Introduction
Termites are key pests of trees, crops, wood and wood products in tropical, subtropical and warm temperate areas of the world, therefore there are many management methods used to limit damage (e.g. Wood, Reference Wood1991; Su & Scheffrahn, Reference Su, Scheffrahn, Abe, Bignell and Higashi2000; GEF, 2005; Verma et al., Reference Verma, Sharma and Prasad2009; Scholz et al., Reference Scholz, Militz, Gascon-Garrido, Ibiza-Palacios and Oliver-Villanueva2010; Rouland-Lefèvre, Reference Rouland-Lefèvre, Bignell, Roisin and Lo2011). Naturally resistant wood species, chemical preservation of non-resistant wood species, chemical dusting and fumigation were the main methods of preventing termite attack until the 1940s and 1950s, but they were largely replaced with the development of soil termiticides after World War II (Ware, Reference Ware1999). Soil termiticides quickly become the dominant termite control method, especially in buildings, due to their low cost and ease of use (e.g. Findlay, Reference Findlay1962; Harris, Reference Harris1971; Hickin, Reference Hickin1971).
Along with all broadcast insecticides used in agriculture, soil termiticides began falling from favour, in part due to harmful effects of insecticides on the public health, environment and other non-target organisms, and in part to their perceived lack of effect on the termite colony (Esenther & Gray, Reference Esenther and Gray1968; Su, Reference Su1994; Grace et al., Reference Grace, Tome, Shelton, Ohsiro and Yates1996; Evans & Iqbal, Reference Evans and Iqbal2015). Compared with soil treatments, baiting is considered relatively environment friendly as it uses small amounts of insecticidal active ingredients with high insect and low mammalian toxicity (Grace et al., Reference Grace, Tome, Shelton, Ohsiro and Yates1996; Evans & Gleeson, Reference Evans and Gleeson2006; Evans, Reference Evans2010). Suppression or elimination of termite colonies by baiting requires non-repellent active ingredients, effective over a 10–100 fold range of concentrations and delayed toxicity (Stringer et al., Reference Stringer, Lofgren and Bartlett1964), which allows the foraging workers to distribute the active ingredient throughout the colony (Esenther & Gray, Reference Esenther and Gray1968; Su, Reference Su1994; Henderson, Reference Henderson2001).
Many baiting systems have been developed, yet these are not universally useful. All commercial termite baiting systems utilize chitin synthesis inhibitors as active ingredients, and all were developed and are now used against the lower termites in the family Rhinotermitidae in buildings, especially in temperate regions of the world (for review see Evans & Iqbal, Reference Evans and Iqbal2015). There are no baiting systems designed specifically to target termites in the family Termitidae. These ‘higher’ termites are more diverse and abundant in urban and agricultural settings in the tropical and subtropical regions of the world (Eggleton et al., Reference Eggleton, Williams and Gaston1994; Eggleton, Reference Eggleton, Abe, Bignell and Higashi2000). The fungus-growing termites in the sub-family Macrotermitinae are important pests in buildings in non-urban and rural areas, and also in forestry and agriculture (Harris, Reference Harris1971; Hickin, Reference Hickin1971; Lee et al., Reference Lee, Vongkaluang and Lenz2007; Rouland-Lefèvre, Reference Rouland-Lefèvre, Bignell, Roisin and Lo2011; Iqbal & Saeed, Reference Iqbal and Saeed2013). In sub- and peri-urban areas, they may become secondary pests in built structures after suppression or elimination of dominant Coptotermes species (Lee et al., Reference Lee, Vongkaluang and Lenz2007).
Part of the reason for the lack of baiting systems against macrotermitid termites in the tropics is the lack of a suitable active ingredient (Evans & Iqbal, Reference Evans and Iqbal2015; Iqbal et al., Reference Iqbal, Evans, Saeed and Khan2016a , Reference Iqbal, Wijdedsas and Evans b ). The chitin synthesis inhibitors used against rhinotermitids are ineffective or less effective against these higher termites (Lee et al., Reference Lee, Vongkaluang and Lenz2007, Reference Lee, Neoh and Lee2014). This is due to developmental differences between these termite families. Chitin synthesis inhibitors kill termites when they attempt to moult. Usually workers of rhinotermitid species (and some termitids in other sub-families) moult many times, hence they are more vulnerable to chitin synthesis inhibitors (Roisin, Reference Roisin, Abe, Bignell and Higashi2000). However, as there is a single instar in the worker caste in the sub-family Macrotermitinae (Neoh & Lee, Reference Neoh and Lee2009), they are less vulnerable to chitin synthesis inhibitors (Lee et al., Reference Lee, Vongkaluang and Lenz2007, Reference Lee, Neoh and Lee2014; Neoh et al., Reference Neoh, Jalaludin and Lee2011). The ‘larvae’ (youngest instars without gut flora) are vulnerable in Macrotermes gilvus (Hagen), because they moult several times, however as larvae are a relatively small proportion of the colony, elimination rarely occurs (Neoh et al., Reference Neoh, Jalaludin and Lee2011; Lee et al., Reference Lee, Neoh and Lee2014). Conditions for high levels of colony suppression include: many larvae, small colony size, and long baiting periods, none of which can be predicted (Dhang, Reference Dhang2011; Lee et al., Reference Lee, Neoh and Lee2014).
Consequently, there is a pressing need for a suitable bait active ingredient for against higher termites, especially macrotermitid species. Neurotoxins are not normally considered as bait active ingredients, yet some may have all the essential properties for a bait active ingredient for higher termites: non-repellent, effective over a wide range of concentrations, and delayed toxicity (Stringer et al., Reference Stringer, Lofgren and Bartlett1964). The organochloride neurotoxin mirex was one of the first active ingredients tested for termite baiting (Esenther & Gray, Reference Esenther and Gray1968; Paton & Miller, Reference Paton and Miller1980), but was abandoned due to environmental concerns (Evans & Iqbal, Reference Evans and Iqbal2015). Two other neurotoxins show some promise: fipronil and imidacloprid (Simon-Delso et al., Reference Simon-Delso, Amaral-Rogers, Belzunces, Bonmatin, Chagnon, Downs, Furlan, Gibbons, Giorio, Girolami, Goulson, Kreutzweiser, Krupke, Liess, Long, McField, Mineau, Mitchell, Morrissey, Noome, Pisa, Settele, Stark, Tapparo, Van Dyck, Van Praagh, Van der Sluijs, Whitehorn and Wiemers2015), both of which are used currently against termites as soil treatments (for review see Hu, Reference Hu and Dhang2011), and as baits against other insects.
Fipronil is a phenylpyrazole insecticide that blocks two ion channels in nerves: gamma-aminobutyric acid gated and glutamate gated chloride channels; causing hyper-excitation of the nerve and death (Cole et al., Reference Cole, Nicholson and Casida1993; Alexander et al., Reference Alexander, Mathie and Peters2011). Fipronil is used in baits against cockroaches and ants, as it is non-repellent at lower doses (i.e. concentration of active ingredient; Kaakeh et al., Reference Kaakeh, Reid and Bennett1997; Collins & Callcott, Reference Collins and Callcott1998; White, Reference White1998; Shelton & Grace, Reference Shelton and Grace2003), and there are laboratory studies suggesting it may be useful as a bait active ingredient for termites (Bagnères et al., Reference Bagnères, Pichon, Hope, Davis and Clément2009; Mao et al., Reference Mao, Henderson and Scherer2011); including transfer and spread among colony members (Saran & Rust, Reference Saran and Rust2007; Spomer et al., Reference Spomer, Kamble, Warriner and Davis2008; Gautam et al., Reference Gautam, Henderson and Davis2012). There are two field studies that suggest efficacy as bait (Huang et al., Reference Huang, Lei and Xue2006; Iqbal et al., Reference Iqbal, Evans, Saeed and Khan2016a ), however neither study showed colony level effects, including colony elimination, as the species used have cryptic underground nests (Thorne & Forschler, Reference Thorne and Forschler2000; Evans & Iqbal, Reference Evans and Iqbal2015).
Imidacloprid is a neonicotinoid insecticide that blocks postsynaptic nicotinic acetylcholine receptors of motor neurons, preventing transmission of nerve impulses, resulting in paralysis and death (Bai et al., Reference Bai, Lummis, Leicht, Breer and Sattelle1991; Mullins, Reference Mullins, Duke, Menn and Plimmer1993). Imidacloprid is a non-repellent insecticide in laboratory studies (Keefer, Reference Keefer2010; Mao et al., Reference Mao, Henderson and Scherer2011; Iqbal & Saeed, Reference Iqbal and Saeed2013), and can be transferred among the colony members (Thorne & Breisch, Reference Thorne and Breisch2001; Shelton & Grace, Reference Shelton and Grace2003; Tomalski & Vargo, Reference Tomalski and Vargo2004). Imidacloprid is used in baits against cockroaches and ants, as it is non-repellent at lower doses (Appel & Tanley, Reference Appel and Tanley2000; Daane et al., Reference Daane, Cooper, Sime, Nelson, Battany, Rust and Rust2008; Brooks et al., Reference Brooks, Nentwig and Gutsmann2009). There are no studies on its potential and feasibility as a bait active ingredient against termites.
In this study, we aimed to test the two insecticides, fipronil and imidacloprid, as a bait active ingredient, against a pest species of fungus-growing, higher termite. We aimed to determine the optimal dose rate (concentration of active ingredient in ppm) of these two insecticides to minimize repellency but maximize the range to be effective. Once the optimal dose rate was determined, we aimed to test the insecticides in baits in the field. The ultimate goal of our study was to identify a novel active ingredient and dose rate for use against pest fungus-growing termites, and thereby provide a new, environmentally benign tool for pest termite management.
Materials and methods
Study site and species
We performed the study at the Singapore Botanic Gardens (Latitude 1.3120511°, Longitude 103.817417°). The SBG is 74 hectares and contains a mixture of habitats, including primary dipterocarp rainforest, various stages of secondary forests, manicured parklands, and specialist gardens. Two Macrotermes species, Macrotermes gilvus and Macrotermes carbonarius (Hagen), are found in the forests and parkland. These are fungus-growing higher termites, both of which build large above ground nests (mounds), often near large trees. We targeted M. gilvus as it can damage trees and building structures in Singapore, Malaysia and Thailand (Lee et al., Reference Lee, Vongkaluang and Lenz2007; Lee, Reference Lee2014), and ignored M. carbonarius as it eats leaf litter (Iqbal et al., Reference Iqbal, Wijdedsas and Evans2016b ). We selected 14 healthy mounds for the study. Studies have shown that M. gilvus and other Macrotermes species have a maximum linear foraging distance of 48 m (Jmhasly & Leuthold, Reference Jmhasly and Leuthold1999; Acda, Reference Acda2004). So, we considered each mounds as a separate and independent colonies as they were more than 50 m apart (Dhang, Reference Dhang2011).
Laboratory repellency study
We collected termites from SBG using underground bait stations filled with wood (Iqbal et al., Reference Iqbal, Wijdedsas and Evans2016b ). We returned the bait stations to the laboratory and separated workers and soldiers from the soil and debris using the methods of Gay et al. (Reference Gay, Greaves, Holdaway and Wetherly1955). We placed the termites in plastic jars (15 × 9 × 6 cm3) with damp paper towelling, to allow time for damaged termites to die (and then be removed), and to hold live termites until their use in the repellency study. The maximum holding time was two days.
We tested the repellency of fipronil (Agenda 10 SC, Bayer Environmental Sciences, Selangor, Malaysia) and imidacloprid (Premise 200SC, Bayer Environmental Sciences) to M. gilvus to discern optimal dose rates (ppm) for baiting. We mixed the following dose rates in distilled water: fipronil 0, 4, 16, 32, 64 ppm, and imidacloprid 0, 20, 40, 80, 160, 250 ppm, with ppm determined as parts per million in water solution; note that we used higher doses for imidacloprid as it is used at higher dose rates in soil treatments against termites). We applied 1 ml of solution of each dose to 3.5 cm2 squares of Whatman filter paper (No. 1), which we air-dried in the laboratory.
We conducted the repellency bioassays in plastic boxes (15 cm long, 9 cm wide × 6 cm high). We placed one square of each dose of treated paper randomly around the edges of each box, but only one insecticide in a box; in other words five squares of fipronil in a box, or six pieces of imidacloprid in a box; note that the paper squares did not cover the base of the plastic box entirely. We placed 50 healthy and active workers and five soldiers of M. gilvus in the centre of each box, and placed the boxes in large plastic trays with moist tissue papers to maintain humidity, then covered the trays with a transparent acrylic sheet. We observed the activity of workers and recorded the numbers of workers on each paper square and on the plastic box after 30, 60, 90, 120 min and then after every 12 h until 48 h. We avoided disturbance to the termites at each sampling time by photographing the boxes (Panasonic digital camera, model DMC-FX75, Kadoma Osaka, Japan); we counted the numbers of workers at each dose from the photographs. We used four replicate boxes with each insecticide and two boxes containing five squares of untreated filter paper squared (wetted with distilled water) as controls.
Field Baiting of M. gilvus
We selected 14 M. gilvus healthy mound-colonies with 0.24 ± 0.035 m height (mean ± SE) and 0.66 ± 0.07 m diameter (mean ± SE) for this experiment. We used these mounds previously in a study of foraging behaviour and station size preferences (Iqbal et al., Reference Iqbal, Wijdedsas and Evans2016b ). We had installed 12 bait stations in the soil around each mound, four small stations (ca. 0.35 L, containing one piece (ca. 74 g) of wood), four medium stations (ca. 3.6 L, containing two pieces (242 g) of wood), and four large stations (11.5 L, containing five pieces (985 g) of wood inside the station (see Iqbal et al., Reference Iqbal, Evans, Saeed and Khan2016a , Reference Iqbal, Wijdedsas and Evans b for details). Installation followed that in previous baiting studies, with the slight variation that station were approximately 2 m from the mounds, and were not connected to the mounds in any way (Peters & Fitzgerald, Reference Peters and Fitzgerald2003; Evans, Reference Evans2010; Dhang, Reference Dhang2011; Neoh et al., Reference Neoh, Jalaludin and Lee2011).
We selected five colonies at random for treatment with fipronil baits, five colonies for treatment with imidacloprid, and four colonies as controls. Based on the results of the repellency study (details below), we used two doses for the baiting experiment: 16 ppm and 64 ppm of fipronil and 40 ppm and 160 ppm of imidacloprid. We used rolls of white toilet paper as the food source, as toilet paper is eaten readily by wood-eating termites, and has been used in many termite foraging studies (e.g. La Fage et al., Reference La Fage, Nutting and Haverty1973; Dawes-Gromadzki & Spain, Reference Dawes-Gromadzki and Spain2003; Evans et al., Reference Evans, Dawes, Ward and Lo2011). We treated the toilet paper rolls with either fipronil or imidacloprid solutions at the above two concentrations. We dunked toilet paper into the treated solutions or water for 30 s, then placed the wet rolls into one station with active M. gilvus termites on 02 July 2013. The toilet papers absorbed ca. 650 ml of solution, thus toilet paper rolls contained either 10.4 mg fipronil (16 ppm solution), 41.6 mg fipronil (64 ppm solution), 26 mg imidacloprid (40 ppm solution), or 104 mg imidacloprid (160 ppm solution). We used large stations whenever possible, as they contained more termites and termites are less likely to abandonment larger stations due to inspections (Evans & Gleeson, Reference Evans and Gleeson2006; Iqbal et al., Reference Iqbal, Wijdedsas and Evans2016b ). If a large station was not active, we used a medium station. We monitored termite activity in bait stations and monitoring stations (i.e. stations that did not receive bait).
We inspected stations on 30 July and 01 August 2013, 4 weeks/28 days after bait placement. We opened all baited and monitoring stations and observed termite presence and activity, and estimated the amount of toilet paper bait removed. We removed a small section of the clay wall of the mound (5 × 5 cm2) and observed termite presence or absence in the mounds. We ascertained colony health with a mound-damage-and-repair manipulation (following Evans, Reference Evans2006, Reference Evans2010; Webb & Mcclintock, Reference Webb and McClintock2015). We used the small opening and replaced the clay pieces in the wall opening of mounds for easier repair. We recorded damage repair after 5 h, and then repeated this process again four weeks later (i.e. 56 days after baits were placed). A ‘full repair’ included completely covering the damage with new layer of clay so that the broken pieces were not visible. A ‘partial repair’ was less complete and involved some building between the broken clay pieces so that clay pieces were visible but (mostly) fixed into their position. ‘No repair’ was when there was no new clay under the broken clay wall pieces, all of which were completely visible and able to be moved. The mounds showing no repair were destructively sampled to see the presence of termites in mounds.
Data analysis
We analysed data recorded from the laboratory repellency experiment, number of workers standing on treated paper, and mortality, using a two way repeated measured ANOVA, with time as the repeated measure with actual time intervals, and Wilks’ lamda distribution for the multivariate (time and interaction) test. We analysed data recorded from the field experiments using Kruskal–Wallis test (nonparametric equivalent of a one-way ANOVA because of the low maxima; following Sokal & Rohlf, Reference Sokal and Rohlf1981). Data were the number of stations per colony baited and infested with M. gilvus; and the estimate of bait removed. We analyzed data from the mound damage-and-repair manipulation with chi squared tests. Data were analyzed with Systat 9.0 (1998) (Chicago, IL).
Results
Laboratory repellency study
For the fipronil treated paper, the worker termites did not settle but instead walked continuously and quickly, whereas in the control boxes the worker termites slowed, often to a standstill, after around 30 min. The termite numbers on each piece of paper had stabilized (i.e. changed little) after 60 min. The numbers of workers did not differ between concentrations of fipronil (F 6,21 = 1.332; P = 0.287) or time (F 5,17 = 0.000; P = 1.000). There was a significant interaction (F 30,70 = 1.749; P = 0.029); however, this was driven by the termites resting on the plastic box and not on the treated paper (fig. 1a).
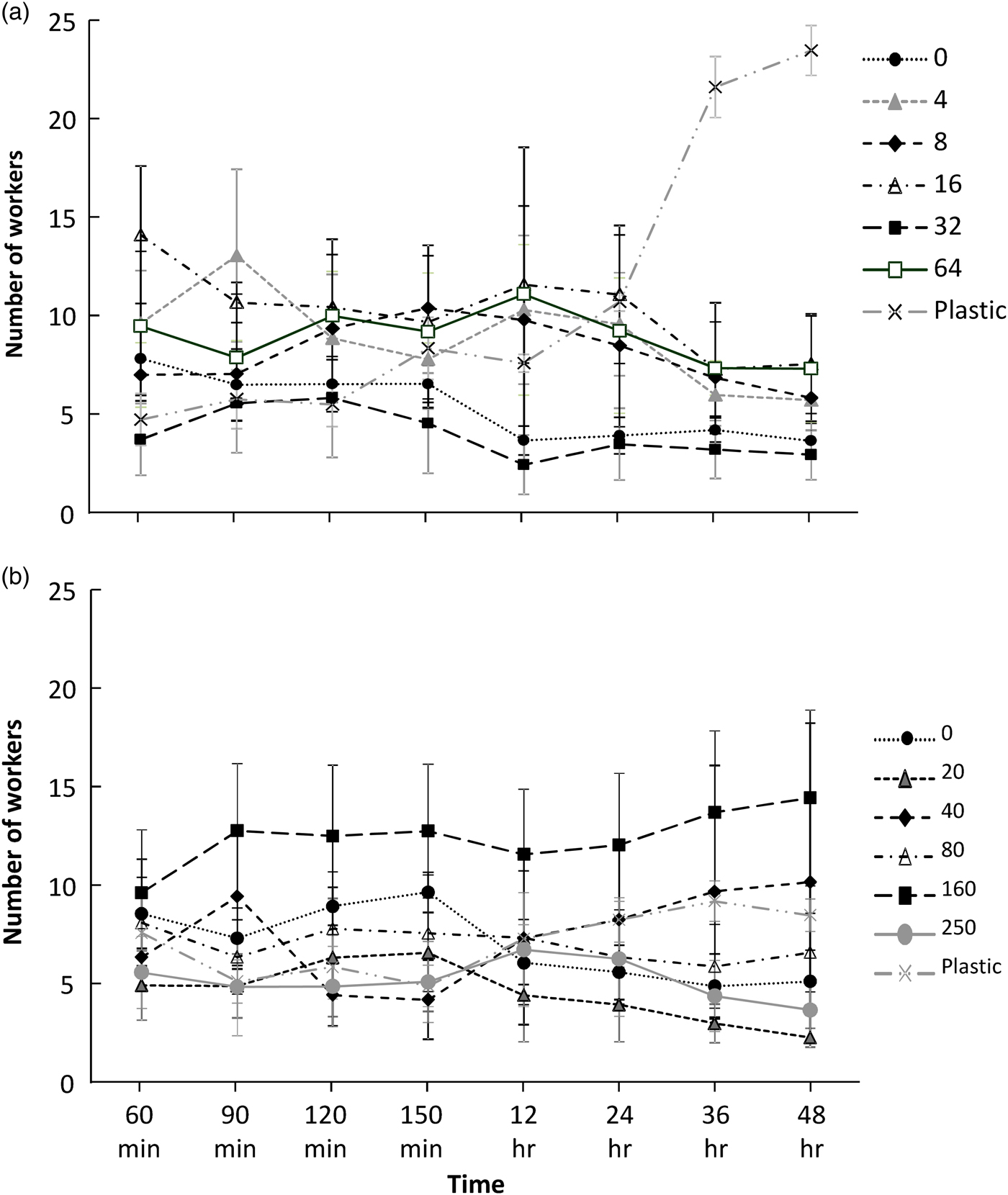
Fig. 1. Numbers of Macrotermes gilvus workers (average ± standard error) on filter paper treated with toxicants over time in a laboratory repellency experiment. (a), fipronil; (b), imidacloprid. ‘Plastic’, termites standing on plastic of the container rather than standing on paper.
The general pattern of results for the imidacloprid treated paper was similar to those of fipronil treated papers, although with no significant changes at all. After an initial fast walking period of 30 min, the worker termites settled down on different doses, with little change in termite numbers on papers after 60 min. The numbers of workers did not differ between concentrations of imidacloprid (F 6,21 = 0.996; P = 0.454) or time (F 5,17 = 0.000; P = 1.000), and the interaction was not significant (F 30,70 = 1.359; P = 0.147) (fig. 1b).
Overall, the number of dead termite workers increased over time, from 12 to 48 h. The mortality rate was lowest in the control treatment, was initially higher in imidacloprid treated paper, but ended but higher in fipronil treated paper. The mean number of dead workers differed significantly between each treatment (F 2,10 = 20.026; P < 0.001) and over time (F 3,8 = 136.255; P < 0.001); and there was a significant interaction, showing that the mortality rate climbed faster over time in the fipronil treated paper (F 6,16 = 14.023; P < 0.001) (fig. 2). Comparing only the active ingredient treated papers, the overall mortality rate did not differ between fipronil and imidacloprid treated papers (F 1,6 = 1.473; P = 0.271), it did increase with time (F 3,18 = 314.170; P < 0.001), and it increased significantly faster in fipronil (F 3,18 = 110.076; P < 0.001) (fig. 2a).
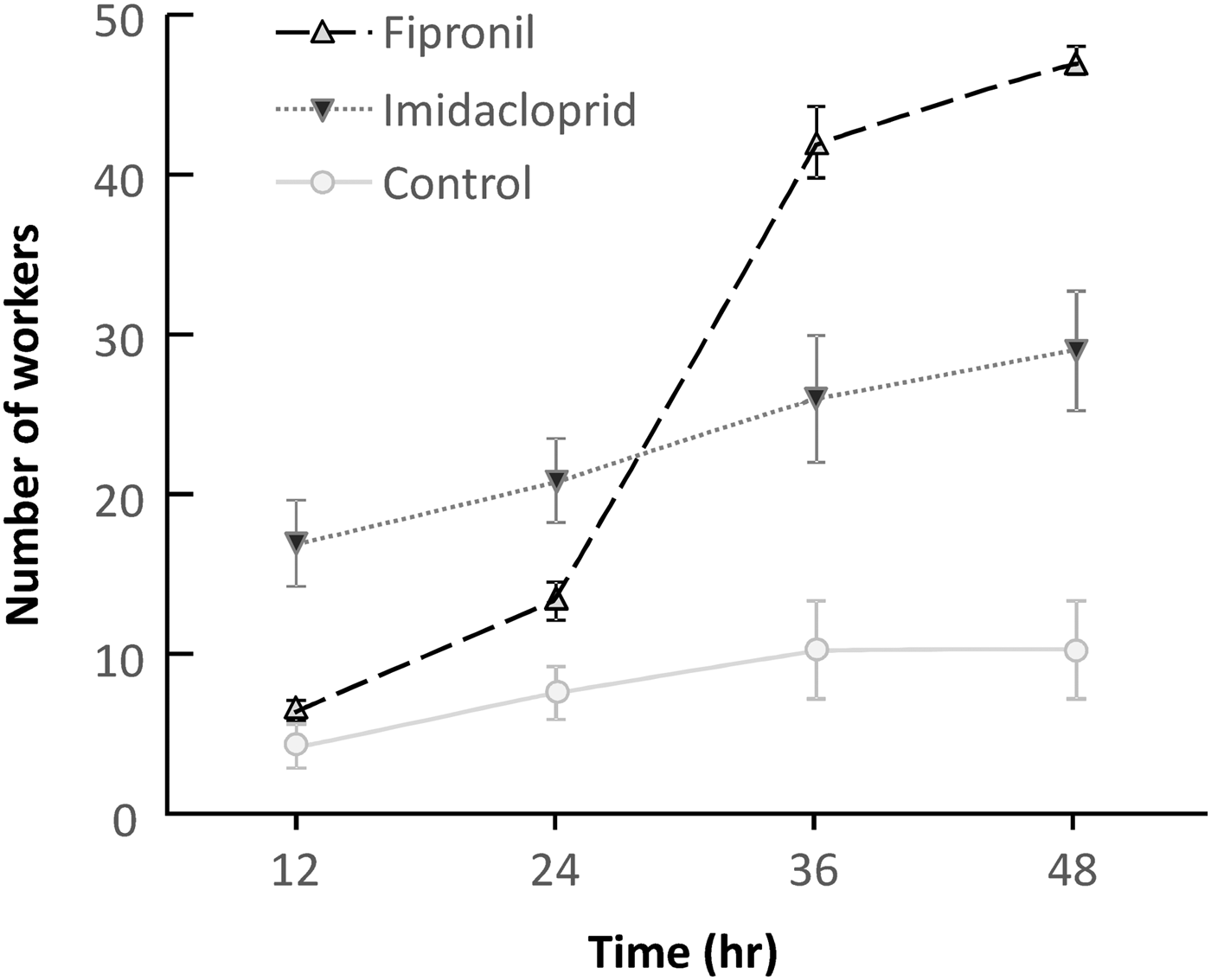
Fig. 2. Numbers of dead Macrotermes gilvus workers exposed to filter paper treated with active ingredient over time in a laboratory repellency experiment.
As there was no significant difference in attraction or repulsion at the various dose rates, we chose the dose rates for the field trial using the highest average number of termites resting on treated paper. We chose 64 ppm fipronil, the highest dose, and 160 ppm for imidacloprid, the second highest dose, because these doses consistently had high average numbers of termites. We interpreted these to be the highest, non-repellent dose. As we were unsure about the effect of high mortality (nearing 100% in the fipronil treated paper, and over 50% in the imidacloprid treated paper) over 48 h, we included lower doses, 16 ppm fipronil and 40 ppm imidacloprid as well, as termites numbers were high on these doses, especially at the end of the experiment (48 h).
Field baiting of M. gilvus
There were 1–3 bait stations and 1–2 untreated monitoring stations per colony in the fipronil and imidacloprid treatments, and 1–3 monitoring stations per colony in the control. There was no difference in the number of stations between treatments (KW 2 = 3.351, P = 0.187), nor was there a difference in the number of toilet paper rolls placed in stations between treatments (KW 2 = 3.900, P = 0.142) (table 1).
Table 1. The field experiment baiting Macrotermes gilvus with two active ingredients over two months in Singapore

Md, mound; Stn, station; a.i., active ingredient; %TPR, percentage of toilet paper roll; dT, dead M. gilvus termites in the station; Data are means, with range in brackets. For Mound repair ‘none’, no repair; ‘part’, partial repair, and ‘full’, mound fully repaired.
On the first inspection after 28 days of treatment, we found termites had removed 5–13% of the fipronil treated toilet paper rolls, none of the imidacloprid treated toilet paper rolls, and 60–70% of the untreated toilet paper rolls, which was a significant difference (KW 2 = 12.216, P = 0.002). We found no M. gilvus termites in any of the stations that received fipronil or imidacloprid treated toilet paper rolls, but found termites in all stations that received untreated toilet paper, which was a significant difference (KW 2 = 13.000, P = 0.002). The number of monitoring stations with M. gilvus did not differ significantly between treatments (KW 2 = 0.039, P = 0.980); although four of the five imidacloprid treated colonies found new stations compared with one of five fipronil treated and one of four control colonies, this difference in new contact was not enough to be significant (KW 2 = 4.534, P = 0.104) (table 1). Species of termite other than M. gilvus infested monitoring stations for two of the fipronil treated colonies, whereas there were no new infestations of this type in either the imidacloprid treated or control colonies.
The presence of live termites in the mounds at 28 days did not differ between treatments, though there were two fipronil treated mounds without termites (χ 4 2 = 4.200, P = 0.122). However, there was a significant difference in repair type in the mound damage and repair manipulation at 28 days, with fipronil treated colonies making no full repairs to their mounds, whereas all imidacloprid treated and control colonies repaired their mounds in full (χ4 2 = 14.000, P = 0.007) (table 1).
On the second inspection after 56 days of treatment, we found that many stations had been emptied of wood, filled with soil, or occupied by other organisms and therefore we did not include data from stations for analysis. However, we found further evidence of the effect of fipronil baits on M. gilvus colonies. Not one of the fipronil treated mounds were repaired after the second mound damage and repair manipulation, whereas all mounds were repaired in the imidacloprid treated and controls. Similarly, all the fipronil treated mounds were empty of termites, whereas those in both other treatments contained live termites. Both of these were significantly different (χ 4 2 = 14.000, P = 0.001). Clearly fipronil treated baits had eliminated colonies, whereas imidacloprid treated baits did not; instead they resembled the control colonies.
Discussion
We found in the laboratory repellency trial that M. gilvus termites displayed no significant preferences among the wide range of doses of fipronil and imidacloprid. However, in this laboratory trial, more termites died and faster when exposed to fipronil compared with imidacloprid. Therefore we had some expectation that fipronil would be too fast-acting to be a good bait active ingredient, whereas imidacloprid would be a suitable choice. We included higher and lower doses of each because of non-significant differences among the doses. In the field experiment, fipronil eliminated M. gilvus colonies whereas imidacloprid did not. This is because fipronil baits were eaten: an average of 4.5% of 16 ppm and 2.5% of 64 ppm of fipronil treated paper over 28 days, which was sufficient to eliminate all the five colonies, two colonies in 28 days and three colonies in 56 days.
It is possible that the workers received a lethal dose of fipronil as a consequence of contact, as seen in soil treatments (Cole et al., Reference Cole, Nicholson and Casida1993; Shelton & Grace, Reference Shelton and Grace2003; Hu, Reference Hu and Dhang2011; Simon-Delso et al., Reference Simon-Delso, Amaral-Rogers, Belzunces, Bonmatin, Chagnon, Downs, Furlan, Gibbons, Giorio, Girolami, Goulson, Kreutzweiser, Krupke, Liess, Long, McField, Mineau, Mitchell, Morrissey, Noome, Pisa, Settele, Stark, Tapparo, Van Dyck, Van Praagh, Van der Sluijs, Whitehorn and Wiemers2015). If so, this is in addition to the dose received by eating the fipronil baits, as shown for cockroaches and ants (Collins & Callcott, Reference Collins and Callcott1998; Buczkowski & Schal, Reference Buczkowski and Schal2001), and suggested for termites (Huang et al., Reference Huang, Lei and Xue2006; Saran & Rust, Reference Saran and Rust2007; Spomer et al., Reference Spomer, Kamble, Warriner and Davis2008; Bagnères et al., Reference Bagnères, Pichon, Hope, Davis and Clément2009; Mao et al., Reference Mao, Henderson and Scherer2011; Gautam et al., Reference Gautam, Henderson and Davis2012; Iqbal et al., Reference Iqbal, Evans, Saeed and Khan2016a ). In comparison, imidacloprid baits were not eaten, and consequently the imidacloprid treated colonies did not appear to be affected, beyond abandoning the baited stations. If there was an effect of imidacloprid on the colonies, we were not able to detect it using our field methods.
The results for fipronil baits from this study match those of three previous termite baiting studies. Fipronil baits had successfully suppressed termite activity of fungus-growing termites in the field in China and Pakistan. In China, two of three baited colonies of Odontotermes formosanus (Shiraki) stopped foraging in trees and bait stations after 120–150 days when fed 40 ppm fipronil baits (Huang et al., Reference Huang, Lei and Xue2006). In Pakistan, three colonies of Microtermes mycophagus (Desnoux) were baited with 10 and 30 ppm fipronil, all of which stopped foraging activity in buildings and bait stations after 45–90 days (Iqbal et al., Reference Iqbal, Evans, Saeed and Khan2016a ). Termites other than fungus-growers have been baited with fipronil as well. Forschler & Jenkins (Reference Forschler and Jenkins2000) baited four colonies of Reticulitermes species with 0.1–10 ppm fipronil baits, with foraging activity in monitoring stations of two colonies reduced to zero in 5–7 months. These termites (O. formosanus, M. mycophagus and Reticulitermes spp.) all make underground nests, therefore accurate assessment of colony elimination (i.e. all termites in the colony killed; after Evans, Reference Evans2010), was difficult (Thorne & Forschler, Reference Thorne and Forschler2000). However, the careful monitoring and complete lack of termite foraging activity is strongly suggestive of colony elimination.
In contrast, none of the colonies baited with imidacloprid was eliminated, possibly none were suppressed. This could be due to reduced palatability of the imidacloprid baits. Various studies on imidacloprid and other neonicotinoid insecticides have reported sub-lethal effects of these insecticides, including disorientation, erratic and unusual behaviours among other effects, especially in honeybees (Bortolotti et al., Reference Bortolotti, Montanari, Marcelino, Medrzycki, Maini and Porrini2003; Yang et al., Reference Yang, Chuang, Chen and Chang2008). Studies on termites had found that imidacloprid can cause cessation of feeding and trophallaxis and mutual grooming (Boucias et al., Reference Boucias, Stokes, Storey and Pendland1996; Tomalski & Vargo, Reference Tomalski and Vargo2005). Even after exposure to small quantities of imidacloprid, exposed workers become ‘confused’ and move erratically until death. Cross et al. (Reference Cross, Maistrello, Henderson, Jones, Zhai and Robinson2002) found that imidacloprid negatively affected foraging Coptotermes formosanus workers. Antennae of treated termites become fixed at right angles to their heads, and the termites displayed abnormal searching patterns. We observed some similar behaviours for M. gilvus in our laboratory trial. In comparison, fipronil did not show any such effects, and the termites showed normal behaviour.
In our present study, M. gilvus workers did not eat the imidacloprid baits, and abandoned bait stations, perhaps as imidacloprid affects the walking ability of termites (Thorne & Breisch, Reference Thorne and Breisch2001; Quarcoo et al., Reference Quarcoo, Appel and Hu2010, Reference Quarcoo, Hu and Appela2012). Hence treated colonies were not eliminated, and therefore imidacloprid is not likely to be an effective bait active ingredient. Previous studies show imidacloprid is an effective soil treatment, with foraging workers acquiring a lethal dose through cuticle contact and absorption instead of by consumption (Parman & Vargo, Reference Parman and Vargo2010; Keefer et al., Reference Keefer, Puckett and Gold2011).
This study demonstrated clearly the colony level effect: that fipronil can eliminate fungus-growing termite colonies, not only suppress foraging activity. Together with large bait station size (>10 litre capacity; Iqbal et al., Reference Iqbal, Wijdedsas and Evans2016b ), low dose fipronil with low density bait matrix (such as toilet paper), we have the essential elements of a successful baiting system for one of the more intractable pests in tropical countries (Harris, Reference Harris1971; Hickin, Reference Hickin1971; Lee et al., Reference Lee, Vongkaluang and Lenz2007; Rouland-Lefèvre, Reference Rouland-Lefèvre, Bignell, Roisin and Lo2011; Iqbal & Saeed, Reference Iqbal and Saeed2013). Furthermore, the components of our baiting system are inexpensive, which should allow a greater uptake in developing countries in the tropical latitudes. Most estimates of the cost of termites as pests come from wealthier countries in temperate latitudes; which gives a false impression that termite damage is more important in these countries. In fact termites are far more important pests in tropical developing countries in relative cost terms; our low cost baiting system is likely to aid pest control at a more affordable price, with a lower cost to the environment as well.
Acknowledgments
The authors thank Jason Nash of Bayer CropScience Singapore for donating the insecticides, and the Singapore National Parks and Singapore Botanical Gardens for granting permission and providing manpower for the study. NI was supported by the Higher Education Commission, Pakistan.






