Introduction
Sugar beet (Beta vulgaris L.) yields 30% of world sugar production (Food and Agriculture Organization, 2012). Moreover, there is growing interest in using sugar beet as an energy crop (Panella, Reference Panella2011). Excellent seed germination and seedling emergence is of crucial importance for optimal field production. To improve seed and seedling performance, seed processing (cleaning, polishing and sizing), priming, pelleting and coating are applied to sugar beet fruits.
Despite the use of mutants and inhibitors of ethylene biosynthesis and action, the role of ethylene during germination remains controversial (Matilla, Reference Matilla2000). In many species, germination runs parallel with ethylene production, i.e. Cicer arietinum (Gallardo et al., Reference Gallardo, Muños De Rueda, Matilla and Sanchez-Calle1994; Gómez-Jiménez et al., Reference Gómez-Jiménez, Garcia-Olivares and Matilla2001), Lactuca sativa (Saini et al., Reference Saini, Consolacion, Bassi and Spencer1989), Oryza sativa (Gianinetti et al., Reference Gianinetti, Laarhoven, Persijn, Harren and Petruzzelli2007) and Pisum sativum (Gorecki et al., Reference Gorecki, Ashino, Satoh and Esashi1991). For some species ethylene production is considered as a cause of germination, i.e. Amaranthus caudatus (Kepczynski and Karssen, Reference Kepczynski and Karssen1985), C. arietinum (Gallardo et al., Reference Gallardo, Muños De Rueda, Matilla and Sanchez-Calle1994), L. sativa (Abeles, Reference Abeles1986) and Nicotiana tabacum (Leubner-Metzger et al., Reference Leubner-Metzger, Petruzzelli, Waldvogel, Vögeli-Lange and Meins1998), while in other species ethylene is a consequence of germination, i.e. Arachis hypogea (Hoffman et al., Reference Hoffman, Fu and Yang1983), O. sativa (Gianinetti et al., Reference Gianinetti, Laarhoven, Persijn, Harren and Petruzzelli2007), Phaseolus vulgaris (De Proft, 1983) and P. sativum (Petruzzelli et al., Reference Petruzzelli, Kunz, Waldvogel, Meins and Leubner-Metzger1999). In Arabidopsis, ethylene affects radicle emergence by decreasing the abscisic acid (ABA) responsiveness (Beaudoin et al., Reference Beaudoin, Serizet, Gosti and Giraudat2000; Ghassemian et al., Reference Ghassemian, Nambara, Cutler, Kawaide, Kamiya and McCourt2000).
In sugar beet, the true seed is surrounded by a protective layer, the pericarp, which consists of a fruit cap (the operculum) and a fruit cavity. The seed surrounded by the pericarp is, in a biological sense, a fruit, referred to as the achene, i.e. the sugar beet dispersal unit (Richard et al., Reference Richard, Raymond, Corbineau and Pradet1989; Hermann et al., Reference Hermann, Meinhard, Dobrev, Linkies, Pesek, Hess, Machackova, Fischer and Leubner-Metzger2007). The seed itself is surrounded by an inner and outer seed coat (testa). The germination process starts with water imbibition and ends with radicle protrusion through the seed coat (Bewley and Black, Reference Bewley and Black1994). In order to obtain better seed quality, commercial sugar beet hybrids are polished, a process by which the soft outer part of the pericarp is removed. Coumans et al. (Reference Coumans, Côme and Gaspar1976) noted 92% germination of true sugar beet seeds (no pericarp present), while fruits without operculum and unpolished fruits had a germination of only 73 and 25%, respectively. The inhibiting effect of the pericarp on germination has several causes. The pericarp is a physical barrier for water and oxygen uptake (Perry and Harrison, Reference Perry and Harrison1974; Lexander, 1981). Furthermore, the pericarp is a source of phenols and salts, compounds that are known to create hypoxia (Coumans et al., Reference Coumans, Côme and Gaspar1976; Richard et al., Reference Richard, Raymond, Corbineau and Pradet1989). The pericarp is also assumed to be a highly selective barrier for the exchange of ACC (1-aminocyclopropane-1-carboxylic acid) and ABA (Hermann et al., Reference Hermann, Meinhard, Dobrev, Linkies, Pesek, Hess, Machackova, Fischer and Leubner-Metzger2007).
Seed quality is a complex trait for improvement by plant breeding. Potential seed vigour markers in sugar beet have been reported, such as enzymes of lipid and starch mobilization (i.e. isocitrate lyase, α-glucosidase), protein synthesis (i.e. elongation factors), the methyl cycle (i.e. S-adenosylmethionine synthetase) and ABA-signalling (i.e. protein phosphatase 2A) (de los Reyes et al., Reference de los Reyes, Myers and McGrath2003; Catusse et al., Reference Catusse, Meinhard, Job, Strub, Fischer, Pestsova, Westhoff, Van Dorsselaer and Job2011). Ethylene production is a good indicator for seed vigour in several species such as lettuce, cabbage, tomato, snap bean, sweet corn and sunflower (Khan, Reference Khan1994; Chojnowski et al., Reference Chojnowski, Corbineau and Côme1997; Siriwitayawan et al., Reference Siriwitayawan, Downie and Geneve2003). This study investigated the kinetics of ethylene evolution as a function of germination to see if ethylene was required for germination, since ethylene has been implicated in the germination process of sugar beet (Hermann et al., Reference Hermann, Meinhard, Dobrev, Linkies, Pesek, Hess, Machackova, Fischer and Leubner-Metzger2007).
Materials and methods
Plant material and seed germination
Diploid monogerm sugar beet (Beta vulgaris L.) fruits of one seed lot (LZD-2386) were obtained from SESVanderHave N.V. (Tienen, Belgium). These fruits were produced in 2010 in France (Nérac) and stored in paper bins at room temperature and a relative humidity of 35% until use.
All fruits used were polished. The experiments were performed on fruits, fruits without operculum (‘deoperculated fruits’) and true seeds. Deoperculated fruits were obtained by exerting pressure on the intact fruit with a pair of tongs. As a consequence, the operculum lifted and could be removed. True seeds were carefully isolated from deoperculated fruits using a dissection needle. Experiments were only executed with undamaged fruits/seeds.
For germination experiments, independent triplicates of 100 fruits or triplicates of 25 true seeds were incubated in the dark at 20°C in polystyrene Petri dishes (90 mm), containing one layer of moist filter paper (Whatman No. 1; 1.5 ml deionized water). Each Petri dish contained 25 fruits/seeds. Where indicated, 1-aminocyclopropane-1-carboxylic acid (ACC; Sigma-Aldrich, St. Louis, Missouri, USA), 2-amino isobutyric acid (AIB; Acros, Geel, Belgium), aminooxyacetic acid (AOA; Sigma-Aldrich) or silver thiosulphate (STS; Sigma-Aldrich) was added to the imbibition medium. Silver thiosulphate was prepared as described by Reid et al. (Reference Reid, Paul, Farhoomand, Kofranek and Staby1980). At specific time intervals, germination was counted. Radicle protrusion of both seed coats was used as the criterion for germination. To compare germination curves with each other, a Gompertz function (y= a*) was fitted. From these fittings, the t10, t25, t50 and t75 values were derived, which indicate the time to reach 10%, 25%, 50% and 75% germination, respectively. The Gompertz function was constructed with the statistical package R, version 2.12.2 (http://www.r-project.org/), and the mean times were compared to each other with Tukey's multiple comparison test (significance level = 0.05).
Ethylene measurements
At specific time intervals, sugar beet fruits (5 times 20 fruits), deoperculated fruits (3 times 20 fruits) or true seeds (3 times 20 seeds) were carefully removed from each Petri dish and incubated at 20°C in a gas-tight 10-ml glass flask sealed with a septum (natural rubber). All repeats were measured independently. The ethylene content was measured after a incubation for 1 h by taking a 1 ml sample of the headspace. A calibration gas mix containing 1 ppm ethylene was used for the determination of the ethylene production rate. Ethylene concentrations were determined via gas chromatography (Shimadzu GC-2014; Shimadzu, ’s-Hertogenbosch, The Netherlands) using a packed column (Porapak R 50/80 mesh, length 3 m, outer diameter 1/8 inch) and a flame ionization detector. The injector, the column and the detector had temperatures of 150, 90 and 250°C, respectively.
Results
Germination and ethylene production
Germination preceded ethylene release in polished sugar beet fruits. Ethylene production began approximately 3 h after germination was first observed (Fig. 1). The first fruits germinated after 45 h of imbibition, while ethylene production was only detected after 48 h. Germination was complete 72 h after the start of imbibition. At the same time, ethylene production reached its maximal value (88 pl h− 1fruit− 1) and declined subsequently.

Figure 1 Germination percentage (black diamonds) and ethylene production in pl h− 1fruit− 1 (grey squares) of intact Beta vulgaris fruits. For the germination experiment each point represents the mean of three independent replicates of 100 fruits. The line represents the fitted values by means of a Gompertz curve (R 2 = 0.997). For the ethylene production, each point represents the mean of five independent replicates of 20 fruits. Mean values ± SD are presented.
Removing the operculum shifted both germination and ethylene kinetics (Fig. 2). Without the operculum, germination started after 20 h, compared to 45 h with intact fruits. Ethylene production started after 32 h, which was 16 h earlier compared to intact fruits (Fig. 1). Maximal ethylene production (73 pl h-1fruit− 1) occurred 65 h after the start of imbibition and was not significantly different compared to the maximal ethylene production of intact fruits (P= 0.0812).

Figure 2 Germination percentage (black diamonds) and ethylene production in pl h− 1fruit− 1 (grey squares) of deoperculated Beta vulgaris fruits. For the germination experiment each point represents the mean of three independent replicates of 100 fruits. The line represents the fitted values by means of a Gompertz curve (R 2 = 0.995). For the ethylene production, each point represents the mean of three independent replicates of 20 fruits. Mean values ± SD are presented.
Germination of true seeds started 8 h after the start of imbibition, whereas ethylene production was only detectable after 30 h (Fig. 3). Maximal ethylene production (68 pl h− 1 seed− 1) was detected after 66 h. This production was comparable with the production obtained with deoperculated fruits (P= 0.607), but was significantly lower than the maximal ethylene production of intact fruits (P= 0.0172).

Figure 3 Germination percentage (black diamonds) and ethylene production in pl h− 1seed− 1 (grey squares) of Beta vulgaris seeds. For the germination experiment each point represents the mean of three independent replicates of 25 seeds. The line represents the fitted values by means of a Gompertz curve (R 2 = 0.986). For the ethylene production, each point represents the mean of three independent replicates of 20 seeds. Mean values ± SD are presented.
Early seedling growth and ethylene production
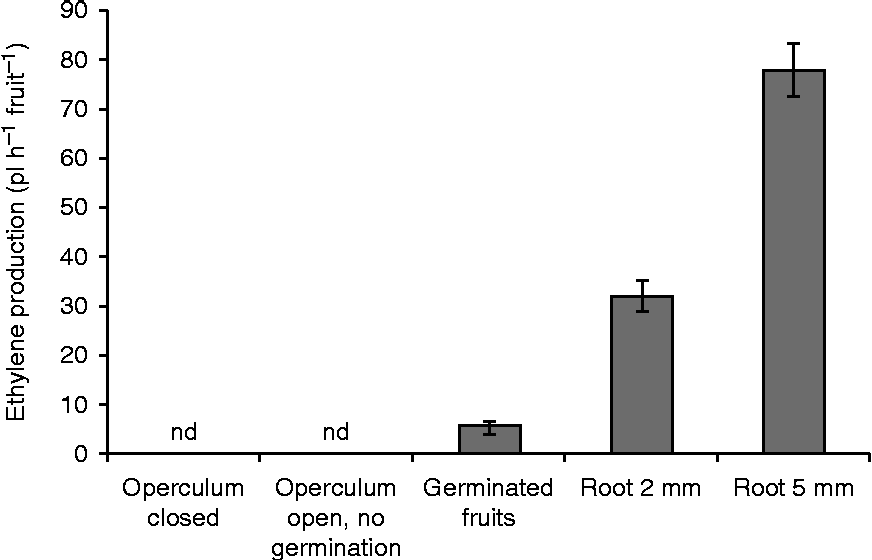
To determine the onset of ethylene production by the fruit, ethylene measurements were executed on well-defined stages during germination and seedling outgrowth (Fig. 4). When the operculum was not lifted, no ethylene could be detected; fruits with a lifted operculum prior to germination did not release ethylene. Ethylene was only detected once the germination of fruits started and this release gradually increased when the roots developed. Although the ethylene increase is partly due to the weight gain of the developing roots, the same pattern was obtained when the ethylene production was expressed per gram fresh weight, although less pronounced (data not shown).

Figure 4 Ethylene production in pl h− 1fruit− 1 at several stages during seedling growth. Each bar represents the mean of five independent repeats of 20 fruits. Mean values ± SD are presented; nd, not detectable.
Application of ethylene precursor and inhibitors
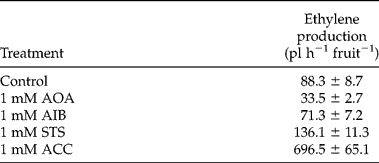
Supplementing the imbibition medium with ACC or ethylene inhibitors influenced the ethylene production of intact fruits (Table 1). Ethylene production was evaluated 72 h after the start of imbibition since this coincided with maximal ethylene release of intact fruits (Fig. 1). AIB, an inhibitor of ACC-oxidase (ACO), resulted in a slight decrease in ethylene production, whereas with AOA, an inhibitor of ACC-synthase, ethylene production was more than halved compared to the control. Further reducing the ethylene production by increasing AIB and AOA concentrations (2–5 mM), was unsuccessful since these levels were proven to be toxic (aberrant seedling growth). When the ethylene signalling was blocked with 1 mM STS, the ethylene production increased more than 50% compared to the control. Addition of 1 mM ACC to the imbibition medium resulted in a strong stimulated ethylene production of almost eight times the control value. Addition of ACC and the ethylene inhibitors had no significant influence on the germination kinetics of the fruits (data not shown).
Table 1 Effect of aminooxyacetic acid (AOA), 2-amino isobutyric acid (AIB), silver thiosulphate (STS) and 1-aminocyclopropane-1-carboxylic acid (ACC) on the ethylene production of intact sugar beet fruits 72 h after the start of imbibition. Each value represents the mean of five independent repeats of 20 fruits, incubated at 20°C. Mean values ± SD are presented

Discussion
Intact sugar beet fruits have a restricted water and oxygen uptake compared to fruits without operculum (Perry and Harrison, Reference Perry and Harrison1974). Removal of the operculum leads to less mechanical resistance for the radicle to emerge and offers improved gas and water exchange. As a result, intact and deoperculated fruits show different temporal patterns of radicle emergence (Figs 1 and 2). In contrast to the findings of Hermann et al. (Reference Hermann, Meinhard, Dobrev, Linkies, Pesek, Hess, Machackova, Fischer and Leubner-Metzger2007), seeds and deoperculated fruits also had different germination behaviour.
Like many other species, germination and ethylene production run parallel to each other in sugar beet fruits (Fig. 1). However, ethylene was only detectable just after protrusion of the radicle (Figs 1–4), suggesting that ethylene is merely a consequence of the germination process. Also, Hermann et al. (Reference Hermann, Meinhard, Dobrev, Linkies, Pesek, Hess, Machackova, Fischer and Leubner-Metzger2007) measured ethylene evolution during germination of sugar beet fruits and found no ethylene production at t50, possibly because of a lack of oxygen required for ACO. In seeds and deoperculated fruits, oxygen uptake was not a limiting factor for ethylene production (Coumans et al., Reference Coumans, Côme and Gaspar1976; Richard et al., Reference Richard, Raymond, Corbineau and Pradet1989), which resulted in an earlier onset of ethylene production compared to intact fruits. Despite the strong induction of the ACO-transcript levels in true seed at t50 (Hermann et al., Reference Hermann, Meinhard, Dobrev, Linkies, Pesek, Hess, Machackova, Fischer and Leubner-Metzger2007), ethylene production in true seeds is only detectable a few hours after t50 (Fig. 3), suggesting that ACC may be limiting at that time. This finding is supported by the results of Hermann et al. (Reference Hermann, Meinhard, Dobrev, Linkies, Pesek, Hess, Machackova, Fischer and Leubner-Metzger2007), since they measured minimal ACC content in seeds at t50. Nevertheless, removal of the operculum or the whole pericarp accelerates radicle emergence more than ethylene production. As a consequence, with true seeds the time span between the start of germination and ethylene release increases (22 h).
An unchanged germination pattern was observed after treatment with ACC, AIB and AOA, indicating that enhancing and reducing ethylene production has no influence on the germination of sugar beet fruits. Also, blocking the ethylene signalling with STS had no influence on the germination pattern, but resulted in an increased ethylene production, indicating a negative feedback control of ethylene production in sugar beet, as already reported in banana fruit, etiolated pea stems and citrus peel (Vendrell and McGlasson, Reference Vendrell and McGlasson1971; Saltveit and Dilley, Reference Saltveit and Dilley1978; Riov and Yang, Reference Riov and Yang1982). In addition, ethylene only becomes detectable after radicle emergence and gradually increases with root elongation (Fig. 4), further showing ethylene to be a consequence of the germination process. Additional seed lots and test conditions are needed to unravel the actual function of ethylene during the germination process of sugar beet. In other species (e.g. lettuce) ethylene is proposed to act by promoting radial cell expansion in the embryonic axis or by increasing the water potential or seed respiration (Kucera et al., Reference Kucera, Cohn and Leubner-Metzger2005; Matilla and Matilla-Vázquez, Reference Matilla and Matilla-Vázquez2008). In Lepidium sativum and Arabidopsis thaliana, ethylene biosynthesis and signalling play an important role during endosperm cap weakening by counteracting ABA inhibition (Linkies et al., Reference Linkies, Müller, Morris, Turecková, Wenk, Cadman, Corbineau, Strnad, Lynn, Finch-Savage and Leubner-Metzger2009). Linkies and Leubner-Metzger (Reference Linkies and Leubner-Metzger2012) proposed, therefore, that in future research on ethylene action during seed germination, tissue-specific mechanisms and interactions with other hormones must be considered.
The role of ethylene during sugar beet germination has been investigated previously by Hermann et al. (Reference Hermann, Meinhard, Dobrev, Linkies, Pesek, Hess, Machackova, Fischer and Leubner-Metzger2007). In apparent contrast with our results, they concluded that ethylene promotes sugar beet germination, on the basis of the effects of applied ethephon (release of ethylene), ACC and the ethylene signalling inhibitor 2,5-norbornadiene. In addition, they reported an accumulation of ACO-transcripts at t50 in fruits and seeds during germination to reinforce their conclusion. In our study, no endogenous ethylene release was detected during the first 48 h after imbibition of intact fruits, in contrast with the ethylene release found afterwards. All this clearly indicates that an increase in ACO-transcripts is not equal to an increase in ethylene production rate. The effects of applied ACC and the ethylene signalling inhibitor STS on the germination pattern in our study are different to those described by Hermann et al. (Reference Hermann, Meinhard, Dobrev, Linkies, Pesek, Hess, Machackova, Fischer and Leubner-Metzger2007). This can be explained by the different genetic background of the analysed sugar beet hybrids (e.g. diploid versus triploid). Genotypic variation on hormonal responses has already been observed in sugar beet callus (Jarl and Bornman, Reference Jarl and Bornman1986).
In conclusion, our study clearly demonstrates that ethylene release in sugar beet results from germination rather than acting as a prerequisite for the germination process. However, also in sugar beet, hormonal responses can differ strongly between different genotypes.