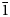Introduction
Rhodonite is a well-known and widespread mineral. It is an important, and quite often the major rock-forming, constituent of metasedimentary manganese ores; in addition, it occurs in some hydrothermal ores and in manganese-rich skarns. This pyroxenoid shows significant chemical variations, especially in the Ca:Mn ratio. This caused a paradoxical situation: until recently, this common mineral was not correctly defined as a mineral species. In different reference books and databases, its idealised formula is written in different ways. The following two formulae were mainly in use: MnSiO3 (Z = 10) and CaMn4Si5O15 (Z = 2). Sometimes both formulae occur in the same publication. For instance, in the 12th Edition of Fleischer's Glossary of Mineral Species (Back, Reference Back2018), the formula of rhodonite is given as Mn2+SiO3 (on page 216) and as CaMn2+4Si5O15 (on page 373, in the list of mineral groups). There are several reasons of such discrepancies: (1) wide chemical variations among samples with the rhodonite-type structure; (2) a confusion in the correct identification of Mn-rich pyroxenoids (rhodonite-, pyroxmangite- and bustamite-type minerals), especially in old publications; and (3) different approaches to writing the end-member formula, i.e. crystal chemical approach (taking into account the repeat unit of the inosilicate chain, Si5O15) vs. simple chemical approach, with aggregate M sites, maximum reduction of stoichiometric coefficients to integer values and formula calculation based on 3 O atoms per formula unit (apfu).
Thus, the definition of rhodonite as a mineral species and the elaboration of the nomenclature of rhodonite-type minerals, in agreement with the current guidelines of mineral nomenclature, were necessary. The nomenclature of the rhodonite group reported here was accepted by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification (IMA–CNMNC) in March 2019 (IMA Proposal 18-I, Miyawaki et al. (Reference Miyawaki, Hatert, Pasero and Mills2019)).
Rhodonite: a retrospective
The term ‘rhodonite’
The term ‘rhodonite’ was first introduced by Germar (Reference Germar1819), on the basis of a proposal by Jasche, who described the same mineral two years earlier (Jasche, Reference Jasche1817). It originates from the Greek word ρόδον, rose (Germar, Reference Germar1819), and it was initially given to a rock formed by pinkish-red Mn-rich silicates (today known as rhodonite and pyroxmangite), quartz, tephroite and manganese oxides. Later the name rhodonite was transferred to the major rock-forming mineral of these rocks, a Mn silicate having typically a pink or red colour. Such rocks and their minerals are common in nature, and during the 19th Century they were described independently with different names from different localities. Many synonyms of ‘rhodonite’ in both the mineralogical and petrological sense were used in the literature of that period: roter braunstein, rothbraunstein or rothbraunsteinerz, rothstein, kieselmangan, rothspat, manganese-spar, manganolite, marcelline, kapnikite, paisbergite or pajsbergite, hermannite and orlets (Chukhrov, Reference Chukhrov1981, and references therein).
Early chemical data and identification problems of Mn pyroxenoids
The first reliable quantitative chemical analyses of rhodonite as a mineral were published in the period from the end of the 19th to the beginning of 20th century. They were collected by Sundius (Reference Sundius1931) who also firstly proposed the triangular diagram CaSiO3–MnSiO3–Fe(Mg)SiO3 and marked separate fields of different ‘pyroxenes’: rhodonite (near the Mn corner, with the subfield ‘iron rhodonites’), bustamite (around the Ca0.5Mn0.5SiO3 point), wollastonite (near the Ca corner), hedenbergite [around the Ca0.5(Fe,Mg)0.5SiO3 point] and ‘sobralite’ [near the Mn0.5(Fe,Mg)0.5SiO3 point; now pyroxmangite–pyroxferroite series minerals].
It should also be noted that the rhodonite field in the diagram by Sundius (Reference Sundius1931) includes only two points corresponding to formulae (recalculated based on 15 O apfu) with Ca < 0.5 apfu and more than ten points having 0.5 < Ca < 1.5 apfu. In general, among chemical data on rhodonite published in the period which could be named ‘pre-structural’, the analyses corresponding to the formulae with Ca content close to 1 apfu (for 15 O apfu) prevail (Larsen and Shannon, Reference Larsen and Shannon1922; Sundius, Reference Sundius1930, Reference Sundius1931; Henderson and Glass, Reference Henderson and Glass1936; Palache, Reference Palache, Berman and Frondel1944; Russel, Reference Russel1946). Analyses of rhodonite with Ca < 0.5 apfu are reported in Sundius (Reference Sundius1931), Ross and Kerr (Reference Ross and Kerr1932) and Hietanen (Reference Hietanen1938).
However, the situation with early analyses of rhodonite is complicated by the possible confusion of this pyroxenoid with the visually indistinguishable and chemically close pyroxmangite (in that period any pink or red pyroxene-like Mn-dominant mineral was referred to as ‘rhodonite’). The name pyroxmangite was introduced in 1913 for a Mn–Fe pyroxene-like mineral (Ford and Bradley, Reference Ford and Bradley1913). It is worth noting that the chemical composition of the original ‘pyroxmangite’ corresponds to the empirical formula (Fe3.52Mn2.59Al0.40Ca0.30)Σ6.81[Si7.00O21] (recalculated based on 21 O apfu) with Fe dominant over Mn, as pointed out by Henderson and Glass (Reference Henderson and Glass1936), and, therefore, corresponding to a Mn-rich variety of pyroxferroite. Ford and Bradley (Reference Ford and Bradley1913) noted that pyroxmangite was considered initially as a highly ferrous rhodonite based on the similarity of most physical properties and chemical composition. At that time, rhodonite and pyroxmangite were distinguished only by optical methods, based on differences in the cleavage angles and 2V values. The 2V value for rhodonite is typically in the range 63–87° whereas for pyroxmangite it is in the range 37–46° (Deer et al., Reference Deer, Howie and Zussman1978). Ноwever, some specimens showed a wide variation in 2V values, filling the gap and ranging from 40 to 72° (Suzaki, Reference Suzaki1963; Aikawa, Reference Aikawa1984). Such intermediate values of 2V are explained by the lamellar structure of pyroxmangite–rhodonite micro-intergrowths in which lamellae are thinner than the wavelength of the light used for optical measurements (Aikawa, Reference Aikawa1984). We report here this situation in detail to underline the great difficulties in the identification of Mn-rich pyroxenoids in the period when X-ray diffraction (XRD) methods were still not widely used. Thus, all data on the mineralogy of these inosilicates from this period must be carefully evaluated.
First XRD data and crystal structure studies
A new era in the mineralogy of Mn pyroxenoids began with the collection of XRD data. The first data on the unit-cell parameters of rhodonite were published by Gossner and Bruckl (Reference Gossner and Bruckl1928): a = 7.79, b = 12.48, c = 6.75 Å, α = 85.10, β = 94.04 and γ = 111.29°, while unit-cell parameters of pyroxmangite were first obtained by Perutz (Reference Perutz1937): a = 6.69, b = 17.38, c = 7.55 Å, α = 113.47, β = 85.25 and γ = 97.32°.
The first powder XRD data for rhodonite were reported by Mikheev and Dubinina (Reference Mikheev and Dubinina1948) for a specimen with the following chemical composition (wt.%): CaO 5.3, MnO 46.9, FeO 1.6, MgO 0.6, SiO2 47.0, total 101.4, corresponding to the empirical formula (Mn4.21Ca0.59Fe0.14Mg0.09)Σ5.03[Si4.98O15]. The specimen was collected from the Maloe Sedel'nikovskoe (alternate spelling: Malosedel'nikovskoe) deposit of rhodonite rock, used as an ornamental stone, at Middle Urals, Russia. Thus, a completely reliable identification of rhodonite in the modern sense became possible only at this period.
The crystal structure of rhodonite was firstly published by Mamedov (Reference Mamedov1958) for a specimen from Switzerland (the exact locality was not reported). The unit-cell parameters are: a = 7.77, b = 12.20, c = 6.70 Å, α = 85.15, β = 94.00 and γ = 111.29°; space group P ![]() $\bar{1}$. It should be noted that this author mentioned the paper by Liebau et al. (Reference Liebau, Hilmer and Thilo1956), who first suggested that rhodonite does not contain three-membered rings of SiO4 tetrahedra, as assumed earlier, but contains chains of SiO4 tetrahedra, like wollastonite. Mamedov (Reference Mamedov1958) found that the rhodonite structure contains chains of tetrahedra with a repeat unit of five tetrahedra, and ribbons of edge-sharing polyhedra М(1), M(2), M(3), M(4) and M(5) (Fig. 1). Unfortunately, no chemical data are given in this paper. All M sites were refined as fully occupied by Mn.
$\bar{1}$. It should be noted that this author mentioned the paper by Liebau et al. (Reference Liebau, Hilmer and Thilo1956), who first suggested that rhodonite does not contain three-membered rings of SiO4 tetrahedra, as assumed earlier, but contains chains of SiO4 tetrahedra, like wollastonite. Mamedov (Reference Mamedov1958) found that the rhodonite structure contains chains of tetrahedra with a repeat unit of five tetrahedra, and ribbons of edge-sharing polyhedra М(1), M(2), M(3), M(4) and M(5) (Fig. 1). Unfortunately, no chemical data are given in this paper. All M sites were refined as fully occupied by Mn.

Fig. 1. General view of rhodonite-type crystal structure. SiO4 tetrahedra are yellow.
Almost at the same time, the crystal structure of rhodonite was independently solved by Liebau et al. (Reference Liebau, Hilmer and Lindemann1959) for a sample with the ideal formula CaMn4[Si5O15] (the locality was not reported) and turned out to be consistent with the structural model by Mamedov (Reference Mamedov1958). However, the data on the cation distribution between the five M sites demonstrated that Ca dominates at M(5) and Mn is dominant at all other [M(1) to M(4)] cation sites. The paper by Liebau et al. (Reference Liebau, Hilmer and Lindemann1959) also contains a table of comparative data on chemically different samples with the rhodonite-type unit cell. The specimens corresponding to the ideal formulae CaMn4[Si5O15] and MnMn4[Si5O15] were joined by Liebau et al. (Reference Liebau, Hilmer and Lindemann1959) under the same name: ‘rhodonite’.
Currently, H-free manganese pyroxenoids with inosilicate сhains [SiO3]∞ include three structure types which differ from each other in the conformation of the silicate chain, the number of SiO4 tetrahedra in the repeat unit of the chain (Si3O9 in bustamite, Si5O15 in rhodonite and Si7O21 in pyroxmangite) and in the number and arrangement of non-equivalent M sites (Fig. 2).

Fig. 2. Fragments of crystal structures of manganese pyroxenoids: (a) bustamite Ca3Mn3[Si3O9]2 (Peacor and Buerger, Reference Peacor and Buerger1962); (b) rhodonite CaMn4[Si5O15] (Peacor and Niizeki, Reference Peacor and Niizeki1963); and (c) pyroxmangite Mn7[Si7O21] (Ohashi and Finger, Reference Ohashi and Finger1975). The colour of polyhedra indicates the predominant M cation: pink for Mn and grey for Ca.
The rhodonite group
In line with the IMA–CNMNC guidelines (Mills et al., Reference Mills, Hatert, Nickel and Ferraris2009), members of the rhodonite group are structurally characterised by chains of Si-centred tetrahedra with the repeat unit Si5O15 and ribbons formed by edge-sharing polyhedra М(1), M(2), M(3), M(4) and M(5) (Fig. 1).
Chemical variability in the rhodonite group
Chemical data for those samples having the rhodonite-type structure that were investigated both chemically and structurally, including the low-Ca species vittinkiite, ideally MnMn3Mn[Si5O15] (IMA2017–082a; Shchipalkina et al., Reference Shchipalkina, Pekov, Chukanov, Zubkova, Belakovskiy, Britvin and Koshlyakova2019b) are given in Table 1.
Table 1. Quantitative chemical data of structurally investigated samples of rhodonite-group minerals.

[1] Peacor and Niizeki (Reference Peacor and Niizeki1963); [2] Peacor et al. (Reference Peacor, Essene, Brown and Winter1978); [3] Shchipalkina et al. (Reference Shchipalkina, Chukanov, Pekov, Aksenov, McCammon, Belakovskiy, Britvin, Koshlyakova, Schafer, Scholz and Rastsvetaeva2017); [4] Shchipalkina et al. (Reference Shchipalkina, Pekov, Chukanov, Zubkova, Belakovskiy, Britvin and Koshlyakova2019b); [5] Nelson and Griffen (Reference Nelson and Griffen2005); [6] Leverett et al. (Reference Leverett, Williams and Hibbs2008).
The approximate compositional fields of minerals belonging to the rhodonite, bustamite and pyroxmangite structure types, plotted based on literature data and our new analyses, and end-member compositions of IMA-approved mineral species, are shown in the Ca–Mn–(Fe + Mg + Zn) triangular diagram (Fig. 3). The field corresponding to rhodonite-group minerals overlaps with the fields of bustamite-group and pyroxmangite-group members in its Ca-richest and Ca-poorest parts, respectively.

Fig. 3. Compositional fields of pyroxenoids in the Ca–Mn–(Fe + Mg + Zn) space, with end-member compositions of minerals marked with red stars: Wol – wollastonite, Bust – bustamite, Mnd – mendigite, Fbust – ferrobustamite, Dln – dalnegorskite (Shchipalkina et al., Reference Shchipalkina, Pekov, Ksenofontov, Chukanov, Belakovskiy and Koshlyakova2019a), Rhd – rhodonite, Frhd – ferrorhodonite, Vit – vittinkiite, Pxm – pyroxmangite and Pxf – pyroxferroite.
As shown in Fig. 3, the end-member pyroxmangite Mn7[Si7O21] may be considered as dimorphous with vittinkiite, Mn5[Si5O15] (the Ca-free rhodonite-type compound is also known among synthetic Mn inosilicates, see Narita et al., Reference Narita, Koto and Morimoto1977; Table 2).
Table 2. Arrangement of M cations in chemically and structurally studied samples of rhodonite-type minerals and an isostructural synthetic compound.

Note: Species-defining cations are given in bold. *Original name given by authors of the cited paper. **For the M(4) site, the Mn:Mg ratio was refined because Mn and Fe (with the atomic numbers 25 and 26, respectively) could not be distinguished by means of XRD; Fe content was added based on the electron-microprobe data. ***Holotype specimen.
Crystal chemistry of the rhodonite group
Currently, crystal structure studies are available for eleven samples having the rhodonite-type structure. Data on the cation distribution between M polyhedra in these samples [including those from the first publication by Mamedov (Reference Mamedov1958) who, unfortunately, did not report any chemical data] are given in Table 2. The numbering of the M sites in structures of rhodonite-type minerals is ‘traditional’, in agreement with the designations used in first publications.
The М(1)-, M(2)- and M(3)-centred polyhedra are weakly distorted octahedra, with mean cation–oxygen distances in the range 2.21–2.23 Å. They are predominantly occupied by Mn2+ in all the samples studied.
The M(4)-centred polyhedron is a strongly distorted octahedron with the shortest and the longest cation–oxygen distances in the ranges 1.95–1.99 and 2.77–2.91 Å, respectively. The M(4) site is typically Mn2+-dominant and can host Fe, Mg and Zn (Peacor and Niizeki, Reference Peacor and Niizeki1963; Ohashi and Finger, Reference Ohashi and Finger1975; Peacor et al., Reference Peacor, Essene, Brown and Winter1978; Nelson and Griffen, Reference Nelson and Griffen2005); in the Fe-richest sample, M(4) is Fe2+-dominant, and for that reason this mineral has been approved by the IMA–CNMNC as a distinct mineral species, ferrorhodonite (Shchipalkina et al., Reference Shchipalkina, Chukanov, Pekov, Aksenov, McCammon, Belakovskiy, Britvin, Koshlyakova, Schafer, Scholz and Rastsvetaeva2017).
The M(5) site has seven-fold coordination and the mean cation–oxygen distance in the range 2.40–2.42 Å. This site preferentially hosts Ca; another constituent at M(5) can be Mn2+. Six out of the eleven samples in Table 2 have Ca > Mn at M(5). Nelson and Griffen (Reference Nelson and Griffen2005) hypothesised the distribution of admixed Ca between several M sites (Table 2). However, they gave no evidence to support this assumption.
Nomenclature of the rhodonite group
The definition of minerals belonging to the rhodonite group is based on the cation distribution over the M sites (Fig. 4).

Fig. 4. Cation ribbons and chains [Si5O15]∞ in the crystal structures of: (a) vittinkiite; (b) rhodonite; and (c) ferrorhodonite. The colour of polyhedra indicates the predominant cation: pink for Mn, grey for Ca and orange for Fe. For site labels and references see Table 2.
In agreement with the currently available crystal chemical data, three mineral species belonging to the rhodonite group can be defined (Table 3).
Table 3. Mineral species belonging to the rhodonite group.

* Holotype specimen
Rhodonite was reported in the official IMA–CNMNC List of Mineral Names (Pasero, Reference Pasero2019) with the formula Mn2+SiO3, which is not compatible with its crystal structure. Thus, the historical name rhodonite required an up-to-date redefinition, and this is a keystone of this nomenclature. As shown in Table 2, the name rhodonite was used in the literature for samples with two end-member formulae, i.e. Mn5[Si5O15] and CaMn4[Si5O15]. Now, the name rhodonite is applied to samples with the ideal formula CaMn3Mn[Si5O15] = CaMn4[Si5O15], for the following reasons: (1) the first reliable chemical analyses of rhodonite belong mainly to samples with 0.5 < Ca < 1.5 apfu (Sundius, Reference Sundius1931); (2) the first published powder XRD data (Mikheev and Dubinina, Reference Mikheev and Dubinina1948) were obtained on a sample with the empirical formula (Mn4.21Ca0.59Fe0.14Mg0.09)Σ5.03[Si4.98O15], i.e. with Ca > 0.5 apfu; (3) the first description of the crystal structure of a rhodonite-group mineral with reported chemical data was for a sample with the ideal formula CaMn4[Si5O15], thus being Ca-dominant at the M(5) site (Liebau et al., Reference Liebau, Hilmer and Lindemann1959); and (4) the rhodonite-group mineral corresponding chemically to the formula CaMn4[Si5O15] (i.e. with 0.5 < Ca < 1.5 apfu) is much more widespread in nature than the mineral with Ca < 0.5 apfu.
Ferrorhodonite was described recently in a specimen from Broken Hill, Yancowinna Co., New South Wales, Australia Shchipalkina et al. (Reference Shchipalkina, Chukanov, Pekov, Aksenov, McCammon, Belakovskiy, Britvin, Koshlyakova, Schafer, Scholz and Rastsvetaeva2017). It is characterised by an unusually high Fe content (the average FeO content is 14.5 wt.%, Table 1). It was named as the analogue of rhodonite with Fe2+ prevailing at the M(4) site. Ferrorhodonite was approved by the IMA–CNMNC (IMA2016–016) as a new mineral species with the ideal formula CaMn3Fe[Si5O15] (Shchipalkina et al., Reference Shchipalkina, Chukanov, Pekov, Aksenov, McCammon, Belakovskiy, Britvin, Koshlyakova, Schafer, Scholz and Rastsvetaeva2017).
Vittinkiite is the rhodonite-group mineral with the ideal formula MnMn3Mn[Si5O15] = Mn5[Si5O15]. The formal border between rhodonite CaMn4[Si5O15] and vittinkiite Mn5[Si5O15] lies at the composition Ca0.5Mn4.5[Si5O15]. Thus the chemical criterion for the definition of vittinkiite is Ca < 0.5 apfu. Taking into account mean cation–oxygen distances, the occurrence of Ca at the M(1–4) sites seems unlikely. The name ‘vittinkiite’ was given for the type locality, the Vittinki (an old Swedish name for Vittinge) iron mines, Isokyrö, Western and Inner Finland Region, Finland. This is the first locality from which a low-Ca rhodonite-type mineral was reported reliably (with 1.3 wt.% CaO: Sundius, Reference Sundius1931). Rhodonite-type minerals with low Ca content (<0.5 Ca apfu) are much rarer than rhodonite s.s. corresponding to the idealised formula CaMn4[Si5O15]. It is worth noting that presumed ‘rhodonite’ from Vittinge was first described by Nordenskiöld (Reference Nordenskiöld1863). Shchipalkina et al. (Reference Shchipalkina, Pekov, Chukanov, Zubkova, Belakovskiy, Britvin and Koshlyakova2019b) studied in detail the mineral from Vittinki using an old specimen from the Fersman Mineralogical Museum of the Russian Academy of Sciences and confirmed that it is Ca-poor, with all M sites being Mn-dominant (Table 2).
Data on two structurally studied specific chemical varieties of rhodonite are also given in Tables 1 and 2. One is a Mg-rich rhodonite – an unusually Mg-rich and low-Fe variety of rhodonite. It occurs in a metamorphosed sedimentary evaporite sequence at the Balmat Mine No. 4, New York, USA. The mineral is associated with the pyroxene donpeacorite, ideally MnMgSi2O6 (Peacor et al., Reference Peacor, Essene, Brown and Winter1978). The second variety is a Zn-rich rhodonite that has a significant Zn content (up to 10 wt.% ZnO: Roberts et al., Reference Roberts, Campbell and Rapp1992). The only known occurrence for this variety is the famous Franklin zinc deposit, Sussex Co., New Jersey, USA. It was initially named ‘fowlerite’ (Shepard, Reference Shepard1832) but was later identified as a Zn-rich variety of rhodonite (Camac, Reference Camac1852).
Available structural data indicate that the M(4) site tends to concentrate cations smaller than Mn2+ (namely, Fe2+, Mg2+ and Zn2+). If rhodonite-type minerals with the predominance of Zn or Mg at one of the M sites [M(4)?] are found in the future, they should be considered as distinct mineral species. By analogy with ferrorhodonite, minerals with cations other than Mn dominant at the M(4) site should be named by adding to the root-name ‘rhodonite’ an appropriate prefix (e.g. ‘zincorhodonite’ or ‘magnesiorhodonite’). The historical name ‘fowlerite’ should not be applied to the hypothetical Zn-dominant mineral species because it was earlier applied to a Zn-rich variety of rhodonite.
Related minerals
Minerals structurally close to rhodonite-group members are babingtonite, HCa2(Fe,Mn)FeSi5O15, manganbabingtonite, HCa2(Mn,Fe)FeSi5O15, marsturite, HNaCaMn3Si5O14(OH), lithiomarsturite, HLiCa2Mn2Si5O15, nambulite, HLiMn4Si5O15, and natronambulite, H(Na,Li)(Mn,Ca)4Si5O15 (Pasero, Reference Pasero2019). These inosilicates have chains of tetrahedra Si5O14(OH) with the same topology as that of rhodonite-group minerals but differ from them: (1) in motifs formed by metal cations (Nagashima et al., Reference Nagashima, Armbruster, Kolitsch and Pettke2014a,Reference Nagashima, Mitani and Akasakab and references therein); and (2) by the presence of hydrogen (the crystal chemical role of hydrogen in these minerals is discussed by Chukanov and Chervonnyi, Reference Chukanov and Chervonnyi2016). This is a reason for considering all these H-bearing inosilicates outside the rhodonite group.
Summary
This report aims at defining the rhodonite group and its constituent mineral species belonging to it.
(1) The rhodonite group contains mineral species having a rhodonite-type structure based on tetrahedral chains with the repeat unit Si5O15 and ribbons formed by edge-sharing polyhedra М(1), M(2), M(3), M(4) and M(5). Their structural formula is VIIM(5)VIM(1)VIM(2)VIM(3)VIM(4)[Si5O15]; the chemical formula is M(5)AM(1–3)B3M(4)C[Si5O15]. The following dominant (species-defining) constituents are currently known: A = Ca or Mn2+, B = Mn2+ and C = Mn2+ or Fe2+.
(2) The nomenclature of the members of the rhodonite group is based on occupancy of the M sites.
(3) Rhodonite is redefined as the mineral species having the end-member formula CaMn3Mn[Si5O15] = CaMn4[Si5O15].
(4) The root-name ‘rhodonite’ is applied to rhodonite-group minerals with M(5) = Ca. A prefix is added in accord with the cation which is dominant at M(4) [if M(4) ≠ Mn], as in ferrorhodonite CaMn3Fe[Si5O15]. Such names could be constructed for potentially new species, e.g. ‘magnesiorhodonite’, CaMn3Mg[Si5O15] or ‘zincorhodonite’, CaMn3Zn[Si5O15] for the hypothetical members of the group with Mg or Zn as a dominant constituents at the M(4) site.
(5) The root-name ‘vittinkiite’ is used for rhodonite-group minerals with M(5) = Mn2+. The IMA-approved species vittinkiite (IMA2017–082a) has the ideal formula MnMn3Mn[Si5O15] = Mn5[Si5O15]. A prefix could be added in agreement with the dominance at M(4) [if M(4) ≠ Mn].
(6) Hydrogen-bearing minerals with the babingtonite- and the nambulite/marsturite-type structures are not included in the rhodonite group.
Acknowledgements
We thank the anonymous referees for their valuable comments. This work was supported by the Russian Foundation for Basic Research, grant 18-05-00332 (in part for crystal chemistry of rhodonite-group minerals).