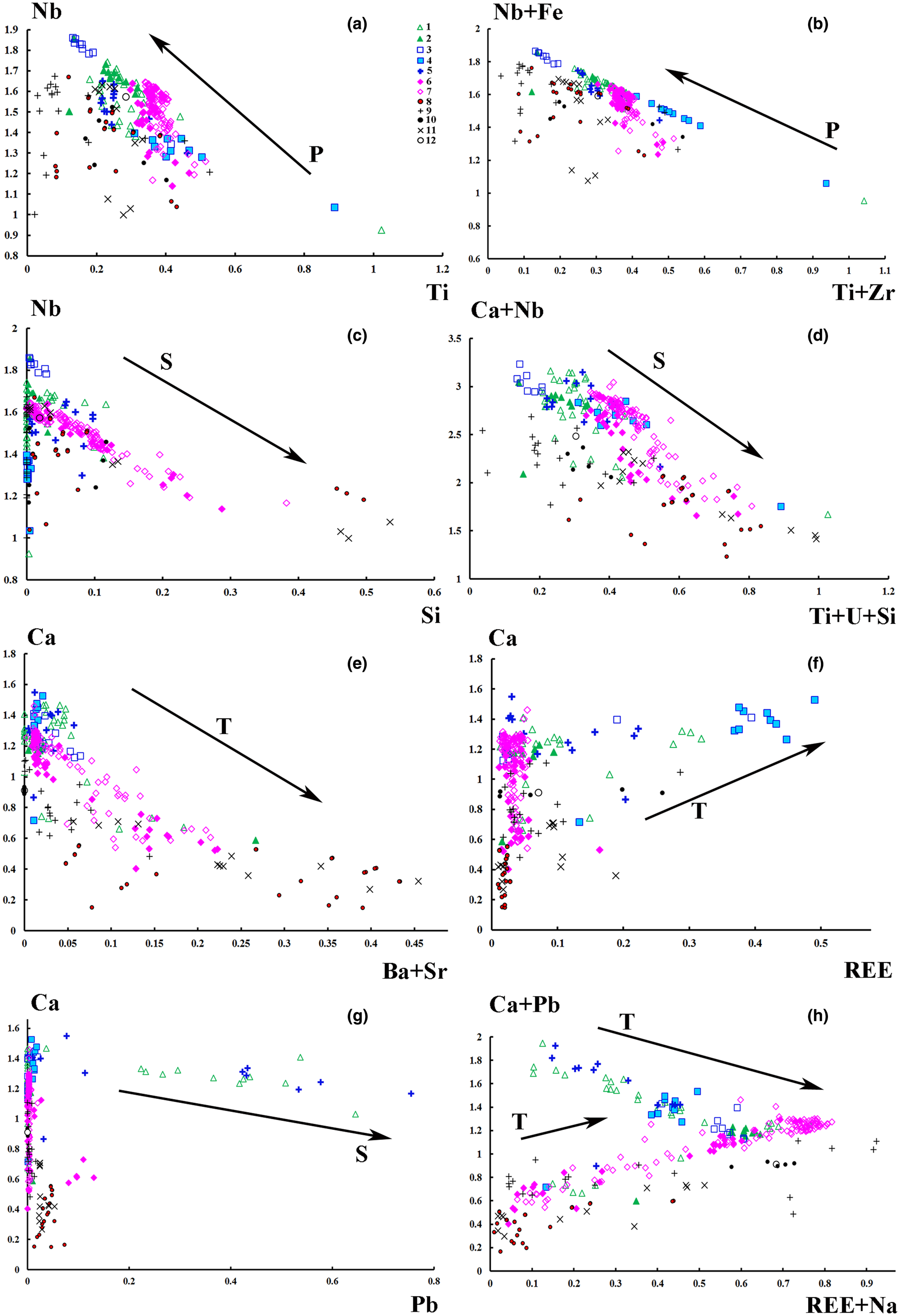
Fig. 4. Correlations between the A- and the B-site cation populations (apfu) in pyrochlore from diverset Indian carbonatites. Compositional variations of pyrochlore from the Amba Dongar carbonatites are shown as arrows pointing towards the progressively more altered compositions: P – primary, T – transitional and S – secondary. The symbols are as in Fig. 3.
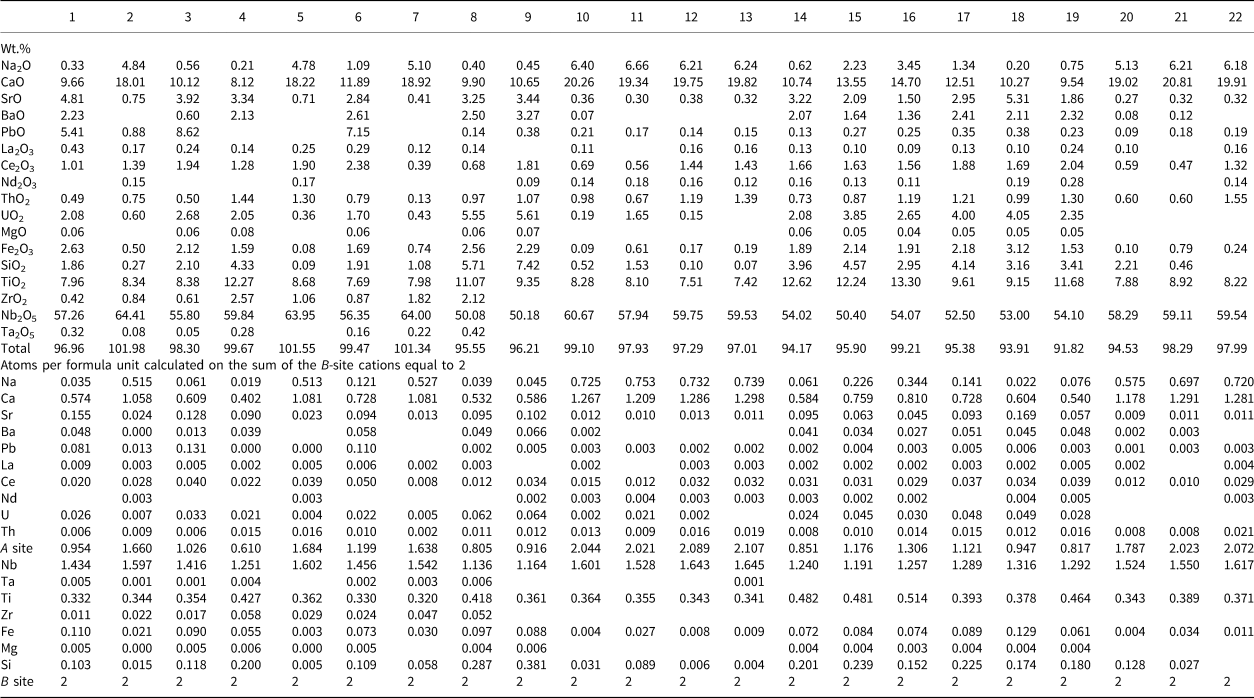
Table 1. Representative compositions of pyrochlore-group minerals from the Amba Dongar calcite carbonatites.*

* Blank cells denote values below the EMPA detection limit. 1–3 – sample 1012, 4–7 – sample 1203 from calcite carbonatites, 8–12 – samples from banded calcite carbonatites, 13–20 – zoned crystals from coarse-grained calcite carbonatites (13, 15 – cores, 14, 16 – rims, 17, 19 – dark and 18, 20 – bright phases in inclusions, respectively).
Table 2. Representative compositions of pyrochlore-group minerals from the Amba Dongar ankerite carbonatites.*
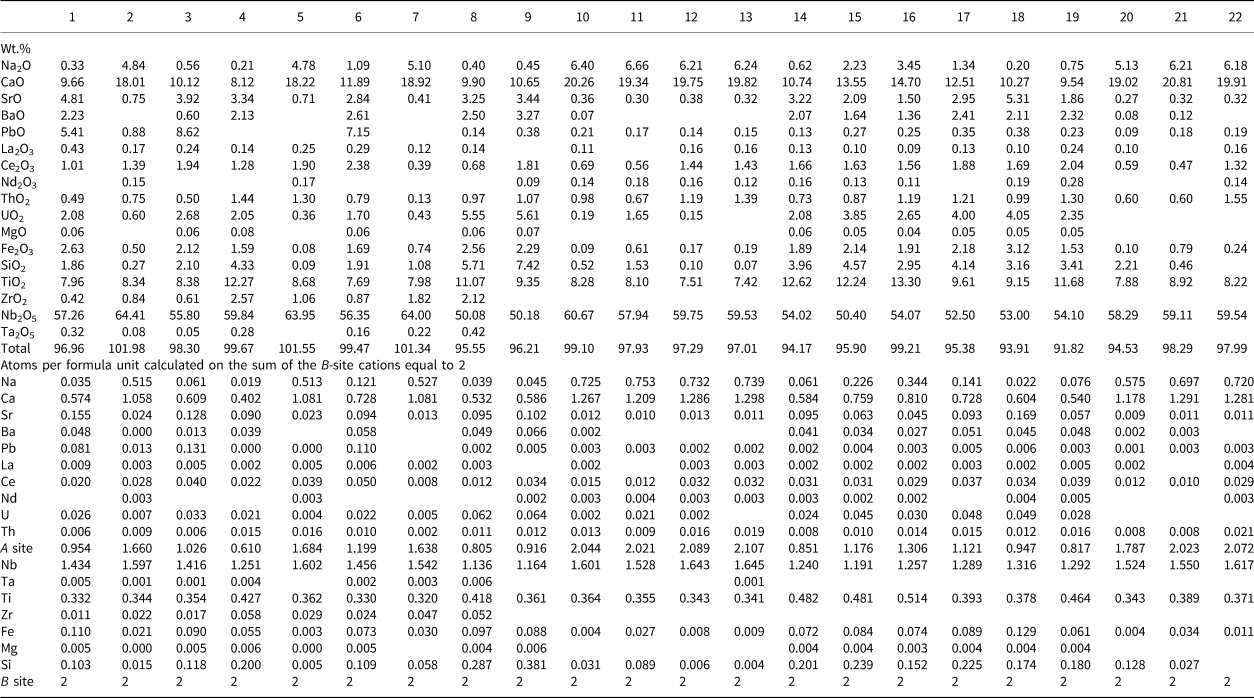
* Blank cells denote values below the EMPA detection limit. The values of F are below the EMPA detection limit. 1–8 – sample 1114, 9–22 – different samples from ankerite plugs, ZrO2 is not defined.
All pyrochlore compositions from the Amba Dongar complex (Tables 1, 2; Fig. 3a) correspond to members of the pyrochlore group. Their composition varies in the calcite and ankerite carbonatites. Most pyrochlore in the calcite and ankerite carbonatites are characterised by a high Nb2O5 content, reaching 70.7 wt.% in banded calcite carbonatites and 64.7 wt.% in ankerite carbonatites (Tables 1, 2). In the marginal parts of pyrochlore crystals from the ankerite carbonatites, the content of Nb2O5 decreases to 47.3 wt.% (Viladkar and Bismayer, Reference Viladkar and Bismayer2010). Compared to pyrochlore compositions from other Indian carbonatite complexes, pyrochlore from both carbonatite types at Amba Dongar is characterised by a lower concentration of Ta2O5 typically ≤ 0.5 wt.%, except for the rim of one zoned crystal, where the Ta2O5 content reaches 12.5 wt.% (Viladkar and Bismayer, Reference Viladkar and Bismayer2010). Typically, Nb in the B site of Amba Dongar pyrochlores is primarily substituted by Ti (Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992; Viladkar and Bismayer, Reference Viladkar and Bismayer2010). However, in most pyrochlore samples from different carbonatite complexes of the world the niobium in the B site is replaced by Ta and less commonly by Ti (Mackay and Simandl, Reference Mackay and Simandl2015). The average content of TiO2 in pyrochlore from the calcite carbonatites is ~6 wt.%, and ~9 wt.% in the ankerite carbonatites. Similar variations in Ti content are typical for pyrochlore from other Indian carbonatites (Figs 3a, 4a). It was found that in some pyrochlore the content of TiO2 is extremely high – up to 25.0 wt.% in pyrochlore from the banded calcite carbonatite (Table 1) and up to 29.4 wt.% in the coarse-grained calcite carbonatite (Fig. 4a,b) (Viladkar and Bismayer, Reference Viladkar and Bismayer2010). The Ti-enriched parts of pyrochlore grains are accompanied by depletion in Nb, Ca and Na and are located in intermediate zones (Fig. 2h,i). Zirconium, Fe, and Si are common impurities at the B site. The maximum content of ZrO2 in zoned pyrochlore is 3.6 wt.% in the calcite carbonatites and 2.6 wt.% in the ankerite carbonatites. According to our (Tables 1, 2) and published data (Viladkar and Bismayer, Reference Viladkar and Bismayer2010; Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020) the concentrations of Fe2O3 range from below detection in unaltered cores to 5.0 wt.% and 3.2 wt.% in grain rims in the calcite and ankerite carbonatites, respectively.
The majority of fresh primary pyrochlore from the calcite and ankerite carbonatites give analytical totals close to 100 wt.%,whereas altered pyrochlore tends to have lower totals, especially in the rim and fractured zones (Tables 1, 2). Altered pyrochlore is characterised by a low total cation content in the A sites and by an increase in the concentration of Si in the B site. In fresh pyrochlore from the calcite carbonatites the content of SiO2 does not exceed 2.1 wt.% (Ghos et al., Reference Ghose, Fialin, Kienast and Viladkar1997); Si can substitute for Nb and Ti in the B site. Our data shows a good inverse correlation between Nb and Si (Fig. 4c). A similar correlation has been found in pyrochlore from alkaline rocks of the Narssârssuk complex, Greenland, metasomatites of the Lovozero and carbonatites of the Khibiny complexes, Kola Peninsula, nepheline syenite of the Mariupol massif, Ukraine, carbonatites of the Kerimasi volcano, Tanzania and many other alkaline intrusions (Bonazzi et al., Reference Bonazzi, Bindi, Zoppi, Capitani and Olmi2006; Chakhmouradian and Mitchell, Reference Chakhmouradian and Mitchell2002; Zaitsev et al., Reference Zaitsev, Williams, Wall and Zolotarev2012; Dumańska-Słowik et al., Reference Dumańska-Słowik, Pieczka, Temp, Olejniczak and Heflik2014; Zaitsev et al., Reference Zaitsev, Spratt, Shtukenberg, Zolotarev, Britvin, Petrov, Kuptsova and Antonov2021). Marginal and fractured parts of zoned pyrochlore from the Amba Dongar ankerite carbonatites are enriched in SiO2 up to 7.4 wt.% (Table 2). Usually these parts of altered pyrochlore are characterised by a deficit of cations at the A site and an increased content of uranium (Fig. 4d). Silica could be present in an amorphous or dispersed state, as was argued for Sr-rich pyrochlore from alkaline rocks of the Lovozero complex (Voloshin et al., Reference Voloshin, Pakhomovsky, Pusharovsky, Nadezhina, Bakhchisaraitsev and Kobyashev1989). Strong enrichment in SiO2 (up to 20.4 wt.%) was found in the altered marginal part of one pyrochlore from the ankerite carbonatites. In addition, this Si-rich area has a high content of CaO, Nb2O5 and TiO2 (up to 15.7 wt.%, 40.3 wt.% and 6.4 wt.%, respectively). The concentration of Na2O and REE 2O3 in the alteration zone does not exceed 1 wt.%. It is possible that the high measured Si content arises from the presence of an unidentified silicate phase in the altered area. Given its low Na content and the predominance of Nb over Ti, the most probable contaminant phases are niocalite (Ca,Nb)4(Si2O7)(O,OH,F)2 and mongolite Ca4Nb6Si5O24(OH)10⋅n(H2O) (Nickel, Reference Nickel1956; Vladykin et al., Reference Vladykin, Drits, Kovalenko, Dorfman, Malov and Gorshkov1985).
Pyrochlore from the ankerite carbonatites displays more significant cation and vacancy variations in the A site than that from the calcite carbonatites (Tables 1, 2). The lowest recorded A-site occupancy in pyrochlore from the calcite carbonatites and ankerite carbonatites is 0.88 and 0.62 apfu, respectively. This cation loss is due to the leaching, mainly of Ca and Na during hydrothermal alteration. The contents of CaO and Na2O in pyrochlore from the calcite carbonatites decrease from 23.0 wt.% (Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020) to 14.2 wt.% and from 5.5 wt.% (Ghos et al., Reference Ghose, Fialin, Kienast and Viladkar1997) to below detection, respectively. The contents of CaO and Na2O in pyrochlore from the ankerite carbonatites range from 20.8 wt.% (Viladkar and Bismayer, Reference Viladkar and Bismayer2010) to 9.5 wt.% and from 6.7 wt.% to 0.2 wt.%, respectively (Table 2). Compared to the composition of the pyrochlore-group minerals from other Indian carbonatites, the A site of pyrochlore from Amba Dongar is predominantly occupied by divalent cations (Fig. 3b).
The average content of minor and trace elements in pyrochlore from the Amba Dongar calcite and ankerite carbonatites does not exceed 0.4 and 0.1 apfu, respectively. The average content of REE in the pyrochlore from the Amba Dongar calcite carbonatites is higher than in pyrochlore from other Indian carbonatites. Significant contents of Ce2O3 and PbO (up to 18.0 wt.% and 33.3 wt.%, respectively) have been observed in alteration zones and rims of pyrochlore in the calcite carbonatites (Table 1, Figs 3c–f, 4f–h). According to literature data, Pb-rich pyrochlore is formed as secondary or late generation phase, and has been described from alkaline granites and laterite weathering crusts on carbonatites (Lottermoser and England, Reference Lottermoser and England1988; Kartashov et al., Reference Kartashov, Voloshin and Pakhomovsky1992; Li et al., Reference Li, Li, Fan, Fan, Zhong, Jahdali, Qin, Jehani, Wang and Nahdi2020).
Viladkar and Wimmenauer (Reference Viladkar and Wimmenauer1992) identified pyrochlore from two coarse-grained calcite carbonatite samples from Amba Dongar that showed significant concentrations of ThO2 (up to 15.3 wt.%). Typically the contents of UO2 and ThO2 in pyrochlore from the calcite carbonatites do not exceed 0.3 wt.%, whereas in strongly zoned pyrochlore from the ankerite carbonatites these elements reach 0.9 and 1.2 wt.%, respectively. Primary magmatic pyrochlore contains insignificant amounts of Th and U. The high levels of UO2 (up to 5.6 wt.%) in pyrochlore from the ankerite carbonatites are accompanied by an increase in silica and a significant cation deficit in the A site (Table 2, Fig. 4d). The contents of BaO and SrO in pyrochlore from the calcite carbonatites rarely exceed 1 wt.% (Table 1), while rim zones of pyrochlores from the ankerite carbonatites were found to contain up to 3.3 wt.% BaO and 6.2 wt.% SrO (Viladkar and Bismayer, Reference Viladkar and Bismayer2010). In general, the average total concentration of Sr and Ba is much lower than in pyrochlore from Newania dolomite–ankerite carbonatite (Viladkar, Reference Viladkar1998; Viladkar and Ghose, Reference Viladkar and Ghose2002; Viladkar et al., Reference Viladkar, Bismayer and Zietlow2017) and pyrochlore from dolomite carbonatite of Sevathur (Viladkar and Bismayer, Reference Viladkar and Bismayer2014) (Figs 3c, 4e).
Of the anions, only fluorine was analysed; this being present in concentrations ranging from below the detection limit to 4.3 wt.% in central zones of pyrochlore grains from the coarse-grained calcite carbonatites (Table 1). Fluorine concentration in pyrochlore from the ankerite carbonatites does not exceed the detection limit.
Discussion: compositional evolution of pyrochlore in carbonatites
The pyrochlore-group minerals are stable in a wide range of physical and chemical conditions from magmatic to hypergene (Hogarth, Reference Hogarth and Bell1989; Lumpkin and Ewing, Reference Lumpkin and Ewing1995; Lumpkin, Reference Lumpkin2001; Nasraoui et al., Reference Nasraoui, Bilal and Gibert1999; Nasraoui and Bilal, Reference Nasraoui and Bilal2000; Zurevinski and Mitchell, Reference Zurevinski and Mitchell2004). The compositional evolution and heterogeneity of pyrochlore reflects changes in temperature, redox potential and chemical activities. Variations in cation and anion composition are typical of pyrochlore from intrusive carbonatites and have been studied, for example, at Oka and Aley in Canada, at Khibiny, Lovozero, Kovdor, Vuoriyarvi and Seblyavr in Russia and in Sokli in Finland (Hogarth, Reference Hogarth and Bell1989; Hogarth et al., Reference Hogarth, Williams and Jones2000; Subbotin and Subbotina, Reference Subbotin and Subbotina2000; Chakhmouradian and Mitchell, Reference Chakhmouradian and Mitchell2002; Chakhmouradian and Williams, Reference Chakhmouradian, Williams, Wall and Zaitsev2004; Chakhmouradian et al., Reference Chakhmouradian, Reguir, Kressall, Crozier, Pisiak, Sidhu and Yang2015; Zurevinski and Mitchell, Reference Zurevinski and Mitchell2004; Zaitsev et al., Reference Zaitsev, Williams, Wall and Zolotarev2012; Lee et al., Reference Lee, Lee, Garcia, Moutte, Williams, Wall and Kim2006; Ivanyuk et al., Reference Ivanyuk, Konopleva, Yakovenchuk, Pakhomovsky, Panikorovskii, Kalashnikov, Bocharov, Bazai, Mikhailova and Goryainov2018).
Primary pyrochlore crystallised in the early stages of carbonatite formation displays compositional variations mainly in B-site cations. There are also significant variations in terms of Ca and U in the A site. Pyrochlore precipitated at late hydrothermal stages varies considerably in terms of cation occupancy at the A site, and is characterised by cation and anion leaching from the A, X and Y sites and incorporation of H2O in the structure (Hogarth, Reference Hogarth and Bell1989; Lumpkin and Ewing, Reference Lumpkin and Ewing1995; Nasraoui and Bilal, Reference Nasraoui and Bilal2000; Zurevinski and Mitchell, Reference Zurevinski and Mitchell2004). Apparently, hydration is followed by cation exchange and controlled by diffusion through a stable B 2X 6 framework (Geisler et al., Reference Geisler, Pöml, Stephan, Janssen and Putnis2005).
The alteration of pyrochlore from carbonatite complexes is described by typical substitutions: A 2+O →AVXV, A 2+O → AVYV, and A +YF– → AVYV, where V represents the A-, X- or Y-site vacancy, respectively (Lumpkin and Ewing Reference Lumpkin and Ewing1995, Lumpkin Reference Lumpkin2001; Hogarth, Reference Hogarth and Bell1989).
Three alteration trends can be distinguished in pyrochlore-group minerals from carbonatites: (1) primary, including magmatic growth, followed by hydrothermal high-temperature alteration (from 600 to 400°C); (2) transitional (hydrothermal alteration at temperature between 300 and 450°C); and (3) secondary, involving pyrochlore alteration below 150°C (Lumpkin and Ewing, Reference Lumpkin and Ewing1995; Lumpkin, Reference Lumpkin2001).
We compared evolution trends of the Amba Dongar pyrochlore to pyrochlore compositions from other Indian carbonatites and these trends are shown as arrows pointing towards altered compositions on the principal classification and discrimination diagrams (Figs 3, 4). Evolution of pyrochlore-group minerals in the Amba Dongar carbonatites involved primarily an increase in the concentration of Ca and a loss of Na at the A site, Nb enrichment in the B site, and, in a few individual zoned pyrochlore, an increase in Ti and F contents (Tables 1, 2). According to the nomenclature scheme of Atencio et al. (Reference Atencio, Andrade, Christy, Gieré and Kartashov2010), these minerals are oxycalciopyrochlore or fluorcalciopyrochlore. Fluorcalciopyrochlore, which contains high amounts of F and Na, was found in the cores of fresh pyrochlore crystals in the calcite carbonatite (Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020). Possible substitutions in the Amba Dongar pyrochlore during the primary stage correspond to the following substitution mechanisms: Ta5+→ Nb5+; 2Nb5+ + Ca2+ → 2Ti4+ + U4+; 2Na+ → Ca2+; Na+ + F- → Ca2+ + O2–; F– → (OH)– (Hogarth, Reference Hogarth and Bell1989; Lumpkin and Ewing, Reference Lumpkin and Ewing1995). As can be seen in Tables 1 and 2, the cores of pyrochlore grains from the ankerite and calcite carbonatites have similar compositions, although pyrochlore from the ankerite carbonatites lacks detectable F. The differentiation of a carbonatite melt from calcite to ankerite carbonatites was studied at Amba Dongar by Viladkar and Wimmenauer (Reference Viladkar and Wimmenauer1992) and Viladkar and Schidlowski (Reference Viladkar and Schidlowski2000). We hypothesise that, primary pyrochlore in both carbonatite types crystallised at the magmatic stage and the early varieties of pyrochlore from the calcite carbonatite appear to be from a relatively undifferentiated magma, rich in F. Primary pyrochlore was later altered by hydrothermal fluids of variable composition, which separated from the carbonatite melt at late stages. In addition, a strong negative correlation between Zr, Ti, Fe and Nb contents was found in the Amba Dongar pyrochlore, which implies a coupled substitution such as Nb5+ + Fe3+ → Ti4+ + Zr4+ (Fig. 4b). Pyrochlore from other Indian carbonatites does not show such a correlation. Perhaps, the extra Nb, Ti and Zr required for substitutions could have been derived by transformation of an early Nb-oxide, for example, zirconolite (Chakhmouradian and Williams, Reference Chakhmouradian, Williams, Wall and Zaitsev2004; Kogarko et al., Reference Kogarko, Sorokhtina, Zaitsev and Senin2009). According to Viladkar and Wimmenauer (Reference Viladkar and Wimmenauer1992), zirconolite occurring in Amba Domgar calcite carbonatites is characterised by a high content of Nb2O5, TiO2 and ZrO2 (up to 10.4 wt.%, 25.1 wt.% and 27.6 wt.%), respectively.
Transitional and secondary alteration is characterised by leaching of Na and F from the A and the Y sites, respectively (Table 1). The content of Na and F in pyrochlore from the calcite carbonatites decrease at the rim and within fractured zones, together with an incease in the proportion of vacancies (Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020). Cation exchange involving Ba, Sr, REE, U, Th, Pb and Fe also occurs and is accompanied by the removal of Na, Ca and F (Figs. 3c–f; 4d–f). In some cases, pyrochlore-group minerals from Amba Dongar, Khamambettu and Sevathur complexes are REE-rich following the substitutional scheme 2Ca2+ → Na+ + REE 3+ (Figs 3d, 4f). Compared to pyrochlore from other Indian carbonatites, a greater enrichment of Amba Dongar pyrochlore in REE is identified in the banded calcite carbonatite, where Ca and Nb is replaced with REE according to the substituton Ca2+ + Nb5+→ REE 3+ + Ti4+, as was demonstrated for the end-member (REE)NaTiNbO6(OH,F) from the Niocan and Bond Zone deposits of the Oka complex (Zurevinski and Mitchell, Reference Zurevinski and Mitchell2004). The concentration of REE in pyrochlore from the ankerite carbonatites increases simultaneously with the concentration of Ba and Sr due to secondary replacement of pyrochlore along grain margins; a common feature of pyrochlore from Indian carbonatites (Fig. 4e). The calcite and ankerite carbonatites of the Amba Dongar complex could be locally enriched in REE (mainly Ce) during differentiation of carbonatite melt (Viladkar and Dulski, Reference Viladkar and Dulski1986; Viladkar and Wimmenauer, Reference Viladkar and Wimmenauer1992). Barium and Sr become enriched in the residual fluid at the hydrothermal stage of carbonatite crystallisation and enter the pyrochlore structure due to cation exchange by secondary alteration.
Finally, hydration and leaching of cations from the A, X and Y sites of the pyrochlore occurs at the latest secondary alteration stage. The cores of the primary Amba Dongar pyrochlore from the calcite carbonatites contain small amounts of Sr, Ba, Si, Al and Zr, whereas the rims and fractured zones have lower Ca, Na and F contents, and elevated concentrations of Si, Pb, U, Th and Fe (Fig. 3e,f). Both U and Si show a positive correlation and were deposited along the rims during hydrothermal reworking. In the intensely zoned pyrochlore from the ankerite carbonatite, rim compositions show a remarkable depletion in Nb, Ca and Na, compensated by enrichment in Si, U, Sr, Ti, Th, Fe and Ba (Fig. 4d). The principal substitution is 2Nb5+ + Ca2+ → Ti4+ + Si4+ + U4+. The increase in SiO2 activity in the hydrothermal fluid at the late stages of carbonatite crystallisation is also confirmed by the replacement of pyrochlore by a Ca–Nb silicate (see above). Previously, Viladkar and Wimmenauer (Reference Viladkar and Wimmenauer1986) reported a Nb-silicate in the Newania ankerite carbonatites. This general trend towards silicates was also described in Kola carbonatites by Chakhmouradian and Williams (Reference Chakhmouradian, Williams, Wall and Zaitsev2004).
Our data show two Pb variation trends in the Amba Dongar pyrochlore. A secondary alteration trend was found for Pb-rich pyrochlore in the calcite carbonatites characterised by a weakly negative correlation between Ca and Pb (Fig. 4g), or Ca with Pb and Na with REE (Fig. 4h). A correlation between these components is observed for pyrochlore in the ankerite carbonatites and can be interpreted to have involved cation exchange in the A site according to the heterovalent substitution: Ca2+ + Pb2+ → REE 3+ + Na+. The higher Pb content in pyrochlore from the calcite carbonatites can be associated with a local secondary enrichment of the hydrothermal fluid in Pb due to the breakdown of Pb-bearing minerals, such as galena.
Conclusions
From our observations and data from the literature (Viladkar and Bismayer, Reference Viladkar and Bismayer2010; Magna et al., Reference Magna, Viladkar, Rapprich, Pour, Hopp and Čejková2020), the petrological and geochemical characteristics of the Amba Dongar calcite and ankerite carbonatites suggest that pyrochlore-group minerals are primary magmatic phases in these rocks. Their extensive compositional variation is due to subsequent post-magmatic and hydrothermal alteration of the host carbonatites.
Pyrochlore-group minerals in the Amba Dongar calcite and ankerite carbonatites show that they are mainly Ca-dominant species, whereas Ce- and Pb-rich species are rare.
Primary Ca-dominant pyrochlore from the calcite and ankerite carbonatites displays variations in the concentration of Ca, Na and, to a lesser extent, REE in the A site, Ta, Nb, Ti and Zr in the Bsite, and F in the Y site. The composition of the primary pyrochlore varies in response to changes in the the compositon of the parental carbonatite melt.
Pyrochlore-group minerals are susceptible to hydrothermal alteration, leading to the formation of hydrated cation- and anion-deficient pyrochlore with a large proportion of vacancies at the A site, together with enrichment in Si, Sr, Ba, Th and REE (Lumpkin and Ewing, Reference Lumpkin and Ewing1995; Nasraoui and Bilal, Reference Nasraoui and Bilal2000). However, the Amba Dongar pyrochlore is characterised by moderate cation- and anion-deficiencies (Tables 1, 2), whereas the recorded variation in Si, Sr, Ba, Th, U and Pb occurs mainly due to transitional and secondary cationic exchange processes.
Secondary pyrochlore is characterised by A-site cation and F deficiency, which can be compensated by the incorporation of OH– or H2O. Concentrations of Ba, Sr, Fe, Pb and Si increase in the altered pyrochlore. Such pyrochlore is formed by reaction of the primary pyrochlore with low-temperature hydrothermal solutions, enriched in alkaline earth elements, and by cation exchange reactions. Enrichment of pyrochlore in divalent cations in the A site does not depend on cation deficiency, whereas the increase in U and Th contents is coupled with the formation of vacancies at this site. During the latest stages, marginal and fractured zones in pyrochlore grains are altered to Pb-, Si-rich and cation-deficient hydrated varieties. Lead might be derived from hydrothermal solutions as a result of galena decomposition.
The analytical data for pyrochlore-group minerals from Indian carbonatites summarised in this study indicate that pyrochlore from Amba Dongar and other complexes differ in the pattern of variation in Zr, Ti, REE, Si and Pb, but show similar variations in Ba and Sr.
The trends of pyrochlore evolution are associated with the primary, transitional and secondary alteration processes that are represented by the following substitution reactions:

Evolution of the pyrochlore composition is interpreted as an interaction between the magmatic pyrochlore and a hydrothermal solution emanating from the carbonatite magma. On the basis of the temperature of hydrothermal fluids and pyrochlore crystallisation (Lumpkin and Ewing, Reference Lumpkin and Ewing1995; Lumpkin, Reference Lumpkin2001; Williams-Jones and Palmer, Reference Williams-Jones and Palmer2002), we believe that the early pyrochlore generations crystallised in a highly alkaline environment at temperatures near 600°C. The secondary alteration of pyrochlore began in a hydrothermal environment with a gradual decrease of pH and the fluid temperatures below 350°C. This process was accompanied by elevated activities of Ba, Sr, U, Th, Fe, Pb and Si in the fluid system.
Acknowledgments
This work was funded by Grants – DST RFBR 2019/120 for Indian and RFBR 19-55-45010 for Russian investigations, respectively. S.G. Viladkar is grateful to the Alexander von Humboldt Foundation for the financial support to carry out this work in 2013, to Prof. T. Geisler of Münster University, Germany, for part of the microprobe analytical work on pyrochlore in sample 1114, and to Prof. U. Bismayer of Hamburg University, Germany. We acknowledge Principal Editor Roger Mitchell for his helpful suggestions and comments. The authors express gratitude to the reviewers and Guest Editors Anton Chakhmouradian and Anatoly Zaitsev for constructive comments on the article. We thank K. Walsh for valuable improvements to the paper.