INTRODUCTION
A common symptom in adults with the human immunodeficiency virus (HIV), especially in those diagnosed with acquired immunodeficiency syndrome (AIDS), is slowed motor and cognitive processing (Hardy & Hinkin, Reference Hardy and Hinkin2002; Llorente et al., Reference Llorente, Miller, D’Elia, Selnes, Wesch, Becker and Satz1998; Sanford, Fellows, Ances, & Collins, Reference Sanford, Fellows, Ances and Collins2018), potentially contributing to a clinical diagnosis of HIV-associated neurocognitive disorder (HAND) (Antinori et al., Reference Antinori, Arendt, Becker, Brew, Byrd, Cherner and Wojna2007; Goodkin, Lopez, Hardy, & Hardy, Reference Goodkin, Lopez, Hardy and Hardy2013). Symbol substitution tests such as the Digit Symbol from the Wechsler Adult Intelligence Scale or the Symbol-Digit Modalities Test (SDMT) are commonly used to assess processing speed in neuropsychological assessment (Lezak, Howieson, Bigler, & Tranel, Reference Lezak, Howieson, Bigler and Tranel2012). Such tests have been shown to be sensitive to HIV infection (Cysique, Maruff, Darby, & Brew, Reference Cysique, Maruff, Darby and Brew2006; Heaton et al., Reference Heaton, Grant, Butters, White, Kirson and Atkinson1995; Llorente et al., Reference Llorente, Miller, D’Elia, Selnes, Wesch, Becker and Satz1998; Sanford et al., Reference Sanford, Fellows, Ances and Collins2018). Symbol substitution tests usually involve a legend or key, pairing a set of test stimuli (such as abstract symbols, as in the SDMT) with another set of response stimuli (various digits). As such, test performance presumably involves an associative learning and memory component. However, since test takers are not explicitly instructed to memorize the stimulus–response pairs (they are told to merely complete the test as quickly and accurately as possible), such learning and memory is often considered to be implicit or incidental.Footnote 1 Although the primary outcome of symbol substitution tests is a measure of psychomotor or processing speed, incidental learning and memory can be measured as well (e.g., Joy, Kaplan, & Fein, Reference Joy, Kaplan and Fein2003; see also Lezak et al., Reference Lezak, Howieson, Bigler and Tranel2012). For instance, after completion of the test, present the first item of each symbol–digit pair and require the recall of each pair’s second item. Better recall indicates greater incidental memory.
Because of the nature of HIV-related neuropathology, including in basal ganglia and other subcortical regions (e.g., Martin, Reference Martin, Grant and Martin1994; Moore et al., Reference Moore, Masliah, Rippeth, Gonzalez, Carey and Cherner2006; Sanford et al., Reference Sanford, Fellows, Ances and Collins2018), there is a reason to expect incidental learning and memory to be impaired in HIV/AIDS. However, research results are mixed. For instance, when using the aforementioned incidental recall measure of the SDMT, Manly et al. (Reference Manly, Smith, Crystal, Richardson, Golub, Greenblatt and Young2011) found a small but statistically significant difference between HIV-positive and HIV-negative women (although this effect disappeared when the covariates of age, education, IQ estimate, and race/ethnicity were included in analyses). However, in another study, incidental recall on the Digit Symbol in HIV-positive and HIV-negative groups was identical (Sassoon et al., Reference Sassoon, Fama, Rosenbloom, O’Reilly, Pfefferbaum and Sullivan2007). Studies using computerized tests also showed mixed results. Using the CogState battery, Cysique and colleauges (Reference Cysique, Maruff, Darby and Brew2006) reported that HIV-positive groups were less accurate and slower on their measure of incidental learning. In contrast, Becker and colleagues (Reference Becker, Dew, Aizenstein, Lopez, Morrow and Saxton2011), using another computerized battery (the Computer Assessment of Mild Cognitive Impairment), found no difference in incidental recall between middle-aged HIV-negative and positive groups (including a subgroup of those with global cognitive impairment). Another kind of incidental or implicit learning and memory is assessed with more procedural tasks (such as the Rotary Pursuit Task) or probabilistic tasks (such as the Weather Prediction Task). Although not the main focus here, even these show variable results in adults with HIV (Gonzalez et al., Reference Gonzalez, Jacobus, Vassileva, Quartana, Amatya and Martin2008; Martin, Gonzalez, Vassileva, & Maki, Reference Martin, Gonzalez, Vassileva and Maki2011; Martin, Heyes, Salazar, Law, & Williams, Reference Martin, Heyes, Salazar, Law and Williams1993). Thus, the overall conclusion across these studies with regard to incidental learning and memory in HIV/AIDS is unclear.
Considering that neurocognitive research on individuals with HIV/AIDS has been taking place for over 30 years, a relevant question is what value is there in determining whether or not incidental learning and memory decrements exist in HIV infection? In reply, we argue that the value lies in the larger goal of determining the cognitive profile of HIV infection. Despite the proliferation of studies, a clear profile or syndrome of the cognitive sequelae of HIV seems to be elusive, with “the main characteristic of HIV cognitive disturbance is its patchy and variable nature” (Grant, Reference Grant2008, p. 37; see also Morgan et al., Reference Morgan, Woods, Delano-Wood, Bondi and Grant2011; Dawes et al., Reference Dawes, Suarez, Casey, Cherner, Marcotte and Letendre2008). Therefore, any discovery or ruling out of a cognitive symptom can potentially help clarify and improve understanding of the impact of HIV on cognitive and nervous system functioning.
In the present study, we examined processing speed and incidental memory in HIV-positive and HIV-negative adults using a novel computerized version of the SDMT. Incidental memory was assessed not by recall of digits (that were paired to symbols), which is a somewhat limited or restricted outcome measure, but with reaction time (RT). Although indirect, the logic of this incidental learning and memory measure is straightforward. To the degree that the association between symbols and digits is learned and retained (implicitly or incidentally, at least to some degree, is the assumption), in the second testing, where symbol–digit pairs are rearranged, there should be evidence of interference with the processing of the new symbol–digit pairs. One advantage of this procedure is that RT, measured in milliseconds, is a sensitive measure of information processing proficiency and impairment (Hardy & Hinkin, Reference Hardy and Hinkin2002), and the range of possible scores is much less restricted. So, if HIV-positive adults exhibit a deficit in incidental learning or memory, then it would be expected that the interference evident with the rearranged symbol–digit pairs would be smaller in HIV-positive adults compared to HIV-negative adults. In addition, because various cognitive or neuropsychological deficits can be a part of the sequela of HIV/AIDS, differing degrees of cognitive impairment will also be examined in relation to our outcome of interest, interference (or the lack thereof) of a learned set of symbol–digit pairs on the processing of a subsequent set of symbol–digit pairs. If general cognitive impairment is associated with the incidental learning of a symbol substitution test, then it would be predicted that HIV-positive individuals with greater cognitive impairment would also show a greater deficit in incidental learning, showing the least amount of interference with the rearranged symbol–digit pairs.
METHODS
Participants
The current study included 135 HIV-positive adults and 28 HIV-negative adults. HIV-positive participants were recruited from an infectious disease clinic at the University of California, Los Angeles, and from local community agencies specializing in services for HIV-positive patients. HIV-negative controls were recruited using posted flyers and referrals from these sites. Recruitment and participation procedures were approved by the UCLA Institutional Review Board, and all participants provided written informed consent and paid 50 dollars for their participation.
HIV-positive participants were divided into three groups with regard to cognitive impairment: unimpaired (n = 89), impaired (n = 24), and most impaired (n = 22). Cognitive impairment was based on a standard neuropsychological battery of tests and determined by a global impairment t-score (see Hinkin et al., Reference Hinkin, Castellon, Durvasula, Hardy, Lam, Mason and Stefaniak2002, for details on tests and procedures). The HIV-positive unimpaired group were those with a t-score of 40 or higher. The impaired group were those with t-scores between 39 and 35. The most impaired HIV-positive group were those with t-scores below 35. The HIV-negative group were all cognitively unimpaired. See Table 1 for group demographics and characteristics. All participants completed a demographics and medical history questionnaire, and a Structured Clinical Interview composed of the mood, psychotic spectrum, and substance use sections of the Structured Clinical Interview for the DSM-IV. There were no group differences in frequency of occurrence of head injury resulting in loss of consciousness (p = .138), history of seizures or neurological problems (p = .124), learning disability (p = .432), mood disorders including major depression (p = .722) and bipolar disorder (p = .482), psychotic disorder (p = .938), current alcohol abuse or dependence (p ≥ .348), and substance abuse or dependence (p ≥ .086). All HIV-positive participants were on self-administered highly active antiretroviral treatment at the time of testing (although not all were equally adherent – see Hinkin et al., Reference Hinkin, Castellon, Durvasula, Hardy, Lam, Mason and Stefaniak2002, et al.,Reference Hinkin, Hardy, Mason, Castellon, Durvasula, Lam and Stefaniak2004] – leading perhaps to various HIV viral load levels evident among HIV-positive groups).
Table 1. Demographics and characteristics of HIV-negative and HIV-positive groups

Note. Standard deviations are inside parentheses. M = mean. For p-values, these pertain to ANOVA or chi-square analyses.
Computerized Symbol-Digit Modalities Test (cSDMT)
A computerized version of the SDMT (Smith, Reference Smith1973) was programmed on SuperLab software (SuperLab Pro Version 2.0, 1999). For the cSDMT, the same stimuli in the same order as in the original SDMT was presented in a trial-by-trial format. Each symbol (black on a white background) subtended approximately 2 × 2.5 °, appeared at the center of the computer monitor and remained on the screen until a response was made (with the dominant forefinger on a rearranged keyboard number pad with 1, 2, 3 in the top row, 4, 5, 6 in the middle row, and 7, 8, 9 in the bottom row). After each response, the next stimulus appeared 500 ms later. A legend showing the symbol–digit relationship was located at the bottom of the computer monitor. As with the original paper-and-pencil SDMT, there were 10 practice trials followed by 110 test trials. After completion of all trials, the same test was completed again but this time with a rearranged symbol–digit relationship (with a new legend replacing the original legend at the bottom of the monitor, see Figure 1). Before the two blocks of cSDMT trials, a baseline task was completed to acclimate participants to responding on the number keypad and to assess baseline processing speed. The digits 1 through 9 were randomly presented at the center of the screen, requiring a corresponding button press on the number pad. There was a total of 27 trials (3 of each digit). All other characteristics of the baseline task were identical to the cSDMT. All participants completed tasks in the same order (baseline task, cSDMT standard, and cSDMT rearranged) and were instructed to respond as quickly and accurately as possible.
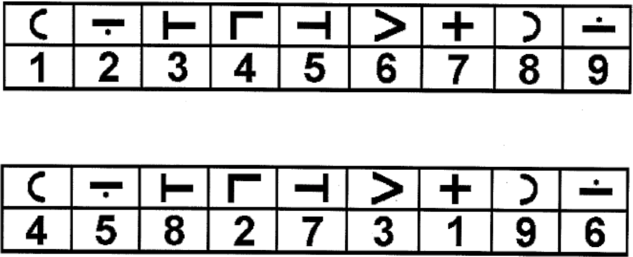
Fig. 1. Legend illustrating symbol–digit pairings for the standard block of trials (top legend) and for the subsequent rearranged block of trials (bottom legend) for the computerized Symbol-Digit Modalities Test (cSDMT).
RESULTS
The primary outcome measure for all analyses was RT in milliseconds for correct response trials. RTs less than 100 ms were considered highly untenable (indicating a premature or false start for those trials) and excluded from analyses. For between-group analyses, the homogeneity of variance assumption was always examined with a Levene test, with no group differences in variability ever evident (ps ≥ .188). Within-subjects analyses employed a Greenhouse–Geisser correction. Effect size was calculated as a partial eta square.
The first set of analyses examined potential group differences on the baseline task. Performance was analyzed with an analysis of variance (ANOVA). There was a significant difference among groups, F(3, 159) = 9.24, p < .001, η 2 = .15.Footnote 2 Mean RT (and SD) was 870 (129) for the HIV-negative group, 895 (99) for the HIV-positive unimpaired group, 941 (137) for the HIV-positive impaired group, and 1025 (142) for the HIV-positive most impaired group. The task was fairly easy with regard to making correct responses, with no significant group differences in accuracy, F(3, 159) = 0.56, p = .640, with mean accuracies of 96.3% (HIV-neagtive), 94.3% (HIV-positive unimpaired), 96.8% (HIV-positive impaired), and 96.3% (HIV-positive most impaired).
Next, performance on the cSDMT was examined with a mixed model 4 x 2 ANCOVA, with participant group (HIV-negative, HIV-positive unimpaired, HIV-positive impaired, and HIV-positive most impaired) as the between-subjects factor, cSDMT task condition (standard and rearranged symbol–digit pairings) as the within-subjects factor, and the baseline task (RT performance) as a covariate to account for basic group differences in response speed on the nine-button keyboard number pad. First, the main effect of participant group was significant, F(3, 158) = 11.18, p < .001, η 2 = .18. Mean RT (and SD) was 1827 (259) for the HIV-negative group, 1879 (258) for the HIV-positive unimpaired group, 2003 (257) for the HIV-positive impaired group, and 2225 (271) for the HIV-positive most impaired group. Note that baseline task RT was a significant and powerful covariate (p < .001, η 2 = .32). The main effect of task condition was also significant, F(1, 158) = 4.58, p = .034, η 2 = .03, with faster mean RT with the standard symbol–digit pairings (M = 1940, SD = 356) compared to the subsequent rearranged pairings (M = 2027, SD = 302). No significant group differences (or interactions) were evident in response accuracy (ps ≥ .365), with accuracy between 96.8 and 97.5% for the HIV-negative group, and between 94 and 95.9% for the HIV-positive groups.
Of most interest was the significant interaction between participant group and cSDMT task condition, F(3, 158) = 2.65, p = .051, η 2 = .05. Mean RT (and SD) for standard and rearranged symbol–digit pairings, respectively, were 1766 (306) and 1888 (259) for the HIV-negative group, 1818 (304) and 1941 (258) for the HIV-positive unimpaired group, 1938 (303) and 2069 (257) for the HIV-positive impaired group, and 2241 (320) and 2210 (272) for the HIV-positive most impaired group. As these means and Figure 2 suggest and post hoc analyses (ANOVA comparisons of mean RT between cSDMT conditions for each group) confirm, mean RT was slower in the rearranged stimulus display condition for all groups (p = .002, .001, .025, respectively) except for the most impaired HIV-positive group (p = .080, with a faster mean RT in the rearranged condition).

Fig. 2. Performance on the standard and rearranged symbol–digit pairings on the computerized Symbol-Digit Modalities Test (cSDMT) for HIV-negative and HIV-positive groups. Error bars are 95% confidence intervals.
DISCUSSION
Looking at the performance across the different tasks or test conditions, one basic finding is slower RTs overall in the HIV-positive groups compared to the HIV-negative group. This is evident in the baseline task, where average RT for the HIV-positive groups was between 25 and 155 ms slower compared to the HIV-negative group. This was an easy task (response accuracy was very high for all groups) merely requiring the identification of a digit and pressing the corresponding button on the computer keyboard. Post hoc pairwise comparisons were not calculated, to reduce inflation of experiment-wise Type I error, but the overall pattern of RTs is straightforward, indicating a progressive slowing in basic processing speed with increased global cognitive impairment across HIV-positive groups (note that global cognitive impairment t-scores were based on common standardized neuropsychological tests and did not include any RT measures). The significant main effect of group in the 4 × 2 ANCOVA, showing an overall group difference in RT on the cSDMT, also indicates a general response slowing in HIV, where mean RT was between 52 and 399 ms slower relative to the HIV-negative group. The fact that this slowing was still statistically significant even with the inclusion of the powerful baseline task covariate suggests that the slower responses in the HIV-positive groups were due mostly to slower central or cognitive processing – response execution requirements were identical in the baseline task and cSDMT, and so variability in RTs due to this should have been covaried out. These findings are compatible with the general finding in many studies over the past few decades of slower cognitive or neuropsychological test performance in adults with HIV/AIDS. They also illustrate the possible progressive nature of this slowing in those with cognitive impairment, although this conclusion is tentative considering results are based on a cross-sectional, not longitudinal, research design.
Of interest are the findings that bear on incidental learning and memory. As is clearly illustrated in Figure 2, for all but the most cognitively impaired HIV-positive group, mean RT was significantly slower in the rearranged cSDMT trials compared to standard cSDMT trials. Degree of slowing was fairly uniform across those groups at 122 ms (HIV-negative), 123 ms (HIV-positive unimpaired), and 131 ms (HIV-positive impaired). A viable candidate to explain the slower RTs in the second block of cSDMT trials is the learning and memory of the symbol–digit pairings in the first block of trials interfering with performance in the second block. Because as usual there were no explicit instructions to participants to learn these symbol–digit associations, it can be argued (as has been in the past by others) that at least some of this learning and memory was incidental. Thus, our results suggest that incidental learning and memory is intact in all but the most cognitively impaired HIV-positive adults. In this group, there was no evidence whatsoever of slowing in the second block of trials where symbol–digit pairings were rearranged. In fact, results suggest a slightly faster mean RT (by 31 ms) in this second block (p = .080).
It is argued that RT was not slower in the second block of the cSDMT (relative to the first block of standard trials) for the most cognitively impaired HIV-positive group because of a deficit in incidental learning and memory. In other words, the incidental learning and memory that normally occurs during a symbol substitution test did not take place for this HIV-positive group during block one on the cSDMT with the standard arrangement of symbol–digit pairings. Because the association between these symbols and digits was never learned during the first block of trials, there was nothing to interfere with the processing of the second block of cSDMT trials where symbol–digit pairings were rearranged. Hence, for this group, mean RT for the second block of trials was comparable or maybe even faster. We realize that, on the face of it, to argue for a deficit (in this case, in incidental learning and memory) based on the absence of a task effect may be counterintuitive. But this argument is based on the design of the cSDMT task and our behavioral results. That said, several potential alternative explanations need to be addressed. One of these is fatigue. Because fatigue typically leads to slower responses, and mean RT for the most cognitively impaired HIV-positive group was comparable or maybe even faster in the second block relative to the first block, fatigue is an unlikely explanatory factor. Another factor to consider is a practice effect. Practice effects, which can occur in RT tasks as the test taker becomes more comfortable with general procedures as well as the particular demands of the task, could account for faster or comparable RTs in the second block of cSDMT trials. This could, in theory, mitigate any detrimental slowing in the second block due to interference from incidental learning and memory of block one stimulus pairs. But this did not occur with the other groups (their mean RTs were significantly slower in the second block), and there is no reason to assume greater practice effects in the most cognitively impaired HIV-positive group. Another possible factor to explain the lack of impact in block two of the cSDMT is overall slowing in processing speed. And indeed, this group reliably exhibited the slowest mean RTs in this study. But it must be pointed out, again, that this group’s mean RT in the rearranged set of symbol–digit pairings was not slower but actually comparable or maybe even faster to their mean RT in block one. Hence, it is difficult to see how in the most cognitively impaired HIV-positive group being generally slower would result in a relatively faster block two RT. You would think mean RT would have been slower, as was the case in the other groups, and perhaps be the slowest among all the groups. Therefore, it is argued that fatigue, practice effects, and a general slowing in processing speed are unlikely viable explanations for this interaction between group and cSDMT task condition.
In conclusion, we propose based on our findings that a deficit in incidental learning or memory is part of the cognitive sequela in HIV-positive adults who experience moderate to severe cognitive impairment. Although results on this topic are mixed in previous studies using standard paper-and-pencil versions of the SDMT or Digit Symbol, we argue that our computerized test utilizing RT as the outcome measure might be a more sensitive assessment tool. And more sensitive not only to deficits in processing speed but to, distinctly, incidental learning and memory, because of the multiple test conditions (in this case, the standard and rearranged symbol–digit pairings, as well as the baseline task) and analytic approach. Whether our cSDMT is a more clinically viable test is an open question and not the main focus of this study. As with most computerized tests of cognition, there are potential advantages (such as greater test sensitivity) and disadvantages (the challenges associated with requiring a computer) and this applies to our cSDMT. We also realize that our cognitively impaired HIV-positive groups were not based on standard clinical cutoff t-scores, such as one or two SDs below the mean (our cutoffs were 1.0 and 1.5 SDs, to preserve sample size integrity). Nonetheless, the pattern of mean RTs across groups and cSDMT conditions is intriguing (see Figure 2). On the face of it, it suggests an initial mild processing speed slowing before any general HIV-related cognitive impairment, followed by an increase in this slowing along with the appearance of some degree of cognitive impairment, ultimately followed by a deficit in incidental learning and memory along with more severe cognitive deficits. The appearance of incidental learning and memory deficits during more severe cognitive impairment is not entirely surprising. In many neurological disorders, it is the incidental or implicit cognitive abilities that are the most robust, showing decline relatively late or in more advanced stages in the syndromic trajectory. We realize this is conjecture based on a cross-sectional design and so caution is warranted, but we argue that this pattern of results may present an interesting question to investigate, either prospectively or in the existing research literature. Our results also illustrate the sensitivity of a computerized cognitive test and information processing approach in the assessment of cognitive functioning. Lastly, with regard to incidental learning and memory deficits in the most impaired HIV-positive group, this is a clinically relevant finding. An admittedly complex component of cognition (Frensch & Rünger, Reference Frensch and Rünger2003), various aspects of implicit or incidental learning and memory have been shown to be related to behaviors, including risky behaviors, in HIV-positive adults (e.g., Stacy, Newcomb, & Ames, Reference Stacy, Newcomb and Ames2000). Therefore, a sensitive assessment of incidental learning and memory can potentially help determine the extent of such a deficit and how it is related to cognitive abilities and behaviors, and to HAND in general, in the HIV/AIDS population.
Acknowledgments
This study was supported by the National Institute of Mental Health Grant No. R01 MH-58552 (awarded to Charles Hinkin) and T32 MH-19535. We gratefully acknowledge (in alphabetical order) for their assistance: Mona Lam, Karen Mason, Marta Stefaniak, and Bryan Zolnikov. Portions of this paper were presented at the 99th Annual Convention of the Western Psychological Association, Pasadena, California, April 2019, and the 31st Annual Convention of the Association for Psychological Science, Washington, D.C., May 2019.
CONFLICTS OF INTEREST
The authors have nothing to disclose.





