Drilling operations of petroleum wells employ drilling fluids. These fluids are responsible for facilitating the transportation of cuttings from inside the wells to the surface, for cooling down the drills and for applying hydrostatic pressure on the formation around the well, among other functions (Bourgoyne et al., Reference Bourgoyne, Millheim, Chenevert and Young1991). Some additives that constitute the drilling fluids provide them with specific features that facilitate the drilling operation and grant stability. Among these additives are viscosity-enhancing agents (American Society of Mechanical Engineers, 2005), filtrate reducers (Bourgoyne et al., Reference Bourgoyne, Millheim, Chenevert and Young1991), bactericides (Fink, Reference Fink2015) and thickeners (Fink, Reference Fink2015). Two clay minerals are used as additives with viscosity-enhancing properties, namely bentonite (Chemeda et al., Reference Chemeda, Christidis, Khan, Koutsopoulou, Hatzistamou and Kelessidis2013; Caenn et al., Reference Caenn, Darley and Gray2016) and palygorskite (Christidis et al., Reference Christidis, Katsiki, Pratikakis and Kacandes2010; Chemeda et al., Reference Chemeda, Christidis, Khan, Koutsopoulou, Hatzistamou and Kelessidis2013; Dino & Thompson, Reference Dino and Thompson2013; Caenn et al., Reference Caenn, Darley and Gray2016). Bentonite is used more commonly because of its more effective thixotropy. However, it coagulates and loses efficiency when in contact with saline formations (Akther et al., Reference Akther, Hwang and Lee2008). Palygorskite can thus be more advantageously employed (Caenn et al., Reference Caenn, Darley and Gray2016), as its properties are less affected by the presence of salt (Christidis et al., Reference Christidis, Katsiki, Pratikakis and Kacandes2010; Caenn et al., Reference Caenn, Darley and Gray2016).
Recently, some investigations have reported on the organophilization of palygorskite for application as a viscosity enhancer in oil-based drilling fluids (Zhuang et al., Reference Zhuang, Wu, Zhang, Zhang, Zhang and Liao2017a, Reference Zhuang, Zhang, Jaber, Gao and Peng2017b). Palygorskite, formerly known as attapulgite, is a hydrated magnesium–aluminium phyllosilicate with the general structural formula (Mg,Al)2Si4O10(OH)⋅4(H2O) (Murray, Reference Murray2000). The structure and shape of palygorskite particles are completely different from minerals such as mica. In palygorskite, clustered ribbon-like structures are separated into individual ribbons when mixed with water (Murray, Reference Murray2000). These structures are characterized mainly by moderate layer charges, moderate base-exchange capacities and large specific surface areas (Murray, Reference Murray2000). The rheological properties of palygorskite suspensions depend on the mechanical interference among the long ribbons, which overcome any electrostatic interactions among the particles. For this reason, palygorskite is an excellent suspension agent in salted water (Caenn et al., Reference Caenn, Darley and Gray2016). The acicular habit of palygorskite and sepiolite, which is another clay mineral, also known as ‘meerschaum’, grant them unique colloidal properties, such as resistance to high concentrations of electrolytes (Murray, Reference Murray2006). The lengths of the palygorskite crystals range between 1 and 10 mm and are ~0.01 mm in diameter (Murray, Reference Murray2006).
Neaman & Singer (Reference Neaman and Singer2004) characterized palygorskite samples collected in the Sacalum mine in Mexico and applied them as viscosity enhancers in water-based mud. The rheological properties of the fluids with 3% (w/v) and 5% (w/v) palygorskite were determined and compared with those of other palygorskite samples from Florida and Georgia in the USA, which are well-known industrial clays. The apparent viscosity (AV) of the fluid containing the Sacalum clay was >15 cP with a clay concentration of 5% (w/v). The yield point (YP) of the Sacalum fluid was comparable with those of the Georgia and Florida clay samples at the 5% (w/v) clay concentration. Therefore, it was proposed that the Sacalum fluid could be used in drilling fluids at a concentration of 5% (w/v).
Baltar et al. (Reference Baltar, Luz, Baltar, Oliveira and Bezerra2009) examined three Brazilian palygorskite samples (dark São Pedro, light São Pedro and Boa Vista) applied in drilling fluids. The authors characterized the palygorskite samples and determined the AV of the fluids by varying the salt concentration and pH of the medium. For all of the samples, the AV increased with increasing KCl concentration in the fluid (0–5000 ppm), and the greatest increase in AV (from 14.5 to 20.2 cP) was observed with the dark São Pedro sample. The São Pedro palygorskite dispersions demonstrated a significant increase in AV in an alkaline medium (pH > 8). The AV of the Boa Vista palygorskite dispersion was not affected by variations in pH.
This study investigates the effects of palygorskite (São Pedro mines, Brazil) and sodium chloride concentration on the properties of salted water-based muds, such as AV, plastic viscosity (PV), YP, pH, density and filtrate volume. Traditionally, the literature only presents studies of palygorskite at low-salt concentrations and does not present comparisons with polymeric fluids. Therefore, the study of all of these parameters together is a worthwhile approach.
Materials and methods
Palygorskite samples
The palygorskite samples were collected from São Pedro mines in the state of Piauí (Brazil). The samples were used as received, without further purification. The chemical composition of the palygorskite samples was determined by X-ray fluorescence (XRF-1800, Shimadzu, Japan). The mineralogical composition was determined by X-ray diffraction (XRD-7000, Shimadzu, Japan) using Cu-Kα radiation. The particle-size distribution was determined using laser diffractometry (Cilas 1090, dry mode; size range: 0.1–500 μm).
Preparation of fluids
Seven aqueous salted drilling-fluid samples were prepared (in duplicate). The first sample was a standard fluid that is already used in the industry. Table 1 shows the functions and concentrations of all of the chemicals used in the preparation of this standard drilling fluid. The viscosity of the fluids was enhanced with xanthan gum and low-viscosity carboxymethyl cellulose (CMC) with a degree of substitution of 0.8 (Hughes et al., Reference Hughes, Jones and Houwen1993). Palygorskite (at concentrations of 3.5 g/350 mL and 17.5 g/350 mL) replaced the xanthan gum and the CMC in the remaining samples. The compositions of the palygorskite samples are given in Table 2. In addition, the concentration of NaCl varied (1.75 g/350 mL, 6 g/350 mL and 12 g/350 mL). All of the chemicals were supplied by Petrobras (Brazil).
Table 1. Composition of the standard aqueous salted drilling fluid (standard fluid) treated with polymers.

Table 2. Composition of the palygorskite samples used for the preparation of fluids.

A Hamilton Beach stirrer was used to prepare all of the fluids (American Petroleum Institute, 2010). Upon the addition of each component, as listed in Tables 1 and 2, the fluid was stirred at 17,000 rpm for 10 min. After final preparation, each fluid was aged in a Fann Roller oven (Model 704ES) for 16 h at 180°C. A 316 stainless steel pressure-proof cell was used for high-temperature ageing. After ageing, the properties of each fluid were measured, and prior to each characterization, the fluid was stirred again at 17,000 rpm for 10 min.
Characterization of the drilling-fluid samples
All of the fluid samples were based on the formulation of 350 mL of fluid. According to the recommended practice of the American Petroleum Institute (2009), the parameters of the drilling-fluid samples were measured following standard procedures (American Petroleum Institute, 2010). The AV, PV and YP were measured with a rotational Ofite viscometer (Model 800). The viscosity was then measured at various shear rates (various stirring velocities) at room temperature (~25°C). The pH of each sample was taken initially with a common digital pH meter. The densities of all of the fluids were determined using a Halliburton Service pressurized densimetric balance. An Ofite American Petroleum Institute press filter was used to measure fluid filtrate volumes at 100 psi for 30 min and at room temperature (~25°C).
Results and discussion
Characterization of original palygorskite samples
The XRD trace indicates the presence of palygorskite and quartz and small amounts of kaolinite (Fig. 1).

Fig. 1. XRD trace of the original clay sample.
The chemical composition of palygorskite is listed in Table 3 as weight percentages. The very low CaO content in the sample suggests the absence of calcite, an accessory mineral that is commonly associated with palygorskite (Neaman & Singer, Reference Neaman and Singer2004). Table 4 shows the particle-size distribution of palygorskite, determined by laser diffraction, with a mean diameter of 28.21 μm.
Table 3. Chemical composition of the palygorskite sample from Piauí (Brazil).

LOI = loss on ignition.
Table 4. Particle-size distribution of the palygorskite sample.

a The d (0.1), d (0.5) and d (0.9) parameters signify the points in the size distribution up to and including which 10%, 50% and 90%, respectively, of the total volume of material in the sample is ‘contained’.
Characterization of the fluid samples
Table 5 shows the results of the characterization of the standard fluid and the samples prepared with palygorskite.
Table 5. Rheological properties of the drilling fluidsa.

a Data are mean values with standard deviations in parentheses.
The filtrate volume decreases with increasing palygorskite concentration (e.g. in Table 5, compare the data for fluid-sample pairs 2 + 5, 3 + 6 and 4 + 7 from Table 2 – these show increasing palygorskite concentrations with the same concentration of NaCl). Palygorskite and sepiolite particles do not flocculate because of the hindered settling of the elongated crystals; therefore, they maintain a constant volume that prevents permeability (Murray, Reference Murray2000, Reference Murray2006). The pH and density of all of the prepared samples were affected slightly by changes in salinity and palygorskite concentration (Table 5).
The YP is related to electrochemical forces, especially the attractive force in the drilling fluid system, and it represents the strength of the fluid structure while in flow (Zhang et al., Reference Zhang, Li, Liu, Li, Guo, Cui and Zhou2016). The YP value is crucial for cutting removal during drilling operations, as drilling fluids with high YP values have optimal wellbore cleaning capabilities. In drilling applications, keeping YP at an appropriate level is critical to preventing the occurrence of a washing wall and to limiting damage to the stability of the borehole (Zhang et al., Reference Zhang, Li, Liu, Li, Guo, Cui and Zhou2016). According to the rheological requirement for well drilling fluids, the YP/PV ratio should be in the range of 0.25–3.00 or 0.75–1.00, while the optimum ratio is 0.36–0.48 (Zhang et al., Reference Zhang, Li, Liu, Li, Guo, Cui and Zhou2016). All of the fluids prepared with palygorskite had low flow resistance and adequate rheological properties.
According to the American Petroleum Institute (2010) Specification 13A, drilling fluids must have a minimal AV of 15 cP. All of the fluids complied with this regulation (Table 5). However, fluids 5 and 7 presented the greatest AV values. Previous work (Baltar et al. Reference Baltar, Luz, Baltar, Oliveira and Bezerra2009) has shown that the dark São Pedro palygorskite, with a concentration of 5% (w/v) with 5000 ppm salinity (1.75 g/350 mL), produced fluid with the best performance and an AV of 20 cP. In the present study, a greater AV (35.5 cP) was obtained even with the high-salt concentration (12 g/350 mL).
Changes in the AV as a function of salinity are given in Fig. 2 (for the palygorskite concentration of 3.5 g/350 mL) and Fig. 3 (for the palygorskite concentration of 17.5 g/350 mL). When the concentration of palygorskite is low (Fig. 2), the amount of NaCl affected the rheological properties of the fluid greatly. A greater AV was obtained only at the low-salt concentration (1.75 g/350 mL). At greater palygorskite contents, there was practically no change in AV with increasing salt concentration (Fig. 3). Even at the NaCl concentration of 12 g/350 mL, the AV was adequate (35.5 cP; cf. fluid 7 with 5% palygorskite).
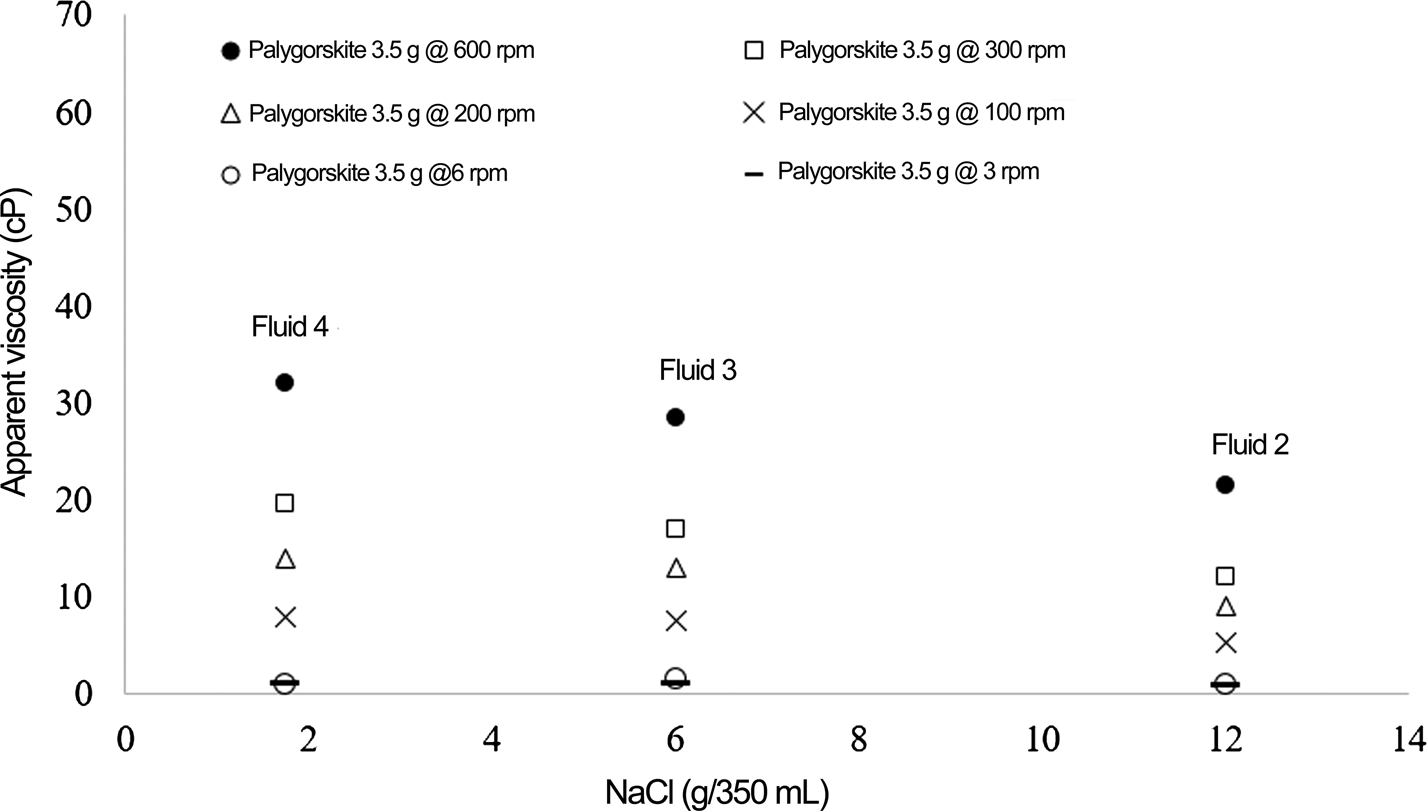
Fig. 2. AV as a function of salt concentration for drilling fluid samples with 3.5 g palygorskite/350 mL.

Fig. 3. AV as a function of salt concentration for drilling fluid samples with 17.5 g palygorskite/350 mL.
Sodium cations may enhance shrinkage of the diffuse double layers when adsorbed in the channels of the palygorskite mineral particles (Zhang et al., Reference Zhang, Li, Liu, Li, Guo, Cui and Zhou2016). Such a phenomenon further promotes face-to-face aggregation, thereby increasing the AV of the fluid. However, the results of the present study show that the sodium cations can only enhance AV when the concentration of palygorskite is low. There would not be sufficient palygorskite channels available for interactions when the salt concentration is high. On the other hand, with greater clay concentrations, few effects on viscosity were observed. As the salt concentration increased, the AV initially decreased, before increasing thereafter as more channels become available for the Na+ cations. In this case, the elongated particles may be more effectively clustered, therefore enhancing the AV.
Conclusions
Palygorskite is an efficient viscosity enhancer for salted water-based mud. When it is used in relatively high concentrations, the fluid properties are not affected significantly by inorganic salts. In this study, drilling-fluid samples were prepared by replacing typical compounds such as xanthan gum and CMC with palygorskite. The AV values of all of the synthetic samples were >15 cP. Analyses of the samples demonstrate that better results are obtained as the concentration of palygorskite increases, as indicated by the optimized AV and filtrate loss volume values. Palygorskite is able to further enhance the viscosity of drilling fluids at very high salt concentrations (approximately six times greater than reported previously). This is an important contribution to the field as it highlights the advantages of using palygorskite in drilling operations.










