INTRODUCTION
Radiotherapy is the use of ionising radiation to treat illness and is an important treatment for cancer. 1 Within the United Kingdom, ~30,000 patients undergo pelvic radiotherapy each year. 1 The most common physical side effects occurring among those undergoing pelvic radiotherapy are gastrointestinal symptoms.Reference Birgisson, Pahlman, Gunnarsson and Glimelius 2 , Reference Henson, Burden, Davidson and Lal 3 These include diarrhoea or constipation, abdominal pain, nausea, steatorrhoea, bloating and weight loss, negatively affecting patients’ quality of life (QOL).Reference Andreyev 4 – 7
The challenge of radiotherapy is to ensure an adequate dose of radiation is delivered to the treatment area, while sparing normal non-cancerous tissue to avoid side effects.Reference Bentzen and Baumann 8 Radiation damage occurs in cells undergoing mitosis resulting in apoptosis, autophagy, senescence and necrosis, all activated to different extents in different tissues and genetically controlled leading to an inflammatory process resulting in side effects.Reference Wouters 9 Cells with a quicker turnover are more vulnerable to the effects of radiation. With the intestinal mucosa repopulating its cells every 5 days, it is vulnerable to radiation damage with gastrointestinal symptoms usually beginning during the second week of treatment.Reference Del Fabbro, Demark-wahnefried and Baracos 10 Chemotherapy also damages the rapidly turning over gastrointestinal tract epithelium, further predisposing patients to gastrointestinal side effects.Reference Beck, Wong and Li 11 The inflammatory response of the gastrointestine to irradiation flattens the intestinal microvilli decreasing enzymatic activity, absorptive surface area and total gut transit time leading to pelvic symptoms and malabsorption of nutrients.Reference Yeoh, Horowitz and Russo 12 – Reference DeWitt and Hegazi 14
Dietary support is one approach in managing some of these patients’ symptoms and is non-invasive and low cost. 15 However, despite this, the current nutritional advice for managing bowel side effects following pelvic radiotherapy is inconsistent and lacks standardised guidelines.Reference McGough, Baldwin, Frost and Andreyev 6 , Reference Henson, Andreyev, Symonds, Peel, Swindell and Davidson 16 Nutritional interventions suggested in the literature include reducing fibre, fat and lactose, and administering probiotics for managing symptoms on the premise that malabsorption of fat, lactose, carbohydrate and small bowel bacterial overgrowth occurs following radiotherapy.Reference Andreyev 4 , Reference McGough, Baldwin, Frost and Andreyev 6 , Reference Webb, Brooke and De Silva 17 , Reference Wedlake, Shaw, Whelan and Andreyev 18
Following an electronic search of databases, no studies were identified that investigated the current dietary advice provided clinically to patients who suffer from gastrointestinal symptoms following radiotherapy. Therefore, it is unknown if the needs of these patients are being met. In the absence of nutrition advice, patients may implement their own coping strategies including decreasing dietary intake or self-imposing restricted diets potentially leading to a nutritionally inadequate diet.Reference DeWitt and Hegazi 14 Minimising food restriction is important because malnutrition is an adverse prognostic factor in most cancers and as up to 33% of patients are malnourished at the start of pelvic radiotherapy minimising the progression of this is important.Reference McGough, Baldwin, Frost and Andreyev 6
In order to develop practical guidance on the use of nutrition support it is important to know what nutritional support is being provided to those who have undergone pelvic radiotherapy. Therefore, the overall aim of this study was to investigate patients’ experience regarding the management of gastrointestinal symptoms during pelvic radiotherapy with a particular focus on nutritional management. The objectives were to
-
∙ establish which patients experienced gastrointestinal side effects;
-
∙ evaluate the service provided by heath care providers regarding nutritional advice to patients who have undergone pelvic radiotherapy;
-
∙ evaluate if patients’ dietary intake has been affected by radiotherapy.
Ethical approval
Ethical approval was obtained from the University of Worcester. As this study was deemed a service evaluation it did not require NHS ethical approval. 19 However, permission was granted by the NHS Trust Research and Development department. This research study was undertaken in line with the Data Protection Act 1998 20 , 21 and anonymity was assured through the use of an anonymous questionnaire.
METHODS
A cross-sectional study was undertaken with participants undergoing pelvic radiotherapy in a single NHS Trust, with the use of purposive sampling. A cross-sectional survey was used to investigate a sample that is representative of a population.Reference Denscombe 22 In this instance, patients undergoing pelvic radiotherapy in the same Trust.
A self-completion postal questionnaire was designed to meet the study’s overall aim (Appendix A). The questionnaire was developed based on the questionnaires used by Henson et al.,Reference Henson, Andreyev, Symonds, Peel, Swindell and Davidson 16 , Reference Henson 23 the Picker Patient Experience Questionnaire-15Reference Jenkinson, Coulter and Bruster 24 and the patient experience questionnaire,Reference Steine, Finset and Laerum 25 which are reliable and validated measures of patient experience.Reference Jenkinson, Coulter and Bruster 24 , Reference Steine, Finset and Laerum 25
The questionnaire was not piloted among those representing the study population due to the likely small sample size and time constraints, however, it was developed and piloted among those with a background in radiotherapy and nutrition to identify any potential problems.
Potential participants were selected according to meeting the inclusion and exclusion criteria.
Inclusion criteria:
-
∙ All patients over 18 years undergoing radical pelvic radiotherapy.
-
∙ Patients willing to participate.
-
∙ Ability to read and write in English.
Exclusion criteria:
-
∙ Those undergoing palliative treatment.
-
∙ Those unwilling to participate.
-
∙ Those considered inappropriate for inclusion by clinical oncology consultant.
-
∙ Age <18 years.
-
∙ Patients unable to complete patient-reported questionnaires.
The principal investigator approached all participants meeting the inclusion criteria towards the end of their treatment outlining the research and why they have been asked to participate. This was carried out from the October 2015 to January 2016. Participants were provided with a pack including the questionnaire, participant information leaflet and stamped addressed envelope. Consent was implied by the return of the questionnaire. During the time of data collection, 65 patients were provided with a questionnaire, 31 were returned giving a response rate of 48%.
The responses were coded, entered and analysed using the statistical package SPSS version 16. Descriptive analysis was undertaken to represent data numerically and provide frequencies, percentages, means and standard deviations. To investigate relationships between variables a Fisher’s exact test was used. A p value ≤0·05 was used. Qualitative data obtained from open-ended questions in each questionnaire were summarised in groups and put into tables. The main points are illustrated below using selected coded extracts and quotations.
RESULTS
Table 1 summarises the characteristics of the 31 participants. The majority of respondents were male, having undergone treatment for prostate cancer, with the other respondents having undergone treatment for endometrial, cervical or bowel cancer. Four of the participants had undergone chemotherapy, three of which had bowel cancer and one cervical cancer. Table 2 outlines the reported incidence of gastrointestinal side effects among each cancer group. Only one respondent stated that he did not suffer from any gastrointestinal side effects. No significant relationships were found between age, gender, diagnosis and chemotherapy and the occurrence of gastrointestinal side effects. Although all female patients (n=5) experienced diarrhoea, this was not statistically significant (p=0·07). Three of the four patients who had chemotherapy-experienced diarrhoea, but this was not statistically significant (p=0·639).
Table 1 Participant characteristics
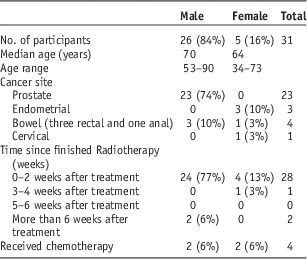
Table 2 Incidence of side effects among respondents

Notes:
a Percentage refers to percentage of those with that cancer.
b Percentage refers to percentage of all patients (n=31).
In total, 18 respondents (58%) made changes to their diet to alleviate bowel symptoms, which involved omitting certain foods, increasing water intake, eating less food and drinking herbal teas. This was a result of experiencing reduced appetite and restricting certain foods which aggravated their symptoms. The following comments are illustrative of this finding.
‘Sometimes not wanting food or just a little’ (66-year-old male with bowel cancer).
‘From week four needed to almost completely remove dietary fibre’ (65-year-old male with prostate cancer).
For the management of diarrhoea, eight (40%) made dietary changes including reducing fibre intake, reducing certain vegetables and caffeine. Of the 24 that suffered with gas 12 (50%) implemented dietary changes with seven stating this helped ease the gas, one stated ‘no’ and four stated ‘a little’. The changes made by patients to alleviate gas and flatulence included drinking herbal teas, reducing fibre, reducing fizzy drinks and omitting pulses from their diet.
‘Reduced fibre, cut out fizzy drinks’ (34-year-old female with cervical cancer), which she stated helped.
‘White bread instead of brown, green veg avoided, tea and coffee avoided, raw fruit avoided, cereals avoided’ (73-year-old female with endometrial cancer), which she stated helped.
One of the objectives of this service evaluation was to evaluate if and what nutritional advice is provided to patients by health care professionals (HCPs). Radiographers were the most frequently cited HCPs from whom patients received dietary information with some respondents stating they got advice regarding management of bowel side effects from a doctor and a nurse.
The dietary advice provided to 13 patients to help alleviate diarrhoea is outlined in Figure 1. Out of the nine respondents who were advised to change their dietary fibre intake six wrote what this advice was, which included both increasing fibre and reducing fibre. The advice provided by HCPs to help alleviate nausea are outlined in Figure 2.

Figure 1 Dietary advice provided by health care professional to alleviate diarrhoea.

Figure 2 Dietary advice provided by health care professional to alleviate nausea.
In total, 17 patients received dietary advice to help alleviate gas. This was a multiple choice question and the dietary advice provided and the frequency of such advice is illustrated in Figure 3. Two received ‘other’ information which was to ‘eat regularly and avoid large meals’ and use ‘peppermint capsules’. Of the six patients who suffered with abdominal pain, four received dietary advice from a HCP who was a radiographer in all cases. The advice provided is shown in Figure 4.

Figure 3 Dietary advice provided by health care professional to alleviate gas.

Figure 4 Dietary advice provided by health care professional to alleviate abdominal pain.
DISCUSSION
Incidence of side effects
In this study, 97% of the participants suffered from gastrointestinal side effects, likewise AndreyevReference Andreyev 26 reports that up to 80% develop early gastrointestinal symptoms. Similar to other studies, gas and flatulence was the most common reported gastrointestinal symptom followed by diarrhoea, abdominal pain and nausea.Reference Andreyev 4 , Reference McGough, Baldwin, Frost and Andreyev 6
Although a small sample (n=4) all of those with gynaecological cancer and 75% (n=3) of those with bowel cancer experienced diarrhoea. As this is a small sample it cannot be assumed that all patients undergoing pelvic radiotherapy for gynaecological and bowel cancer experience diarrhoea. However, with a larger area of the gastrointestine irradiated in those with gynaecological and bowel cancer this further causes gastrointestinal symptoms.Reference Mitchell 27 In addition, those with gynaecological and bowel often receive chemotherapy, as was the case in the current study, and therefore consideration should be made to ensure these patients are closely monitored for side effects and early intervention implemented as appropriate as these side effects can negatively impact patients’ QOL.Reference Gami, Harrington and Blake 5
Dietary interventions implemented by patients
In total, 18 (58%) of the patients in the present study made dietary changes to alleviate bowel symptoms. These findings as listed above are similar to other research.Reference Gami, Harrington and Blake 5 , Reference Wouters 9 , Reference Dunberger, Lind, Steineck, Waldenström, Onelöv and Avall-Lundqvist 28 , Reference Jakobsson, Ekman and Ahlberg 29 Although in the present study the majority of patients stated the dietary changes helped alleviate symptoms one limitation was that respondents could select a number of dietary changes implemented and therefore it was not possible to identify which dietary change improved symptoms.
Dietary advice provided by HCPs
Oncology practice advises a low-fibre diet during the course of radiotherapy to manage gastrointestinal symptoms,Reference McGough, Baldwin, Frost and Andreyev 6 as the findings in the present study show. Although there was some contrast, with some patients advised to increase fibre and others advised to decrease it the majority were advised to reduce fibre intake. Those with cancer receive a wide range of advice from many sources about foods they should eat and avoid, and often this advice is conflicting.Reference Doyle, Kushi and Byers 30 This highlights the need for the development of definitive guidance on the nutritional management of gastrointestinal side arising from radiotherapy.
Although reducing fibre intake is commonly advised to patients, omitting all fibre may worsen patients’ diarrhoea because the intake of dietary fibre can help alleviate diarrhoea by increasing faecal mass and modulating gastrointestinal motility.Reference Roberfroid 31 It has also been shown that increasing soluble fibre intake reduces the incidence and severity of diarrhoea during radiotherapy.Reference Murphy, Stacey, Crook, Thompson and Panetta 32 However, this fibre used was psyllium seed husk, a supplementary soluble fibre and not food soluble fibre. Although psyllium is not routinely provided in the department where this study was undertaken fibre supplements are recommended in the information booklet by Macmillan Cancer Support 33 to manage bowel problems after pelvic radiotherapy and therefore patients may take supplemental soluble fibre as it is readily available over the counter.
During radiotherapy, a pilot study undertaken among 60 patients showed a statistically significant reduction in the incidence of diarrhoea in those who took one to two tsp of psyllium daily.Reference Singh 34
Low-residue diets can be nutritionally inadequate and are not recommended for long periods of time. 35 Soluble fibre is an essential nutrient for gastrointestinal health because it is fermented by colonic microbiota to produce short-chain fatty acids, one of which is butyrate.Reference Hamer, Jonkers, Venema, Vanhoutvin, Troost and Brummer 36 Butyrate has immunemodulatory and anti-inflammatory actions which may negate some of the effects of radiotherapy, suggesting that fibre should not be completely eliminated.Reference Hamer, Jonkers, Venema, Vanhoutvin, Troost and Brummer 36 , Reference Cook and Selin 37 This is illustrated whereby the American Cancer Society 38 does not recommend excluding all fibre but recommends an increase in soluble fibre foods.
Among the respondents, decreasing fibre intake was achieved by decreasing certain vegetables, pulses, fruits and wholegrains. The vegetables most commonly reduced included fibrous and raw vegetables. McGough et al.Reference McGough, Baldwin, Frost and Andreyev 6 from their review concluded that reduced intake of raw vegetables is beneficial in preventing acute gastrointestinal symptoms. This is because these foods can aggravate the lining of the bowel, which has become inflamed following radiotherapy.Reference Wedlake, Thomas, McGough and Andreyev 39
Reducing caffeine, omitting alcohol, increasing water intake and avoiding spicy foods is in agreement with other dietary advice provided to alleviate diarrhoea as they stimulate the bowel. 35 , 38 Ensuring adequate fluid intake is important for those who experience diarrhoea to avoid dehydration and maintain electrolyte balance.Reference Shaw and Taylor 40
As illustrated above reducing dairy and lactose was recommended to participants in this study. Avoiding dairy and lactose for the management of diarrhoea is suggested by many authors.Reference McGough, Baldwin, Frost and Andreyev 6 , 7 , 35 , 38 Although a small sample, three of those who underwent chemotherapy (75%) suffered from diarrhoea. One study illustrated that 5-fluorouracil-based chemotherapy, which is a standard chemotherapy regime for colorectal cancer exacerbated the incidence of diarrhoea and bloating on the premise that these patients may have developed lactose intolerance.Reference Österlund, Ruotsalainen and Peuhkuri 41 Therefore, in clinical practice recommending the avoidance of dairy and or lactose may help alleviate diarrhoea in some individuals undergoing pelvic radiotherapy and/or chemotherapy.
To help alleviate gas and flatulence the advice followed by patients in this study is in line with a typical anti-flatulent diet suggested by other sources. 42 , Reference Oates, Schneider and Lim Joon 43 Including reducing caffeine, avoiding excessive dairy intake, reducing hot and spicy foods, reducing gas-forming vegetables (onions and brassica vegetables) and eating cooked vegetables warm.Reference Oates, Schneider and Lim Joon 43 Oates et al.,Reference Oates, Schneider and Lim Joon 43 although a small study with 30 participants showed that an anti-flatulent diet during radiotherapy reduces rectal volume variability. However, this study also used psyllium husk, which can minimise abdominal distension, gas, and bloating because it delays gastric emptying and reduces the acceleration of colon transit.Reference Singh 34 This finding further highlights the benefit of including soluble fibre in these patients’ diet. However, caution should be taken with patients who have decreased gut motility and/or taking opioid pain medications to prevent intestinal blockage.Reference Stubbe and Valero 44
Although this study focusses on an important area for concern, it has some limitations. First, the majority of patients who were included in this service evaluation had prostate cancer, however, with prostate cancer being the most common cancer among men in the United Kingdom, 45 in a study involving all patients undergoing pelvic radiotherapy it is likely there will be an over representation. In addition, the presentation of gastrointestinal side effects depends on the type of radiotherapy given, the dose given and treatment time,Reference Andreyev 46 therefore it would be beneficial if these treatment details were known.
CONCLUSION
Overall, pelvic radiotherapy affected patients’ dietary intake. The most common dietary advice offered by HCPs and implemented by participants in this study included restricting fibre intake, altering fruit and vegetable intake, increasing water and decreasing caffeine. It is clear that more dietary interventions aimed at decreasing symptoms are needed, especially for the management of diarrhoea and gas and flatulence as these were the most common occurring side effects. Due to the suggested benefits of psyllium seed husk in the management of gastrointestinal side effects further research in this area is needed.
With the findings showing some conflicting dietary advice being provided to patients it is recommended that clear guidelines regarding dietary advice for these patients are developed. With radiographers most frequently giving nutritional advice to these patients they must be supported and provided with guidance regarding clinical and dietary management to support those undergoing pelvic radiotherapy.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Appendix A















