INTRODUCTION
Coccidiosis is a widespread disease caused by several Eimeria species. This apicomplexan parasite is most notable in the chicken and turkey farming industry. Coccidiosis often runs a subclinical course with a negative effect on the performance of farmed poultry and clinical outbreaks of the most pathogenic species can cause significant economic losses. According to the literature, 7 species that infect the turkey host (Meleagris gallopavo) are recognized – E. adenoeides, E. dispersa, E. gallopavonis, E. innocua, E. meleagridis, E. meleagrimitis and E. subrotunda (for review see Chapman, Reference Chapman2008). However, there are doubts about the taxonomic integrity of E. meleagrimitis (Cook et al. Reference Cook, Higuchi, McGowan, Schrader, Withanage and Francis2010) and due to the inconsistency of data in the literature and the lack of molecular characterization, correct species identification can be difficult.
Eimeria adenoeides was first described by Moore and Brown (Reference Moore and Brown1951). It is considered the most pathogenic turkey coccidia and heavy infections can cause 100% mortality in young turkey poults (Clarkson, Reference Clarkson1960). Low doses of oocysts lead to depressed body weight gains and the increasing doses progressively influence severity of infection (Hein, Reference Hein1969). The pathogenic effect of E. adenoeides is greater than that of E. meleagrimitis in birds fed with an equivalent number of oocysts (Clarkson, Reference Clarkson1958, Reference Clarkson1959).
Traditionally, Eimeria species are distinguished by oocyst morphology, pre-patent period, site of infection, pathogenic effects or minimum sporulation time based on the assumption that these parameters are fixed for each species. Oocyst morphology was always considered a reliable discriminating parameter to distinguish between species that differ significantly in oocyst size or shape.
Here we report strain variation in the oocyst morphology of E. adenoeides that is outside the traditional limits of strain variation observed in any Eimeria species. While the oocysts of the E. adenoeides reference strain, KR, are large and ellipsoidal we have found a strain (KCH) whose oocysts are small and ovoid. Other biological parameters including pathogenicity measured as a body weight gain depression after infection were similar. We also confirmed the cross-protection between these 2 strains and compared the sequences of the small ribosomal subunit (18S), the internal transcribed spacer 1 (ITS1) and the mitochondrial cytochrome c oxidase subunit I gene (COI).
MATERIALS AND METHODS
Parasites
Fecal samples were collected during coccidiosis outbreaks at small turkey farms in the Czech Republic where E. adenoeides was suspected according to macroscopic lesion diagnosis. These farms were not using in-feed anticoccidials. Oocysts were isolated using the modified salt flotation method (Long et al. Reference Long, Millard, Joyner and Norton1976) and were stored in 2·5% potassium dichromate at 4°C. Single oocyst isolations were completed with a micromanipulator (Narishige, Japan) that was mounted to an inverted microscope (Nikon, Japan) using glass microcapillaries filled with silicone oil. Single oocysts were selected, aspirated into a micropipette, moved between droplets of PBS and then embedded into agar cubes that were subsequently used for inoculating the turkeys. The isolated strains were passaged in British United Turkeys (BUT) Big 6 turkeys. Commercial vaccines Immucox®-T (Vetech, Canada) and Coccivac®-T (Intervet, USA), both of which contain multiple species of turkey coccidia, served as a source of DNA for comparison with our strains of E. adenoeides.
Biological characterization
The oocyst size and shape of each isolated strain were determined by measuring 100 oocysts under the microscope. The longest and the shortest perpendicular dimensions were recorded, a histogram was generated in Microsoft Excel and the average dimensions were determined. To measure the pre-patent period, turkeys in 2 groups of 5 birds per group were infected at 23 days of age with 10 000 sporulated oocysts per bird with either KR or KCH strain, and feces were collected at 6-h intervals between 0 and 120 h post-infection. Oocysts isolated from feces were counted using the standard McMaster method (Long et al. Reference Long, Millard, Joyner and Norton1976). To examine macroscopic lesions, turkeys at 30 days of age in 2 groups of 5 birds per group were infected with 200 000 sporulated oocysts of either KR or KCH strain and intestinal lesions were inspected 6 days after infection.
Pathogenicity comparison
The pathogenic effect on body weight gain was studied using 17-day-old turkeys arranged into 8 groups of 5 birds per group, which were infected with various doses of sporulated oocysts (5, 10 and 20 thousand) of either KR or KCH strain. The control groups were not infected. Turkeys were individually weighed at days 0, 3, 6 and 9 after infection. Relative body weight gains among groups were compared using the ANOVA and Dunnett's multiple-comparison test with NCSS 2001 software. P<0·05 was considered significant.
Cross-immunization experiment
The first cross-immunization experiment included immunization with the KCH strain and a challenge with the KR strain. In this experiment 45-day-old turkeys arranged into 2 groups of 5 birds per group were either immunized with 500 sporulated oocysts of the KCH strain or not immunized and both groups were challenged 14 days later with 10 000 sporulated oocysts of the KR strain. Single droppings were collected from each bird after massage of the cloaca at days 0, 5 and 6 after the challenge, and the oocysts were counted using the standard McMaster method. Oocyst counts were expressed as the number of oocysts per gramme of feces (OPG). The second cross-immunization experiment included immunization with the KR strain and challenge with the KCH strain. Here the 14-day-old turkeys were again arranged into 2 groups of 5 birds per group, and the birds were either immunized with 1000 sporulated oocysts of the KR strain or not immunized and both groups were challenged 14 days later with 10 000 sporulated oocysts of the KCH strain. The OPG was counted using all feces collected between days 4 and 8 after the challenge. The homologous challenge was tested in a separate experiment where the 10-day-old turkeys were arranged into 4 groups of 5 birds per group. Two groups were immunized with 500 sporulated oocysts of each strain (KR and KCH strains, respectively) and the remaining 2 groups were not immunized. Each group was challenged 20 days later with 50 000 sporulated oocysts of the corresponding homologous strain. Single droppings used for OPG calculation were collected from each bird after massaging of the cloaca 6 days after the challenge.
Sequencing of 18S, ITS1 and COI
Oocysts for isolation of DNA were washed in PBS, disrupted in a Mini-BeadBeater-16 (BioSpec, USA) using 0·5 mm glass beads, and the DNA was extracted from the lysate using the DNeasy Blood & Tissue Kit (Qiagen, Germany) according to the manufacturer's instructions. Full-length small subunit ribosomal DNA was amplified using ERIB1 and ERIB10 primers (Barta et al. Reference Barta, Martin, Liberator, Dashkevicz, Anderson, Feighner, Elbrecht, Perkins-Barrow, Jenkins and Danforth1997) and the ITS1 region was amplified using custom degenerate primers designed according to the comparison of sequences from multiple Eimeria species using Clustal W software (Larkin et al. Reference Larkin, Blackshields, Brown, Chenna, McGettigan, McWilliam, Valentin, Wallace, Wilm, Lopez, Thompson, Gibson and Higgins2007, Table 1). Mitochondrial cytochrome c oxidase subunit I gene was amplified using primers KM204 and KM205 (Schwarz et al. Reference Schwarz, Jenkins, Klopp and Miska2009). PCR reactions to amplify the 18S rDNA and COI gene were carried out using high-fidelity Phusion Hot Start DNA polymerase (Finnzymes, Finland). These reactions contained 200 μM dNTP each, 500 nM forward and reverse primers, 1 μl of template DNA and 0·02 U/μl of Phusion enzyme in Phusion HF buffer. The thermal cycling program for 18S consisted of an initial denaturation at 98°C for 1 min followed by 25 cycles of denaturation at 98°C for 15 s, annealing at 69°C for 20 s and extension at 72°C for 1 min. The program for COI consisted of an initial denaturation at 98°C for 1 min followed by 30 cycles of denaturation at 98°C for 15 s, annealing at 67°C for 20 s and extension at 72°C for 30 s. PCR reactions for ITS1 were carried out using Taq polymerase premixed in 2×Blue Master Mix (Top-Bio, Czech Republic) with 500 nM forward and reverse primers and 1 μl of template DNA. The thermal cycling program for ITS1 consisted of an initial denaturation at 95°C for 1 min followed by 25 cycles of denaturation at 95°C for 15 s, annealing at 55°C for 30 s and extension at 72°C for 1 min. PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Germany). Taq-generated PCR products were blunted and all ribosomal DNA fragments were phoshorylated and cloned into EcoRV digested dephoshorylated pBluescript vector (Stratagene, USA) using home-made (Inoue et al. Reference Inoue, Nojima and Okayama1990) chemically competent DH5α™ cells (Invitrogen, USA). Minipreps were prepared with the QIAprep Spin Miniprep Kit (Qiagen, Germany). Amplified COI genes were sequenced directly from the PCR products using the same PCR primers and the ribosomal targets (18S and ITS1) were sequenced from the purified plasmids (Macrogen, Korea). Eight independent PCR, cloning and sequencing reactions were done for the 18S and ITS1 of both E. adenoeides strains. Two COI PCR reactions from each strain were bidirectionally sequenced. Consensual sequences were manually created in BioEdit software (Hall, Reference Hall2005). Vaccine Immucox®-T was analysed by the sequencing of 48 clones of each 18S and ITS1, and Coccivac®-T was analysed using 96 clones of each 18S and ITS1.
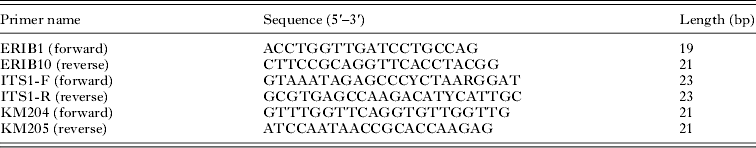
Table 1. Sequences of primers used for amplification of 18S, ITS1 and COI
RESULTS
Isolation and biological characterization of E. adenoeides strains
We have isolated 2 strains of E. adenoeides that differ significantly in oocyst morphology. The strain which has the standard oocyst morphology attributed to this species (large and ellipsoidal) was named E. adenoeides KR and the strain with small and oval oocysts was named E. adenoeides KCH. Size difference was easily seen under the microscope because the average oocyst length of the smaller strain is approximately the width of the larger strain (Fig. 1, Table 2). The pre-patent period was measured to be the same for both strains and it corresponds to the published data about E. adenoeides (Table 2). After experimental infection, both strains of E. adenoeides displayed typical macroscopic lesions for this species, most notably caecal caseous plugs. Pathological signs included caecal deformation and loss of flexibility, ulceration of the tubular part of the caeca, liquid or creamy caecal content with the presence of white caseous and fibrinous necrotic material. There were no notable differences in macroscopic lesions between these two strains.

Fig. 1. Sporulated oocysts of Eimeria adenoeides KR (A) and E. adenoeides KCH (B).
Table 2. Biological characteristics of isolated strains of E. adenoeides

Pathogenicity comparison
Comparison of the pathogenic effect on body weight gain after infection did not reveal any significant difference in pathogenicity between the KR and KCH strains (Fig. 2). The pathogenic effect on body weight gain was clearly dose-dependent at least in the range of 5 to 20 thousand sporulated oocysts used for infection.

Fig. 2. Pathogenicity measured as a weight gain reduction after infection with various doses of Eimeria adenoeides sporulated oocysts.
Cross-immunization experiment
Data from the cross-immunization experiment indicate that the KR and KCH strains of E. adenoeides do induce cross-protection, i.e. immunization of turkey with the one strain protects against a challenge with the other strain. When immunized with the KCH strain, the oocyst output after the challenge with the KR strain was reduced by 95% and 79% on days 5 and 6 post-challenge, respectively. When immunized with the KR strain the oocyst output after the challenge with the KCH strain was reduced by 77% (Fig. 3). In the homologous immunization and challenge experiment the oocyst output was reduced by 98% in both strains (Fig. 4).

Fig. 3. Oocyst output results in experimental groups employing different immunization and challenge strains of Eimeria adenoeides.

Fig. 4. Oocyst output results from the homologous immunization and challenge experiment.
Sequencing of 18S and ITS1 rDNA and COI gene
The sequences of small ribosomal subunits of the KR and KCH strains were found to be almost identical differing in only 2 transversions but the ITS1 sequences share only 77% similarity. The 18S sequences of our E. adenoeides strains share 99·8% similarity to the sequence in GenBank named Eimeria adeneodei (Accession no. AF324212, Zhao and Duszynski 2001, unpublished). We also found the 18S and ITS1 sequences of our KR strain to be present in the Coccivac®-T vaccine and the sequences of KCH strain in the Immucox®-T vaccine. The sequences of 18S from our strains were identical to the sequences in these vaccines and the ITS1 sequences shared more than 99% similarity. The sequences of 18S and ITS1 were deposited into GenBank (Accession nos FR745913 – FR745916). Comparison of our ITS1 sequences with ITS1 sequences from the published work concerning diagnostic PCR (Cook et al. Reference Cook, Higuchi, McGowan, Schrader, Withanage and Francis2010) revealed that while the sequence of the KR strain corresponds to the published E. adenoeides sequence, the sequence of the KCH strain is absent and there is no sequence in that paper with a high homology to this sequence. The mitochondrial COI sequences of our E. adenoeides strains share 97·7% identity at the DNA level and 100% identity at the amino acid level, meaning that all nucleotide substitutions are synonymous. COI sequences of these two strains were also deposited into GenBank (Accession nos FR846201 and FR846202).
DISCUSSION
We have isolated and characterized 2 strains of E. adenoeides that differ remarkably in oocyst size and shape. This is the first description of such a substantial strain variation of oocyst morphology in any Eimeria species. The size of the oocysts was always considered as a fixed and relatively reliable parameter for distinguishing among Eimeria species but our findings indicate that this might not be true for all coccidian species. Although diagnostics according to oocyst morphology is well established in chicken coccidia it seems that such diagnostics will be problematic or even impossible in turkey coccidia.
Although strain variation of oocyst morphology was already described in E. meleagrimitis (Long et al. Reference Long, Millard and Shirley1977) it was not to such an extent as we have observed in E. adenoeides. While the described variability of the length of E. meleagrimitis oocysts was 19% we have encountered a 43% difference in oocyst length between E. adenoeides strains. Such a difference is also readily seen under the microscope and it is easy to mistake the smaller strain of E. adenoeides for E. meleagrimitis which has oocysts of the same size and shape.
We have shown that the other biological parameters of the KR and KCH strains are similar and that their pathogenic effect also does not differ significantly, therefore their virulence (strength) can be considered equal. The cross-protection between the two strains was shown to be very high (77–95%) although not as high as in the homologous challenge experiment (98%), still suggesting, however, that there is no need to include both strains in a live turkey coccidiosis vaccine because immunization with one strain protects against a challenge with another strain.
Sequencing of the 18S ribosomal DNA revealed that both strains share almost the same small subunit sequence, which is also homologous to the sequence in GenBank incorrectly named E. adeneodei. However, sequences of ITS1 differed substantially, which might indicate a distant relation of the two strains. The ITS1 sequence divergence between these two strains is approximately as high as the ITS1 sequence divergence between chicken Eimeria species. Therefore, when looking only at ITS1 these two strains could be considered as two different species but it is known that ITS1 varies strongly both between strains as well as between species, and it is not a reliable marker for inferring phylogenetic relationships of chicken Eimeria (Lew et al. Reference Lew, Anderson, Minchin, Jeston and Jorgensen2003). Moreover, there can be more variants of an internal transcribed spacer present within a single genome and these copies can also differ significantly (Lew et al. Reference Lew, Anderson, Minchin, Jeston and Jorgensen2003; Cantacessi et al. Reference Cantacessi, Riddell, Morris, Doran, Woods, Otranto and Gasser2008; Cook et al. Reference Cook, Higuchi, McGowan, Schrader, Withanage and Francis2010). The recently published diagnostic PCR for turkey coccidia (Cook et al. Reference Cook, Higuchi, McGowan, Schrader, Withanage and Francis2010) that is based on ITS1 sequences includes only sequence homologous to the KR strain, and the alignment of the sequences of primers developed for E. adenoeides to ITS1 of the KCH strain indicates that this PCR will probably fail to detect the KCH strain. Comparison of COI sequences revealed that these genes are translated to the identical protein although the DNA sequences differ by multiple synonymous substitutions. These differences in the DNA sequence might also indicate a relatively distant relation of the two strains. Nevertheless, the identity at amino acid level (100%) is still higher than the identity shared between the closest chicken coccidia species (99·3%). The closest COI amino acid sequences found in chicken coccidia are those of E. tenella and E. necatrix and these sequences differ at 2 amino acid positions.
By analysis of the 18S and ITS1 sequences, which are present in the commercial live virulent turkey coccidia vaccines Immucox®-T and Coccivac®-T, we have revealed that each vaccine contains a different strain of E. adenoeides and that these strains correspond to our KR and KCH strains. Coccivac®-T contains a strain of E. adenoeides that is similar to the KR strain, and Immucox®-T contains a strain similar to the KCH. Furthermore, we have confirmed by microscopy that Immucox®-T does not contain large and ellipsoidal oocysts typical for the KR strain. Such microscopical evaluation was possible since Immucox®-T contains only 2 species (E. adenoeides, E. meleagrimitis), and E. meleagrimitis has small oocysts as well. Owing to the fact that Coccivac®-T contains 4 different species that are similar in oocyst morphology, we could not evaluate the oocyst morphology of an included E. adenoeides strain.
Our findings show that diagnostics of turkey coccidia according to oocyst morphology have to be carried out with caution and the same should be valid for other coccidian species that are much less studied. Currently, the best approach to fast and reliable diagnostics is to develop a PCR-based molecular method, which will cover all strains and species, as was completed for chicken coccidia. However, care should be taken when using polymorphic targets such as ITS1 or ITS2.
ACKNOWLEDGEMENTS
The authors would like to thank Katka Hodacova, Gabina Tylsova, Alena Kudejova and Ruzena Krizikova for their technical support. The authors would also like to give thanks to Dr Petr Bedrnik for his invaluable advice. We would also like to thank Etienne Effenberger (BIOPHARM, Czech Republic) for his critical comments on this paper.
FINANCIAL SUPPORT
This work was supported by BIOPHARM, Research Institute of Biopharmacy and Veterinary Drugs, a.s., Czech Republic.








