Introduction
Neospora caninum is the causative agent of neosporosis, a disease associated with infectious abortion in livestock and neonatal mortality in beef and milk cattle (Dubey and Schares, Reference Dubey and Schares2011). This veterinary pathogen is responsible for significant economic losses estimated to be more than one billion dollars annually worldwide (Reichel et al., Reference Reichel, Ayanegui-Alcerreca, Gondim and Ellis2013).
Actin is an abundant and highly conserved protein ubiquitously found in eukaryotes, with an intrinsic ability to form filaments (F-actin) from the organization of monomers (G-actin). This protein is responsible for many cellular processes including motility (Pollard and Borisy, Reference Pollard and Borisy2003; Blanchoin et al., Reference Blanchoin, Boujemaa-Paterski, Sykes and Plastino2014), maintenance of cell structure and polarity (Matsudaira, Reference Matsudaira1991) and transportation of vesicles and organelles (Kübler and Riezman, Reference Kübler and Riezman1993; Frölich and Wallach, Reference Frölich and Wallach2015). A large number of regulation factors are known to modulate actin functions, such as actin-binding proteins (ABPs). The ABPs play important roles in the dynamic regulation of actin (Lappalainen, Reference Lappalainen2016) as well as small molecules such as ATP and Mg+2 (Kang et al., Reference Kang, Bradley, Elam and De La Cruz2013; Kudryashov and Reisler, Reference Kudryashov and Reisler2013). Toxins including jasplakinolide (JAS), which provoke actin polymerization (Bubb et al., Reference Bubb, Spector, Beyer and Fosen2000), and cytochalasin D (CYTD), an actin depolymerizing substance (Cooper, Reference Cooper1987), can regulate actin dynamics. Moreover, actin is also regulated by post-translational modifications (PTMs), i.e. the covalent modifications of protein amino acids. PTMs are responsible for equilibrating the organization and proportion of actin monomers and polymers (Terman and Kashina, Reference Terman and Kashina2013).
Organisms from the Apicomplexa phylum have unconventional actins (Sahoo et al., Reference Sahoo, Beatty, Heuser, Sept and Sibley2006; Skillman et al., Reference Skillman, Ma, Fremont, Diraviyam, Cooper, Sept and Sibley2013), similarly to other protozoan such as Amoeba, Trypanosomatids and Giardia (Gupta et al., Reference Gupta, Thiyagarajan and Sahasrabuddhe2015). Apicomplexan parasites comprise veterinary pathogens such as Neospora, Theileria and Babesia, zoonotic agents such as Toxoplasma gondii and Cryptosporidium parvum, and human pathogens represented by the Plasmodium genus. Apicomplexans can infect host cells due to their ability to travel through tissues and penetrate cells using gliding motility (Soldati and Meissner, Reference Soldati and Meissner2004). Toxoplasma gondii and Plasmodium spp. have been the central organisms employed for elucidation of apicomplexan motility (Tardieux and Baum, Reference Tardieux and Baum2016). In the current model of gliding motility, the dynamic association of F-actin and myosin A, integrated into the complex of specialized proteins named glideosome, powers the parasite to move forward (Opitz and Soldati, Reference Opitz and Soldati2002; Baum et al., Reference Baum, Papenfuss, Baum, Speed and Cowman2006). Actin has other important roles in mechanisms such as cell replication, motility of dense granules, apicoplasts replication and endocytic trafficking (Smythe et al., Reference Smythe, Joiner and Hoppe2008; Haase et al., Reference Haase, Zimmermann, Olshina, Wilkinson, Fisher, Tan, Stewart, Tonkin, Wong, Kovar and Baum2015; Heaslip et al., Reference Heaslip, Nelson and Warshaw2016; Periz et al., Reference Periz, Whitelaw, Harding, Gras, Del Rosario Minina, Latorre-Barragan, Lemgruber, Reimer, Insall, Heaslip and Meissner2017; Whitelaw et al., Reference Whitelaw, Latorre-Barragan, Gras, Pall, Leung, Heaslip, Egarter, Andenmatten, Nelson, Warshaw, Ward and Meissner2017).
Here, we investigated N. caninum actin (NcACT) using in silico and proteomic approaches. NcACT is encoded by one single gene, although different isoforms were identified in one-dimensional (1D) and 2D SDS-PAGE. Additionally, NcACT was visualized in N. caninum tachyzoites, being sensitive to JAS and CYTD effect.
Materials and methods
NcACT sequence selection and in silico analysis
The open-reading frame encoding for NcACT was searched using the keyword ‘actin’ in ToxoDB 6.0 (Gajria et al., Reference Gajria, Bahl, Brestelli, Dommer, Fischer, Gao, Heiges, Iodice, Kissinger, Mackey, Pinney, Roos, Stoeckert, Wang and Brunk2008). The selected actin ID was chosen considering the sequence with higher identity with the apicomplexan parasite T. gondii actin (TgACT) ACT1 (Dobrowolski et al., Reference Dobrowolski, Niesman and Sibley1997), access TGME49_009030. The NcACT primary sequence was aligned with other homologous actin of apicomplexan and non-apicomplexan cells. The alignments were performed using MegAlign (LaserGene, DNASTAR, Madison, USA) and edited by GeneDoc version 2.7.000.
Some PTM sites were predicted in NcACT using computational tools. Ubiquitination prediction was carried out in UbiSite (http://csb.cse.yzu.edu.tw/UbiSite/), s-nytrosylation prediction in GPS-SNO 1.0 (Xue et al., Reference Xue, Liu, Gao, Jin, Wen, Yao and Ren2010) and K-acetylation prediction in GPS-PAIL 2.0 (Deng et al., Reference Deng, Wang, Zhang, Xu, Zhang, Liu and Xue2016) using high threshold as confidence parameters. The prediction of phosphorylation was performed by NetPhos 3.1 (Blom et al., Reference Blom, Gammeltoft and Brunak1999), sumoylation by SumoPlot Analysis Program (http://www.abgent.com/sumoplot) and S-glutathionylation by GSHSite (http://csb.cse.yzu.edu.tw/GSHSite/), considering high confidence level (score ⩾0.75). Carbonylation was predicted by PredCar-Site (Hasan et al., Reference Hasan, Li, Ahmad and Molla2017).
Neospora caninum culture
Neospora caninum tachyzoites NC1 (Dubey et al., Reference Dubey, Hattel, Lindsay and Topper1988) were cultivated in Vero cells as described previously (Pereira et al., Reference Pereira, Candido-Silva, De Vries and Yatsuda2011). The tachyzoites were purified by exclusion chromatography in previously equilibrated PD10 columns (Sephadex G-25, GE Healthcare, Buckinghamshire, UK) in phosphate-buffered saline (PBS).
Protein extraction
Depending on the aim of the experiment, total protein from N. caninum or Vero cells was extracted using two different buffers. One method was extraction with the actin-stabilizing buffer (60 mm PIPES, 25 mm HEPES, 10 mm EDTA, 2 mm MgCl2, 125 mm KCl, pH 7.2) containing 10% glycerol, 1% Triton X-100 and 1X protease inhibitor (Complete Mini EDTA free, Roche, Mannheim, Germany) in tachyzoites and Vero cells pellets. The suspension was incubated for 1 h on ice. The total protein extraction was also performed by sonication of the pellet with a sample buffer (7 M urea, 2 M thiourea, 4% CHAPS). The suspensions of both extraction buffers were centrifuged at 9500 × g for 10 min at 4 °C. The supernatants were quantified by the Bradford method (Bradford, Reference Bradford1976) and analysed by SDS-PAGE/Western blot.
One-dimensional and 2D SDS-PAGE
The total protein extracts were resolved by SDS-PAGE in 1D or 2D. For 1D SDS-PAGE, 10 or 12.5% acrylamide gels were run in Tris-glycine buffer (25 mm Tris, 192 mm glycine, 0.1% SDS) with 120–150 V and stained with Coomassie G-250. For 2D SDS-PAGE, a buffer exchange was necessary, from the actin-stabilizing buffer to a sample buffer (7 M urea, 2 M thiourea, 4% CHAPS) using Microcon concentrator (Millipore, Bedford, USA). Subsequently 5 µL of protease inhibitor (Sigma-Aldrich, St. Louis, USA), 0.2 mm DTT (Thermo Fisher Scientific, Waltham, USA), 5 µL immobilized pH gradient (IPG) buffer 3–11 NL (GE Healthcare, Uppsala, Sweden) and rehydration buffer (7 M urea, 2 M thiourea, 4% CHAPS, traces of bromophenol blue) were added to 50 µg total protein. The samples were loaded on a 7 cm IPG strip (Rehydrate Immobiline DryStrip gel 3–11 NL, GE) and rehydrated for 18 h at room temperature. The isoelectric focusing (IEF) was conducted in the Ettan IPGPhor 3 (GE Healthcare) at the following conditions: step and hold, 300 V, 0.2 kVh; gradient, 1000 V, 0.3 kVh; gradient, 5000 V, 4 kVh; step and hold, 5000 V, 2.0 kVh (total of 6.5 kVh); at 20 °C. After IEF, the IPG strips were equilibrated for 15 min with DTT 10 mg mL−1 in equilibration buffer (50 mm Tris, 6 M urea, 30% glycerol, 2% SDS), followed by 25 mg mL−1 iodoacetamide incubation in the same conditions. The strips were run in SDS-PAGE 12.5% using 10 mA for 15 min and 20 mA for the rest of the electrophoresis run. The gels were digitalized using LabScan v.5.0 software on a flatbed scanner (Image Scanner, GE).
Western blot
Western blots were performed as described by Pereira et al. (Reference Pereira, Candido-Silva, De Vries and Yatsuda2011) with modifications. The SDS-PAGE contents were transferred to polyvinylidene difluoride membranes (0.45 µm Immobilon, EMD Millipore) and the membranes were blocked with PBS-GT [0.8% swine gelatin, Sigma-Aldrich, 0.05% (v/v) Tween-20 in PBS]. Monoclonal antibody anti-β-actin C4 (C4 antibody) (Santa Cruz Biotechnology, Dallas, USA) was used for protein detection and anti-mouse IgG-peroxidase (Sigma-Aldrich) as secondary antibody. Membranes were exposed to radiographic film after being incubated with Luminol (SuperSignal West Pico Chemiluminescent Substrate, Thermo Fisher Scientific, Rockford, USA) for 5 min. Images were obtained as described for SDS-PAGE.
The anti-β-pan-actin C4 monoclonal (Lessard, Reference Lessard1988) is a broad specificity antibody able to detect actin in monomeric and filamentous forms. Its detected epitope is situated in a highly conserved region of the actin sequence (Fig. 1). Here, a C4 antibody was used to detect NcACT in tachyzoites and Vero cells (control) protein extract.
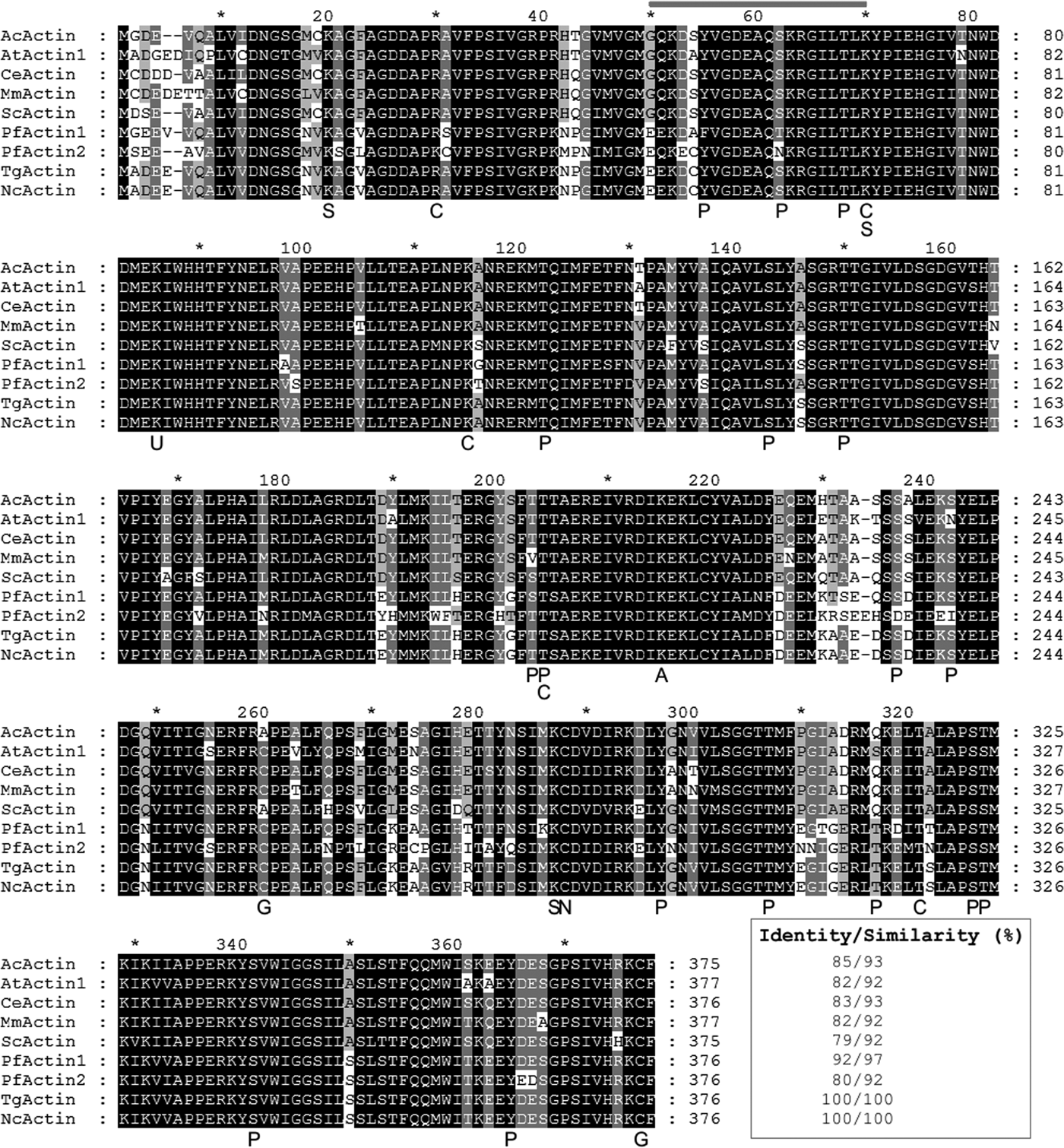
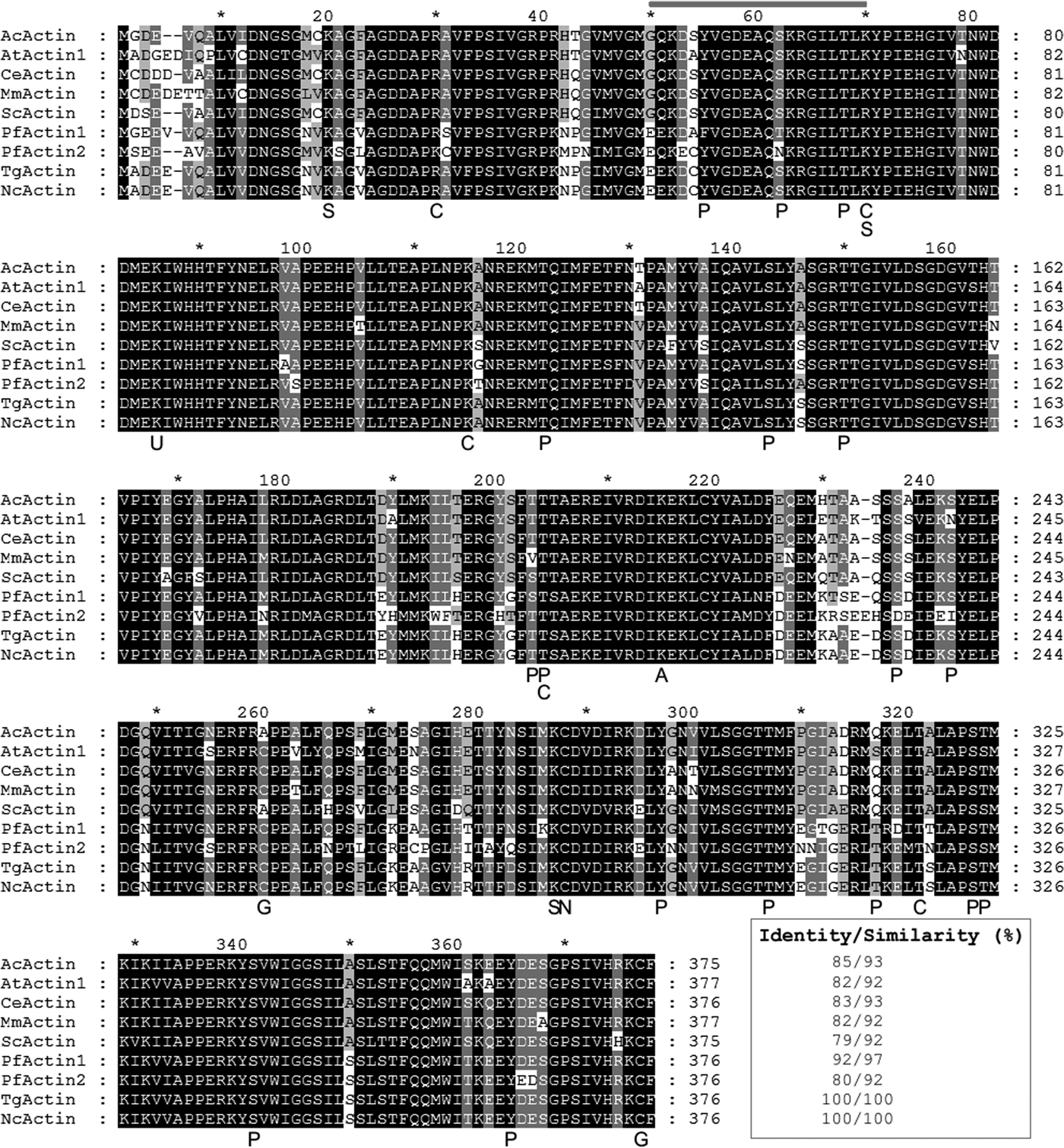
Fig. 1. Multiple alignment of NcACT with representative homologous actins. The sequences are: Acanthamoeba castellanii actin (AcActin; NCBI ID CAA23399), Arabidopsis thaliana actin 1 (AtActin1; NP850284), Caenorhabditis elegans actin (CeActin; CAA34718), Mus musculus actin (MmActin; AAA37164), Saccharomyces cerevisiae actin (ScActin; P60010), Plasmodium falciparum actin 1 (PfActin1; AAA29465), P. falciparum actin 2 (PfActin2; AAA29467); Toxoplasma gondii (TgActin; XP002369663) and Neospora caninum actin (NcActin; ToxoDB ID NCLIV_003440). The primary protein sequences of actin were aligned using Clustal W algorithm. In grey line, the epitope recognized by the C4 antibody. The predicted PTMs are signalized below NcActin sequence. S, sumoylation; C, carbonylation; P, phosphorylation; U, ubiquitinylation; A, k-acetylation; G, s-glutathionylation; N, s-nytrosylation.
Mass spectrometry (RP-nanoLC-MS/MS) of actin bands and spots
The bands (1D SDS-PAGE) or spots (2D SDS-PAGE) were excised from acrylamide gels and analysed by nano LC-MS/MS as described (Pollo-Oliveira et al., Reference Pollo-Oliveira, Post, Acencio, Lemke, van den Toorn, Tragante, Heck, Altelaar and Yatsuda2013). The resulting data were searched against N. caninum predicted protein database ToxoDB version 28 and Swissprot (The UniProt Consortium, 2017), using Mascot software version 2.6.1 (Matrix Science, London, UK). The search was performed using carbamidomethylation as fixed modification of cysteine and oxidation of methionine as variable modification. The peptide tolerance was set to 50 ppm and 0.6 Da of ion tolerances, allowing for two missed cleavages. Proteins and peptide identifications >95% probability were accepted.
Immunofluorescence
Tachyzoites, purified from 5–7 days old cultures, were divided into four (5 × 106 tachyzoites each) 1.5 mL tubes. The samples were incubated with agitation in a total volume of 500 µL of Roswell Park Memorial Institute (RPMI) 1640 containing 5 µ m JAS (Santa Cruz Biotechnology), 2 µ m CYTD (Santa Cruz Biotechnology), 1% dimethyl sulfoxide (DMSO) (estimated concentration of this vehicle present in drug incubations) or only RPMI for 15 min at 37 °C. DMSO and RPMI were the negative controls. The tachyzoites were transferred to eight-well chamber slide (Thermo Fisher Scientific, Rochester, USA) and rinsed in PBS, followed by fixation with 3.6% formaldehyde (Sigma-Aldrich) in PBS for 1 h at 25 °C. After rinsing, the tachyzoites were permeabilized with 0.2% Triton X-100 in PBS for 40 min at 25 °C and blocked in PBS with 3% bovine serum albumin (BSA) and 20 mm glycine for 18 h at 10 °C. The samples were rinsed and incubated with monoclonal antibody anti-β-actin C4 (1:500) diluted in PBS containing 0.3% BSA and 2 mm glycine for 45 min at 25 °C, followed by incubation with goat anti-mouse AlexaFluor 488 conjugated antibody (2.5 µL mL−1; Thermo Fisher Scientific, Oregon, USA). The nuclei were stained with 0.5 µ m propidium iodide in PBS (Santa Cruz Biotechnology). Images were obtained on a confocal microscope (Leica TCS SP5 Laser Scanning Confocal Microscope, Leica Microsystems, Heidelberg, Germany – Multi-user Laboratory of Confocal Microscopy LMMC-FMRP-USP) using 100× objective in oil immersion, Ar laser at 488 nm for Alexa Fluor 488 and HeNe laser at 543 nm for propidium iodide. The captured images were processed in ImageJ 1.46r (National Institute of Health, USA).
Results
NcACT is sequentially identical to TgACT1
Searches on N. caninum Liverpool database in ToxoDB using word ‘actin’ resulted in four identification numbers (ID) annotated as actin: NCLIV_003440 (actin, related), NCLIV_061200 (actin, putative), NCLIV_064780 (actin, putative) and NCLIV_004950 (actin, putative). BLAST searches with the four predicted primary protein sequences indicated that NCLIV_064780 might correspond to actin-related protein 1 (ARP1) homologue, while NCLIV_061200 and NCLIV_004950 might be actin-like proteins 1 (ALP1) and 4 (ALP4) homologues, respectively. Although ARPs share 17–60% identity with conventional actins and are all recognizable as homologous to actin, they are a diverse group with very different functions in cells (Schafer and Schroer, Reference Schafer and Schroer1999). As previously proposed, ARPs are present in many different organisms, while ALPs are exclusive from the Apicomplexa phylum (Gordon and Sibley, Reference Gordon and Sibley2005; Gordon et al., Reference Gordon, Buguliskis, Buske and Sibley2010). The sequence NCLIV_003440 shared actin homology with various species, including T. gondii and other apicomplexan parasites. Furthermore, a BLAST search of NCLIV_003440 in N. caninum database indicated that N. caninum has one single gene copy for actin, like T. gondii. This ID, referred to as NcACT, was aligned with multiple homologous actins from representative and apicomplexan organisms and showed 100% identity with TgACT, followed by 92% identity with Plasmodium falciparum actin 1, and 85% identity with Acanthamoeba castellanii actin (Fig. 1).
C4 antibody detected two bands in 1D SDS-PAGE
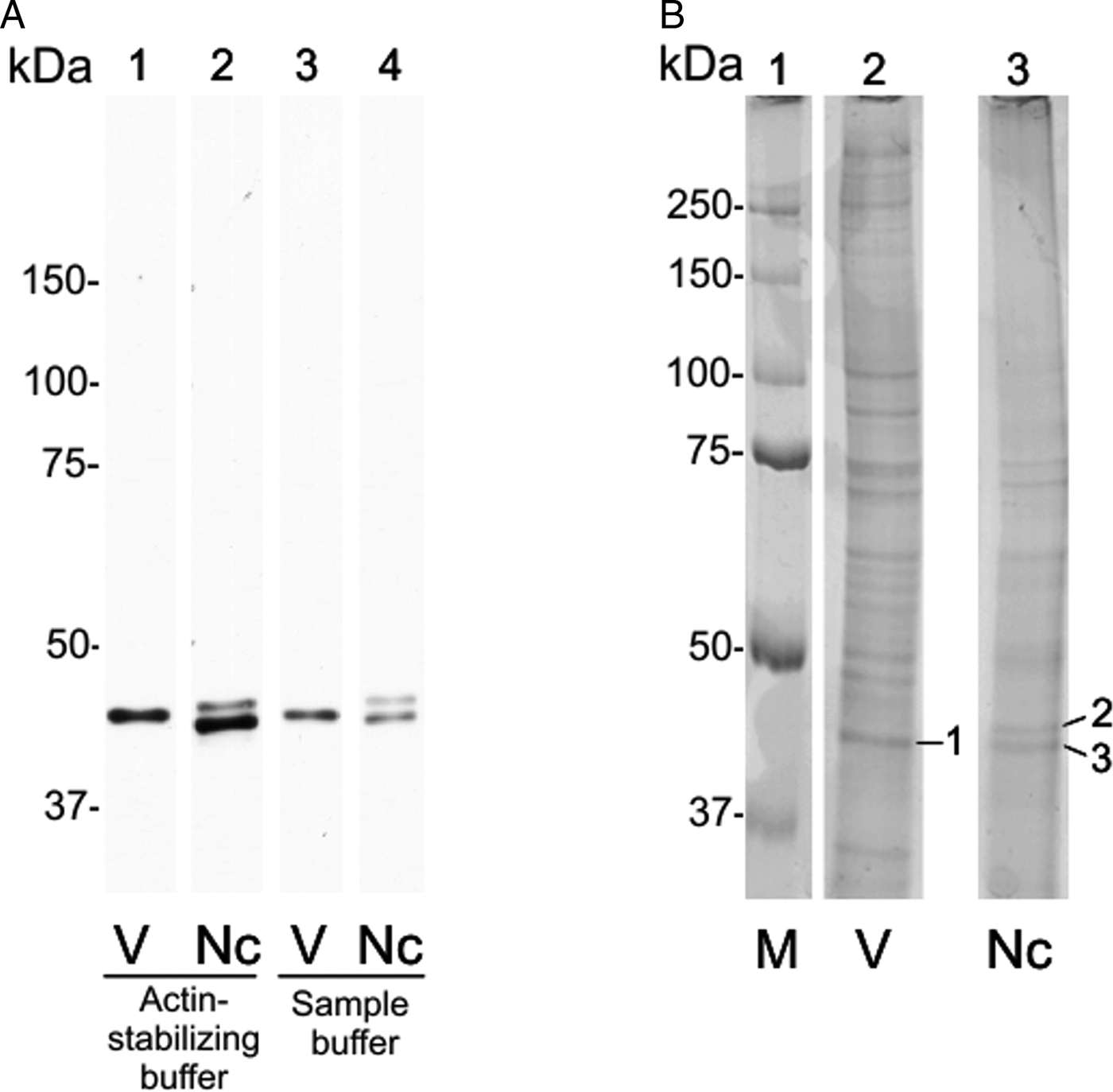
On 12.5% SDS-PAGE, the C4 antibody detected a single ~43 kDa band in both extracts of N. caninum and Vero cells (unpublished results). When N. caninum total extracts was run on a 10% SDS-PAGE subjected to a longer run, the single band was interestingly divided in two bands of ~42 and ~44 kDa (Fig. 2A, wells 2 and 4). In the control, a single ~43 kDa band in Vero cells remained (Fig. 2A, wells 1 and 3). The same result was achieved in two different conditions: using Vero cells extract supernatants in actin-stabilizing buffer (Fig. 2A, wells 1 and 2) or in a denaturing buffer (Fig. 2A, wells 3 and 4).
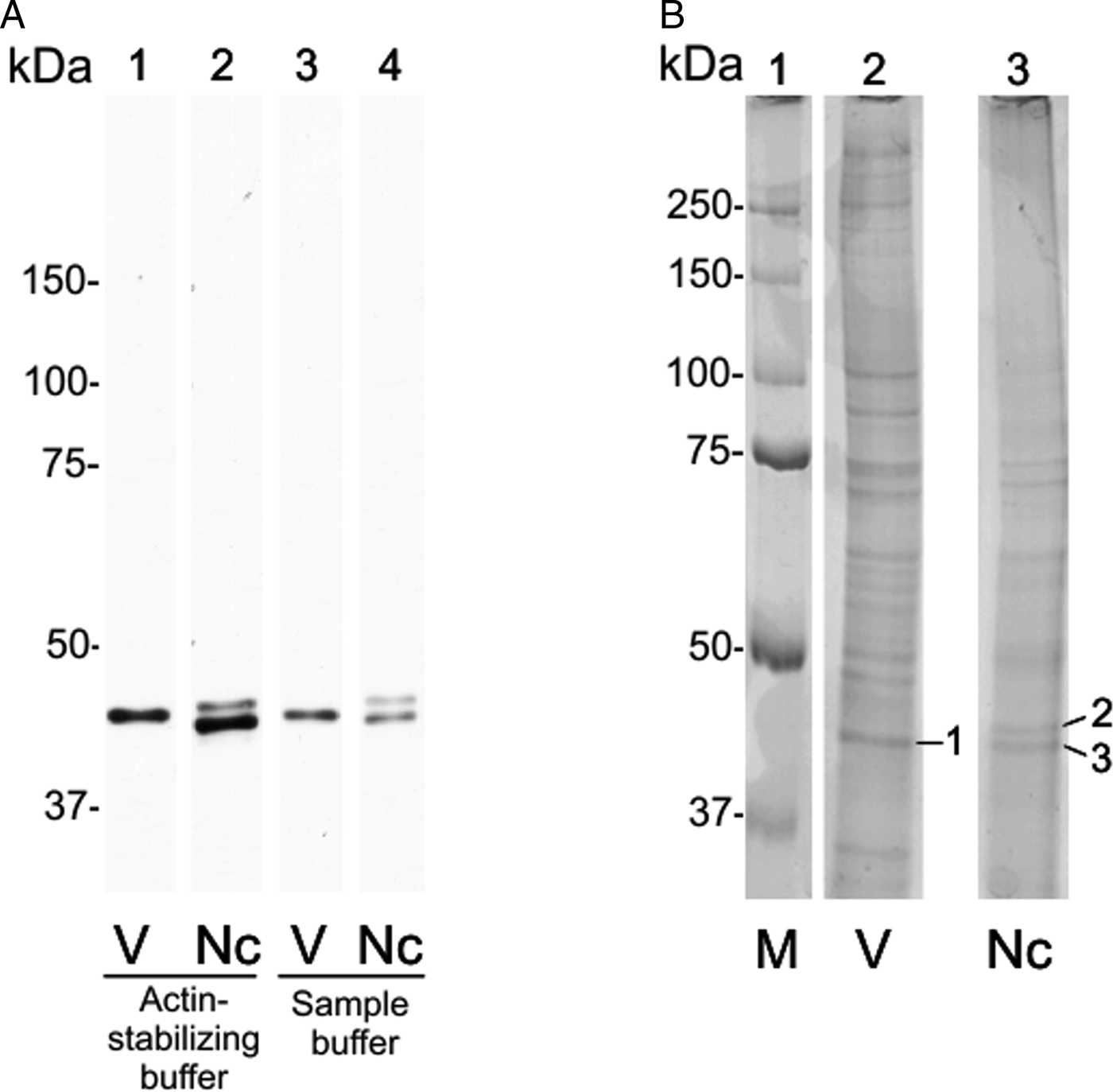
Fig. 2. Detection of NcACT by the C4 antibody and its correspondence in stained gel. A 10% SDS-PAGE was run with Neospora caninum or Vero cells extracts until the 25 kDa marker overpassed the gel. Actin was detected by Western blot. (A) Western blot using an anti-actin antibody 1:2000 and anti-mouse antibody 1:8000. Lanes 1 and 3: Vero cell total extract in actin-stabilizing buffer and sample buffer, respectively. Lanes 2 and 4: Neospora caninum total extract in actin-stabilizing buffer and sample buffer, respectively. (B) SDS-PAGE stained with Coomassie G-250. Lane 1: marker. Vero cells (lane 2) and N. caninum (lane 3) extracts were obtained in actin-stabilizing buffer. The bands corresponding to anti-actin detection were excised (1–3) and analysed by mass spectrometry. Nc, N. caninum; V, Vero cells; M, marker.
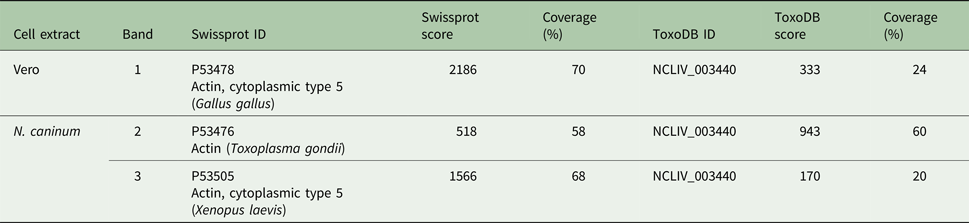
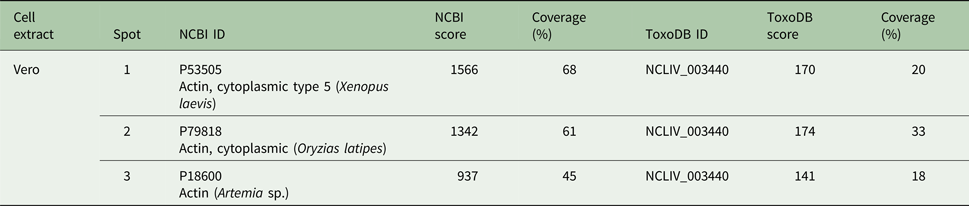
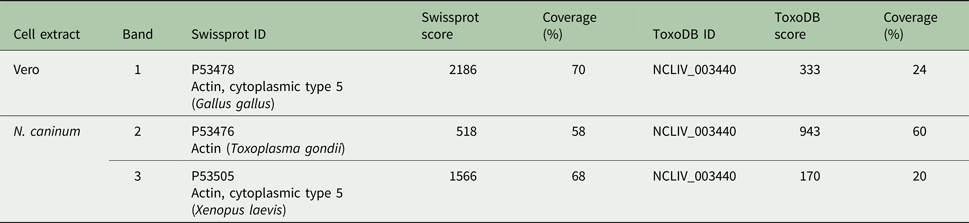
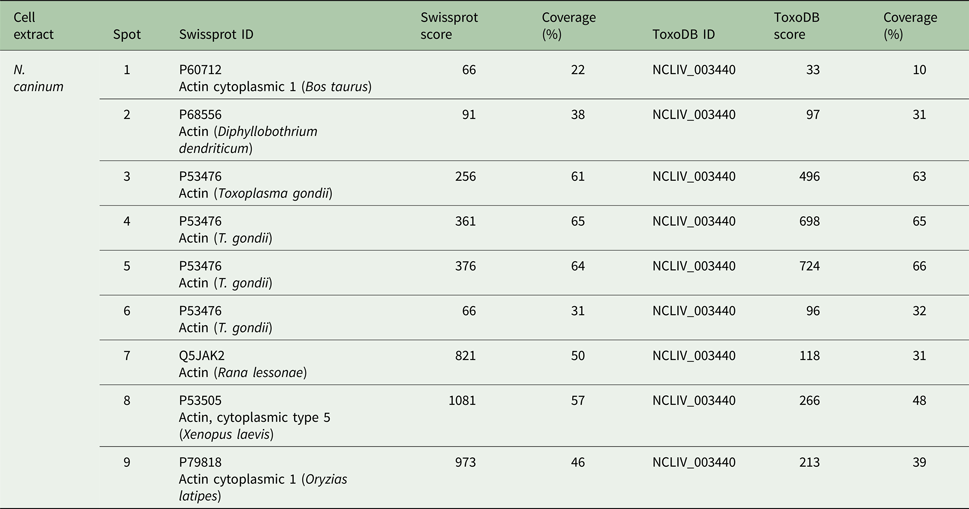
The SDS-PAGE bands containing N. caninum and Vero cells extracts were submitted to nanoLC-MS/MS (numbered as indicated in Fig. 2B). The three bands exhibited hits for actin (Table 1, ranked per the probability score). In ToxoDB, the ID NCLIV_003440 (NcACT) was identified in the two bands (2 and 3) that contained N. caninum extract. The same ID for NcACT at the Vero cells extract band 1 was obtained due to the presence of conserved peptides (Table 1). The search at Swissprot identified vertebrate cytoplasmic actins in the Vero cells and N. caninum bands (1 and 3, respectively) but also in TgACT (band 2, Table 1). The cross IDs between both extracts was expected, not only because of Vero cells debris that may have remained after tachyzoite purification, but also due to a high sequence identity between NcACT and Vero cell β-actin (83%, unpublished results). The significant and top-ranked IDs showed that all peptides from the ToxoDB searches of Vero cells band matched the Vero cell β-actin sequence (Supplementary Material 5). On the other hand, peptides from band 3 did not match Vero cell β-actin, apart from one present at NcACT (bold peptide, Supplementary Material 5; Table 1). In addition, the mass spectrometry allowed the identification of several other proteins, despite the presence of actin in every analysis (Supplementary Material 1–4). Overall, the MS results showed that bands 1 and 3 are composed of vertebrate actin, and band 2 is composed of NcACT. However, the evidence of NcACT was found in band 3, indicating the presence of isoforms.
Table 1. Actin hits from mass spectrometry analysis of Neospora caninum and Vero cells extract. Western blot bands detected by the C4 antibody were localized with their correspondent bands at 1D SDS-PAGE for excision and analysis by MS/MS. Hits with the higher scores for actin identification in Swissprot and ToxoDB are shown
NcACT has different isoforms in 2D SDS-PAGE
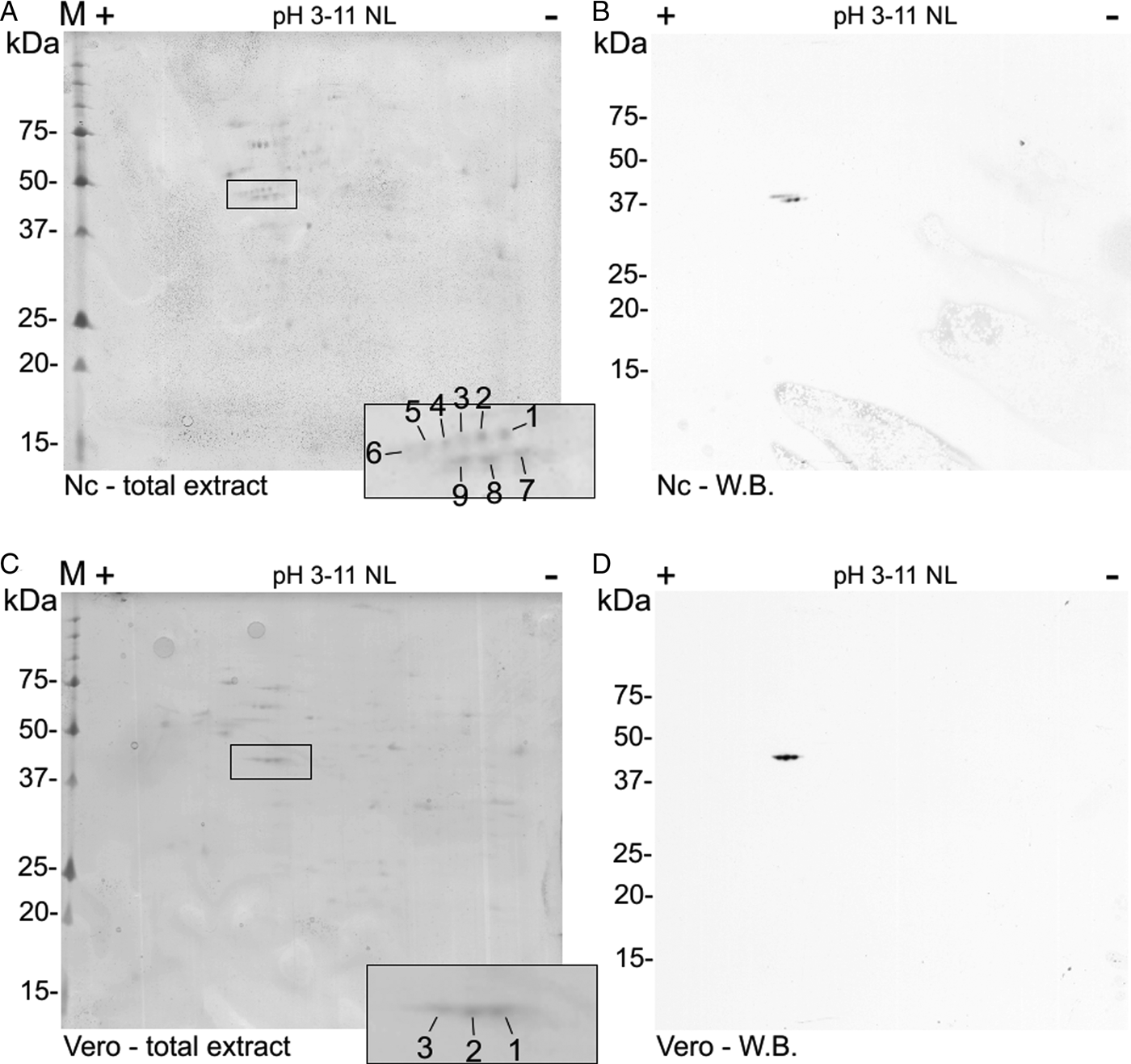
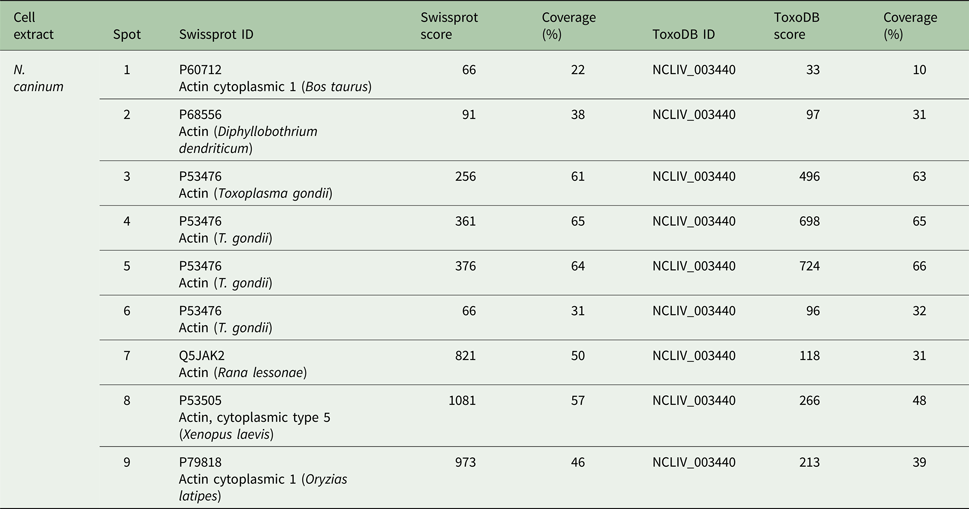
A double row was observed at the N. caninum extract 2D Western blot (like the two bands at the 1D Western blot). The spots were numbered 1–9 (Fig. 3A) in comparison to the control Vero cells extract with three spots (pI 5.2–5.7) (Fig. 3D). Nine spots (with pI 5.2–5.8) were detected by the C4 antibody in N. caninum extract, co-localized at a Coomassie-stained SDS-PAGE and submitted to nanoLC-MS/MS (Fig. 3A and B).
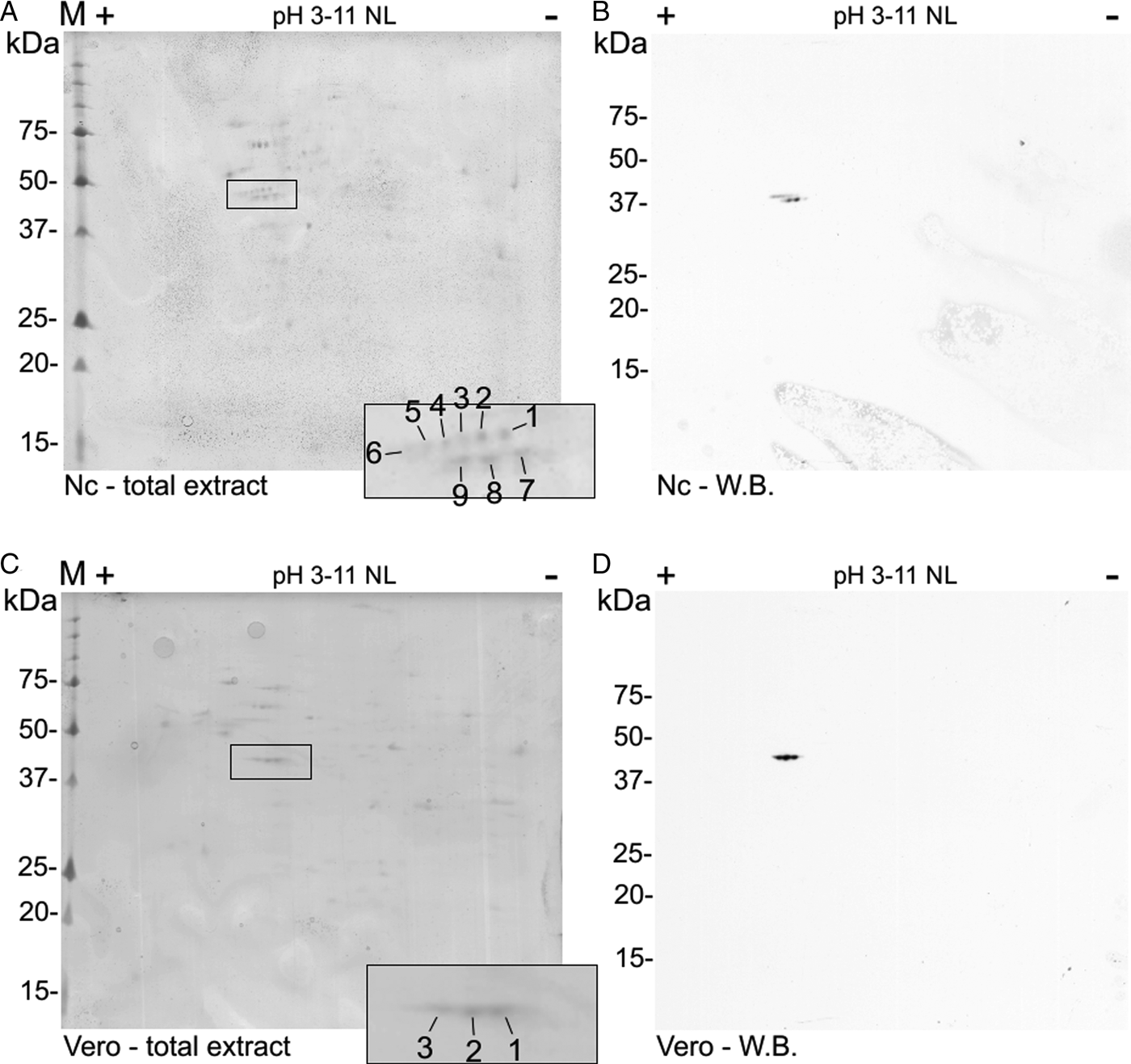
Fig. 3. Two-dimensional detection of NcACT. Two-dimensional SDS-PAGE was performed using gel strips with non-linear pH range 3–11 previously focused with Neospora caninum (A and B) or Vero cell (C and D) total extracts. The strips were run in 12.5% SDS-PAGE. (A) and (C) Coomassie-G250 stained gels containing N. caninum and Vero cells extracts, respectively. The correspondent spots detected by Western blot were numbered and highlighted inside black rectangles. (B) and (D) Western blot from SDS-PAGE containing N. caninum and Vero cell extracts, respectively. The actin isoforms were detected with anti-actin antibody 1:2000 and anti-mouse antibody 1:8000. W.B., Western blot.
The three Vero cells spots presented hits for actin in Swissprot and ToxoDB (Table 2). Nevertheless, all significant and top-ranked peptides from ToxoDB searches were identical to both NcACT and Vero cell β-actin respective sequence regions (Supplementary Material 6). Spots from N. caninum extract were also identified as actin in both databases (Table 3) but in contrast to the Vero cells spots, presented at least one NcACT exclusive peptide when compared with Vero cell β-actin at ToxoDB searches (Supplementary Material 7). Except for spot 1, all N. caninum spots presented hits for TgACT in Swissprot searches (Supplementary Material 3). Additionally, the higher scores of protein accessions in Swissprot for N. caninum spots 3, 4, 5 and 6 were hits for TgACT (Table 3). Although N. caninum spot 1 did not have hits for TgACT in Swissprot searches, the peptide identifications to NcACT in ToxoDB matched exclusively with NcACT sequence when compared with Vero cell β-actin (Supplementary Material 7).
Table 2. Actin hits from mass spectrometry analysis of Vero cells extract. Western blot-correspondent spots detected by the C4 antibody were localized in their correspondent spots at 2D SDS-PAGE for excision and analysis by MS/MS. Hits with the higher scores for actin identification in Swissprot and ToxoDB are shown
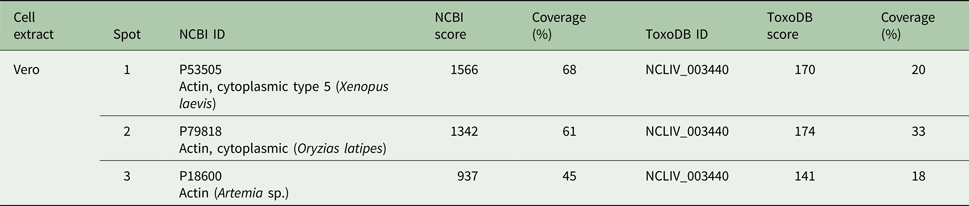
Table 3. Actin hits from mass spectrometry analysis of Neospora caninum extract. Western blot spots detected by C4 antibody were localized with their correspondent spots at 2D SDS-PAGE for excision and analysis by MS/MS. Hits with the higher scores for actin identification in Swissprot and ToxoDB are shown
Collectively, these results provided evidence that the C4 antibody is able to detect actin in N. caninum extract and that NcACT presents nine different isoforms, varying in molecular weight and isoelectric points between them.
PTMs are computationally predicted in NcACT
The analysis of NcACT by computational tools revealed possible target sites for PTMs. One site was predicted for ubiquitination, s-nitrosylation and K-acetylation, 17 sites for phosphorylation, two sites for s-glutathionylation, five sites for carbonylation and three sites for sumoylation (Fig. 1 and Supplementary Material 8).
NcACT is located peripherally on the cytoplasm
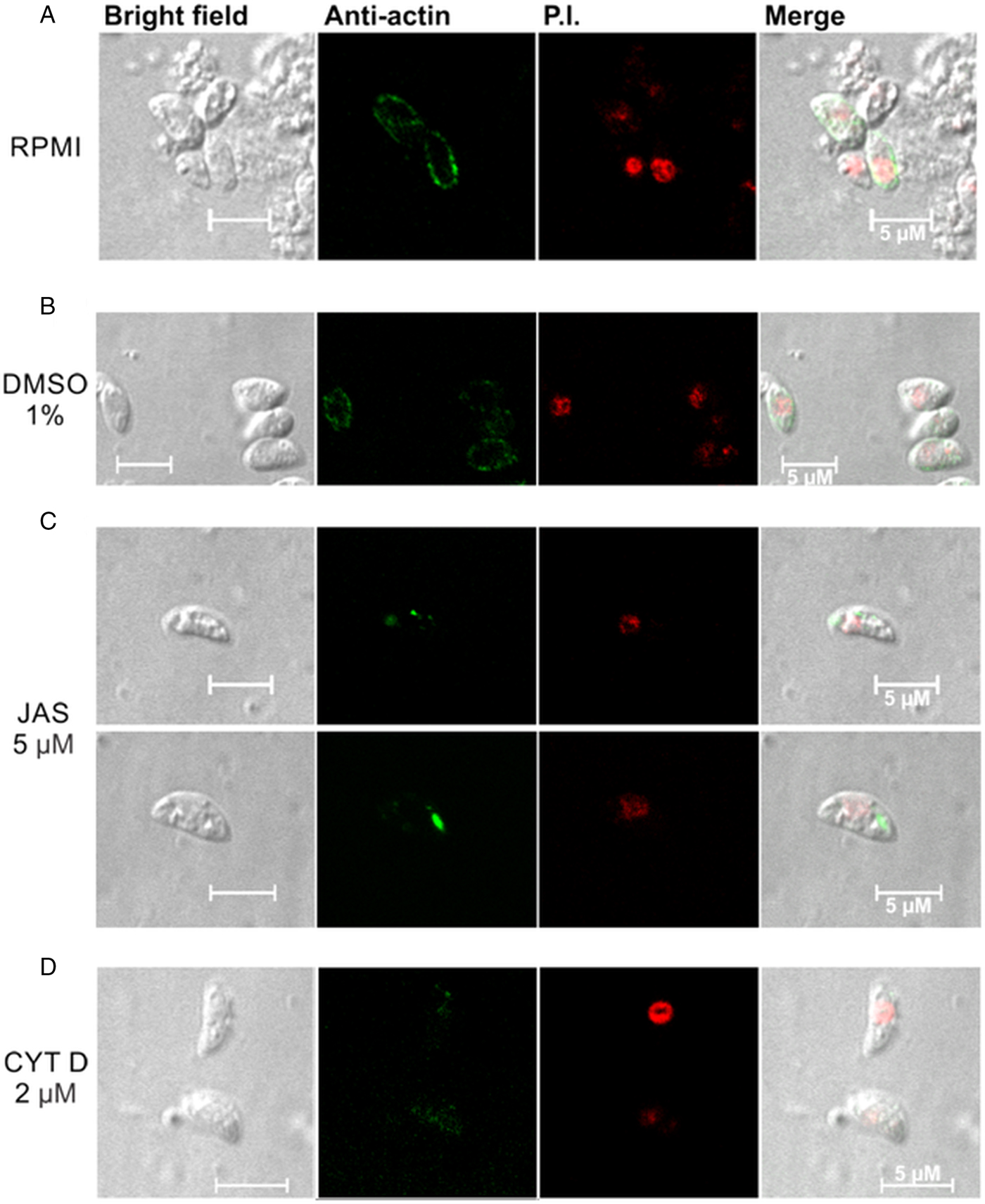
The tachyzoites incubated with RPMI presented a well-defined peripheral location of NcACT in the cytoplasm (Fig. 4A). A similar pattern was observed when control tachyzoites were incubated with 1% DMSO (Fig. 4B). However, in tachyzoites incubated with JAS, NcACT was found concentrated in localized dots. The actin dots were located predominantly in apical and/or posterior regions (Fig. 4C). CYTD-treated tachyzoites presented a weak diffuse cytoplasmic staining of NcACT (Fig. 4D).
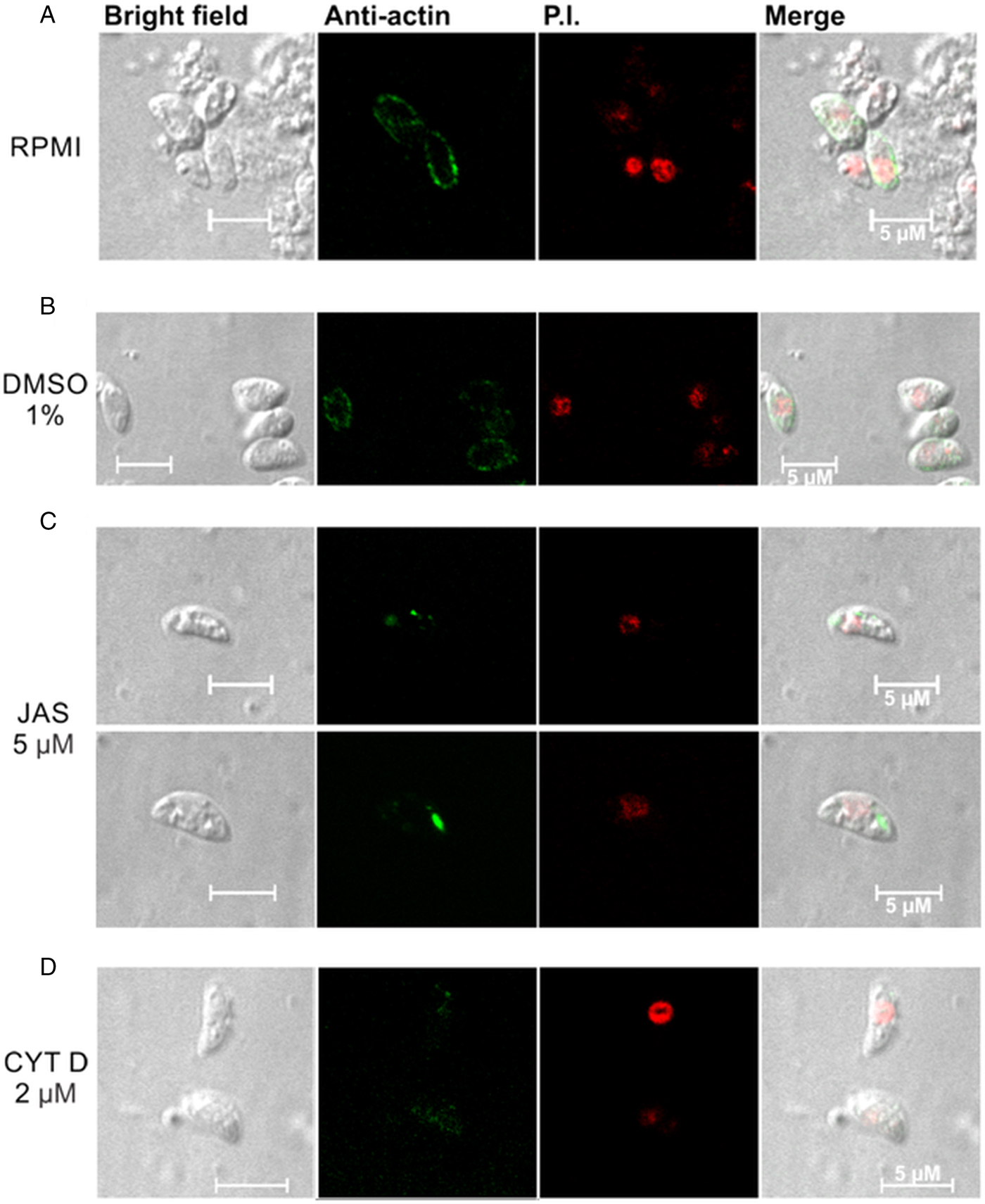
Fig. 4. Immunolocalization of NcACT from Neospora caninum tachyzoites. Tachyzoites were treated for 15 min at 37 °C with drugs that affect the cytoskeleton. The C4 antibody (1:500) was used to detect NcACT. The detection was visualized with the anti-mouse Alexa Flour 488 conjugated antibody and the nucleus was marked with propidium iodide (P.I.). (A) The tachyzoites were incubated with only RPMI. (B) Incubation with 1% DMSO in RPMI. (C) Incubation with 5 µ m jasplakinolide (JAS). (C) Incubation with 2 µ m cytochalasin D (CYTD). The white bars indicate 5 µm.
Discussion
The characterization of actin from the apicomplexan N. caninum (NcACT) was performed by computational analysis, in-gel identification and localization in tachyzoites treated with toxins that affect actin. The mechanisms of actin regulation have been extensively studied as this protein has great importance for cellular functions. Apicomplexan organisms have one of the most divergent and primitive actin (Vahokoski et al., Reference Vahokoski, Bhargav, Desfosses, Andreadaki, Kumpula, Martinez, Ignatev, Lepper, Frischknecht, Sidén-Kiamos, Sachse and Kursula2014), known for their unconventional characteristics (Dobrowolski and Sibley, Reference Dobrowolski and Sibley1996; Wetzel et al., Reference Wetzel, Håkansson, Hu, Roos and Sibley2003). Consequently, it is difficult to conciliate in silico observations of TgACT isodesmic polymerization (Skillman et al., Reference Skillman, Ma, Fremont, Diraviyam, Cooper, Sept and Sibley2013) with the recent in vivo observation of critical concentration during filaments formation (Periz et al., Reference Periz, Whitelaw, Harding, Gras, Del Rosario Minina, Latorre-Barragan, Lemgruber, Reimer, Insall, Heaslip and Meissner2017). Additionally, Plasmodium actin 1 (PfACT1) was reported to exhibit a polymerization-dependent nucleation (Kumpula et al., Reference Kumpula, Pires, Lasiwa, Piirainen, Bergmann, Vahokoski and Kursula2017). We demonstrated that NcACT is composed of different isoforms. The computational analysis showed NcACT as sequentially identical to TgACT, thus they may present similar in vitro polymerization characteristics. In contrast, the detection of actin by the C4 antibody showed significant differences. While actin from T. gondii, Plasmodium berghei and P. falciparum are detected by the C4 antibody as a single predicted actin band in 1D Western blot (Dobrowolski et al., Reference Dobrowolski, Niesman and Sibley1997; Angrisano et al., Reference Angrisano, Riglar, Sturm, Volz, Delves, Zuccala, Turnbull, Dekiwadia, Olshina, Marapana, Wong, Mollard, Bradin, Tonkin, Gunning, Ralph, Whitchurch, Sinden, Cowman, McFadden and Baum2012b), NcACT is detected by the same antibody as two distinct bands with different lysis conditions. This is a unique observation for an apicomplexan actin so far. The possibility of a non-specific reaction or exclusive detection of Vero cells actin in N. caninum extract by the C4 antibody was excluded, as both bands were identified as NcACT proteins by MS/MS. Moreover, no hits for ALPs or ARPs were obtained within the analysis. However, hits for the ALP 1 and ARP 1 (NCLIV_061200 and NCLIV_064780, respectively), identified here in BLAST searches, were previously identified by a quantitative approach in N. caninum secretome (Pollo-Oliveira et al., Reference Pollo-Oliveira, Post, Acencio, Lemke, van den Toorn, Tragante, Heck, Altelaar and Yatsuda2013). The same approach also detected other members of an ALP family (Pollo-Oliveira et al., Reference Pollo-Oliveira, Post, Acencio, Lemke, van den Toorn, Tragante, Heck, Altelaar and Yatsuda2013).
Searches in the N. caninum genome at the ToxoDB database confirmed that NcACT is expressed by a single-copy gene. These observations indicated that the nine different isoforms described represent PTMs. In multicellular organisms, the actin isoforms are encoded by separate genes. In mammals, the variation of N-terminal residues and intracellular localization of the six isoforms may determine their function (Perrin and Ervasti, Reference Perrin and Ervasti2010). Plants have a variable number of actin family genes that may be more diversified in plant organisms with complex tissues (Slajcherová et al., Reference Slajcherová, Fišerová, Fischer and Schwarzerová2012). Additionally, the actins of the apicomplexan Plasmodium spp are encoded by two genes (I and II), being actin I expressed throughout the parasite life cycle, and actin II expressed by the reproductive forms (Wesseling et al., Reference Wesseling, Snijders, van Someren, Jansen, Smits and Schoenmakers1989; Deligianni et al., Reference Deligianni, Morgan, Bertuccini, Kooij, Laforge, Nahar, Poulakakis, Schüler, Louis, Matuschewski and Siden-Kiamos2011; Andreadaki et al., Reference Andreadaki, Morgan, Deligianni, Kooij, Santos, Spanos, Matuschewski, Louis, Mair and Siden-Kiamos2014). Nevertheless, TgACT is encoded by one gene (Dobrowolski et al., Reference Dobrowolski, Niesman and Sibley1997), and the presence of multiple isoforms were detected in T. gondii global proteome analysis, even though specific actin findings were not discussed in the study (Xia et al., Reference Xia, Sanderson, Jones, Prieto, Yates, Bromley, Tomley, Lal, Sinden, Brunk, Roos and Wastling2008). Additionally, actin was identified in two spots of approximately pI 5 in a general 2D electrophoresis map of N. caninum (Lee et al., Reference Lee, Kim, Shin, Shin, Suh, Kim, Kim, Kim and Jung2003). Multiple actin isoforms with stage-specific pattern were detected in Trypanosoma cruzi extracts in 2D electrophoresis by anti-T. cruzi actin antibody (Cevallos et al., Reference Cevallos, Segura-Kato, Merchant-Larios, Manning-Cela, Alberto Hernández-Osorio, Márquez-Dueñas, Ambrosio, Reynoso-Ducoing and Hernández2011). The 2D SDS-PAGE is an appropriate approach to detect covalent modifications in side chains of proteins, i.e. PTMs, carried out either during or after translation. The PTMs of actin are important regulatory mechanisms to the function of actin in a cell (Terman and Kashina, Reference Terman and Kashina2013). A known PTM of actin is acetylation, performed by aminopeptidases that remove the N-terminal methionine and modify the immediate subsequent amino acids (Rubenstein and Martin, Reference Rubenstein and Martin1983). The ubiquitination of actin (arthrin) has been reported in arthropods (Bullard et al., Reference Bullard, Bell, Craig and Leonard1985; Burgess et al., Reference Burgess, Walker, Knight, Sparrow, Schmitz, Offer, Bullard, Leonard, Holt and Trinick2004) and in PfACT1 (Field et al., Reference Field, Pinder, Clough, Dluzewski, Wilson and Gratzer1993; González-López et al., Reference González-López, Carballar-Lejarazú, Arrevillaga Boni, Cortés-Martínez, Cázares-Raga, Trujillo-Ocampo, Rodríguez, James and Hernández-Hernández2017). Other PTMs such as arginylation (Karakozova et al., Reference Karakozova, Kozak, Wong, Bailey, Yates, Mogilner, Zebroski and Kashina2006), phosphorylation (Kishi et al., Reference Kishi, Clements, Mahadeo, Cotter and Sameshima1998; Gu et al., Reference Gu, Zhang, Chen and Chen2003), carbonylation (Castro et al., Reference Castro, Ott, Jung, Grune and Almeida2012), s-nitrosylation (Lu et al., Reference Lu, Katano, Okuda-Ashitaka, Oishi, Urade and Ito2009), methylation (Nyman et al., Reference Nyman, Schuler, Korenbaum, Schutt, Karlsson and Lindberg2002), glutathionylation (Sakai et al., Reference Sakai, Li, Subramanian, Mondal, Bajrami, Hattori, Jia, Dickinson, Zhong, Ye, Chang, Ho, Zhou and Luo2012) and sumoylation (Alonso et al., Reference Alonso, Greenlee, Matts, Kline, Davis and Miller2015) were characterized in actin. Among the previously characterized actin PTMs, the study computationally predicted sites of ubiquitination, phosphorylation, carbonylation, s-nitrosylation, glutathionylation, K-acetylation and sumoylation in NcACT. The present data indicated that NcACT may have its function regulated by mechanisms other than ABPs.
The distribution of actin in N. caninum tachyzoites was different from T. gondii and Plasmodium. Dobrowolski et al. (Reference Dobrowolski, Niesman and Sibley1997) observed, in extracellular T. gondii marked with the C4 antibody, a more intense peripheral localization and a cytoplasmic diffuse signal. Plasmodium berghei sporozoites also had a peripheral concentration of actin, although a more uniform fluorescence was localized throughout the cytoplasm in merozoites and ookinetes (Angrisano et al., Reference Angrisano, Riglar, Sturm, Volz, Delves, Zuccala, Turnbull, Dekiwadia, Olshina, Marapana, Wong, Mollard, Bradin, Tonkin, Gunning, Ralph, Whitchurch, Sinden, Cowman, McFadden and Baum2012b). Fixed P. berghei ookinetes presented actin concentrated at the extreme apical pole (Siden-Kiamos et al., Reference Siden-Kiamos, Pinder and Louis2006). The peripheral presence of NcACT is coincident with the localization of the glideosome between the inner membrane complex and the plasmatic membrane of the tachyzoite (Heintzelman, Reference Heintzelman2015). JAS and CYTD are valuable tools that have been extensively used and have enabled useful insights into the characterization of actin from Apicomplexa (Dobrowolski et al., Reference Dobrowolski, Niesman and Sibley1997; Wetzel et al., Reference Wetzel, Håkansson, Hu, Roos and Sibley2003; Angrisano et al., Reference Angrisano, Riglar, Sturm, Volz, Delves, Zuccala, Turnbull, Dekiwadia, Olshina, Marapana, Wong, Mollard, Bradin, Tonkin, Gunning, Ralph, Whitchurch, Sinden, Cowman, McFadden and Baum2012b; Drewry and Sibley, Reference Drewry and Sibley2015; Whitelaw et al., Reference Whitelaw, Latorre-Barragan, Gras, Pall, Leung, Heaslip, Egarter, Andenmatten, Nelson, Warshaw, Ward and Meissner2017). The current study demonstrated that NcACT is sensitive to these toxins since the JAS and CYTD treatments modified the localization of actin in the tachyzoites. The dots of actin localized in the cytoplasm after incubation with JAS, specifically in the apical and/or posterior regions, most likely represent the induction of the polymerization. A similar pattern was observed in fixed P. berghei ookinetes incubated with a monoclonal anti-actin antibody (Siden-Kiamos et al., Reference Siden-Kiamos, Pinder and Louis2006) or expressing constitutive GFP-Pb actin (Angrisano et al., Reference Angrisano, Delves, Sturm, Mollard, McFadden, Sinden and Baum2012a). The apical protrusions observed in JAS-treated T. gondii tachyzoites (Shaw and Tilney, Reference Shaw and Tilney1999; Wetzel et al., Reference Wetzel, Håkansson, Hu, Roos and Sibley2003; Angrisano et al., Reference Angrisano, Delves, Sturm, Mollard, McFadden, Sinden and Baum2012a) and P. falciparum merozoites (Mizuno et al., Reference Mizuno, Makioka, Kawazu, Kano, Kawai, Akaki, Aikawa and Ohtomo2002) were not detected in our experimental conditions. The N. caninum tachyzoites treated with CYTD presented a weak and diffuse signal for actin, indicating actin filaments disruption. Intracellular T. gondii tachyzoites had the actin networking collapsed after CYTD treatment (Periz et al., Reference Periz, Whitelaw, Harding, Gras, Del Rosario Minina, Latorre-Barragan, Lemgruber, Reimer, Insall, Heaslip and Meissner2017), whereas in P. falciparum, the localization of actin remained similar to the control when treated with the same substance (Smythe et al., Reference Smythe, Joiner and Hoppe2008).
In summary, our findings demonstrated that the actin localization on N. caninum tachyzoites and detection in Western blot differed from the findings previously described for the related coccidian T. gondii. In addition, the data strongly suggest that post-translational processing occurs in NcACT. The PTMs add complexity to the analysis of important cellular functions such as those determined by the actin dynamics. The study of PTMs in apicomplexan parasites may explain the specificities of their unconventional actin dynamics and complement the information of the functional singularities caused by divergences in amino acids sequences between species.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018000872
Acknowledgements
We would like to thank Elizabete Rosa Milani (M.Sc.) for the specialized technical support during the use of the confocal microscope and Multi-user Laboratory of Confocal Microscopy (LMMC-FMRP-USP-Fapesp 2004/08868-0).
Financial support
LB received an M.Sc. fellowship from Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) and a Ph.D. fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grant number 2012/22772-2). LPO received a Ph.D. fellowship from CAPES and a short Ph.D. fellowship from Utrecht University. The proteomics analyses were supported by the Netherlands Proteomics Centre, embedded in the Netherlands Genomics Initiative.
Conflict of interest
None.
Ethical standards
Not applicable.