Introduction
Congenital aural atresia is characterised by deformity of the auricle and/or external auditory canal, and is often associated with external and middle-ear anomalies, with rare inner-ear and other temporal bone anomalies.Reference De la Cruz, Teufert, Brackmann, Shelton and Arriaga1 This disorder has an incidence of 1.5 in 10 000–15 000, with a male predominance.Reference Qin, Zhang, Dai and Yang2 It is usually an isolated anomaly; however, rarely congenital aural atresia occurs as a part of syndromes such as Goldenhar syndrome, Treacher Collins syndrome and Crouzon syndrome. Certain risk factors have been documented in the literature to be possibly involved in the causation of congenital aural atresia, including low birth weight, higher maternal parity, advanced maternal age, maternal diabetes mellitus, low maternal education, maternal acute illness (rubella), and teratogens such as retinoids, thalidomide, the immunosuppressant mycophenolate mofetil and alcohol.
Patients with congenital aural atresia have hearing impairment present since birth, and this may lead to delayed and impaired language development, especially in case of bilateral and severe affliction, thereby adversely affecting a child's global development.Reference Luquetti, Heike, Hing, Cunningham and Cox3,Reference Kelley and Scholes4
Clinically, in patients with congenital aural atresia, the auricle is usually small in size (known as microtia), or may be completely absent (known as anotia). In addition, the external auditory canal is frequently malformed (stenotic or atretic), which precludes examination of the external and middle ear, and hinders the delineation of abnormalities.Reference Ishimoto, Ito, Karino, Takegoshi, Kaga and Yamasoba5
The management of congenital aural atresia comprises non-surgical (hearing aids) and surgical options, with the choice largely depending on the presence or absence of associated temporal bone anomalies, in addition to other patient factors such as the degree of hearing loss, unilateral or bilateral involvement, and so on.Reference Gao, Wang, Fan, Ai, Zhang and Xue6,Reference Schuknecht7
This study aimed to: obtain a profound knowledge of the temporal bone anatomy, delineate the various temporal bone anomalies in patients with congenital aural atresia, and establish any possible associations of microtia grade with mean hearing loss and other anomalies found on computed tomography (CT).
Materials and methods
This was a cross-sectional study comprising 56 ears of 40 Indian patients with congenital malformation of the auricle and/or external auditory canal, conducted over a one-year period. Relevant clinical details and local examination findings were recorded, including the grade of microtia (grades I–IV based on Marx's classification of microtia,Reference Marx, Henke and Lubarsh8 modified by RogersReference Rogers, Tanzer and Edgerton9) (Table 1), and the status of the external auditory canal and tympanic membrane. Microtic ears were classified into two subgroups: a minor microtia subgroup consisting of ears with grades I and II microtia, and a major microtia subgroup comprising ears with grades III and IV microtia. Auditory function (type and degree of hearing loss) was assessed using pure tone audiometry in children aged over five years and brainstem-evoked response audiometry in children aged less than five years. Ethical clearance was obtained from the institutional ethics committee (approval code: F. NO. 17/IEC/MAMC/2017/RADIO-D11).
Table 1. Distribution of ears according to microtia grade

Grades I–IV based on Marx's classification of microtia,Reference Marx, Henke and Lubarsh8 modified by Rogers.Reference Rogers, Tanzer and Edgerton9 *Total n = 56
All patients underwent high-resolution (non-contrast) CT of the temporal bone using a 128-slice multi-detector CT scanner (Siemens Somatom® Definition Adaptive Scanning (AS+) equipment). Infants and very young children were sedated before the scan. Axial scans through the temporal bone were acquired (voltage, 120 kVp; current, 230 mAs; matrix size, 512 × 512; and pitch, 0.85); these scans were reconstructed in planes parallel to the lateral semi-circular canal, from the top of the petrous apex to the inferior tip of the mastoid bone. The coronal images were reconstructed from the anterior margin of the petrous apex to the posterior margin of the mastoid. Sagittal images and volume-rendered images were also obtained. The images were analysed in terms of the parameters described below.
External auditory canal anomalies were categorised according to Schuknecht's classificationReference Schuknecht7 (Figure 1), with patients having been assessed for the presence or absence of soft tissue within the ear, expansion of the external auditory canal, and scalloping of the external auditory canal wall. In cases of bony atresia, the thickness and status of pneumatisation of the bony atretic plate were noted.
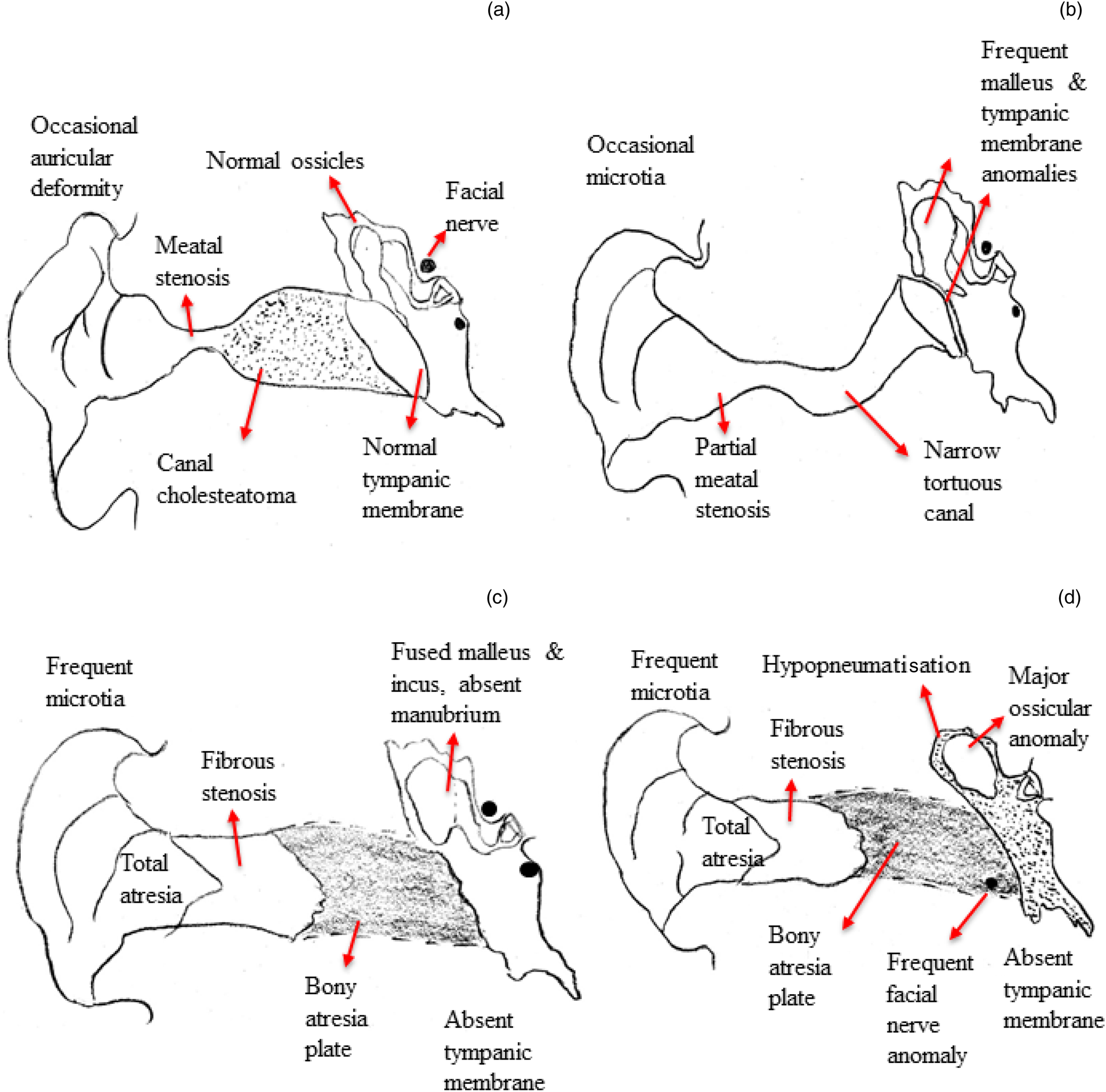
Fig. 1. Classification of external auditory canal anomalies (adapted from SchuknechtReference Schuknecht7): (a) type A – stenosis of cartilaginous external auditory canal; (b) type B – stenosis of cartilaginous and bony external auditory canal; (c) type C – atretic external auditory canal with well-developed pneumatisation of the tympanic cavity; and (d) type D – atretic external auditory canal with reduced pneumatisation of the temporal bone.
The tympanic cavity was evaluated for size. It was measured in the mediolateral dimension, in a coronal plane, from the promontory to the atretic plate, at the level of hypotympanum. The cavity was considered small when it was less than 3 mm. The presence or absence of soft tissue, and ossicular status was also evaluated.
All segments (labyrinthine, tympanic and mastoid segments) of the facial nerve canal were evaluated for size, number and course.
The oval and round windows were evaluated for atresia and stenosis. The oval window size was measured in a coronal image, and the round window size was measured in an oblique reconstructed image reformatted along the posterior semi-circular canal. Stenosis of the oval and round windows was considered when their size was less than 1 mm, whereas atresia was defined as bony obliteration of the oval and round windows.
The inner ear was evaluated for malformations of the cochlea, vestibule, semi-circular canals, internal auditory canal, and vestibular and cochlear aqueducts.
Mastoid air cells were assessed for pneumatisation. Mastoid pneumatisation was classified as: well-pneumatised, hyper-pneumatised, diploic, sclerotic or mixed. The mastoid was considered: (1) well-pneumatised when the antrum was well-aerated, with at least some extension into the mastoid; (2) hyper-pneumatised when the aeration extended beyond the mastoid, to the petrous apex and squamous part of the temporal bone; (3) diploic when it had a diploic bone texture, with no air cells; (4) sclerotic when it had an increased density, with an absence of air cells; and (5) to have a mixed pattern of pneumatisation when it consisted of a combination of pneumatic, diploic and sclerotic pneumatisation types.
The images were searched to identify the possible presence of various carotid canal and jugular bulb vascular anomalies. The jugular bulb was assessed for position, morphology and size. The jugular bulb was considered high-riding when it reached to the level of the internal auditory canal floor, the inferior rim of the round window niche, the external auditory canal floor or the basal turn of the cochlea. A jugular bulb larger than 1 cm was considered a mega jugular bulb.
The temporomandibular joint was assessed to examine the condylar process and articular fossa morphology.
The tegmen mastoideum was assessed for inferior displacement and to determine the degree of displacement. Inferior displacement of the mastoid tegmen was considered mild when it was 25–50 per cent of the middle-ear height (i.e. the vertical distance between the roof of the epitympanum and the inferior aspect of the hypotympanum), and was considered severe when it was more than 50 per cent of the middle-ear height.
Scoring was carried out using the Jahrsdoerfer grading scale (designed to determine candidacy for surgery in patients with congenital aural atresia),Reference Jahrsdoerfer, Yeakley, Aquilar, Cole and Gray10 based on nine high-resolution CT scan parameters, namely: normal stapes, open oval window, middle-ear space, facial nerve position, malleus–incus complex, mastoid pneumatisation, incus–stapes connection, open round window and external ear appearance. Each parameter was assigned 1 point, except for the stapes which was allotted 2 points, totalling 10 points.
Statistical analysis
Data were collected and entered into a pre-designed proforma. The data were analysed and statistically evaluated using SPSS software, version 17 (SPSS, Chicago, Illinois, USA). High-resolution CT imaging findings were expressed in the form of percentages. For qualitative variables, the chi-square test was used for analysis. For quantitative data, the student's t-test was used for two-group comparisons, and analysis of variance followed by a post-hoc test was used for comparisons of more than two groups. A p-value of less than 0.05 was considered statistically significant.
Results
In our study, congenital aural atresia was seen to have a male predominance, with a male to female ratio of 2.07:1. Patients’ age ranged from 6 days to 36 years. Most of the patients (32.5 per cent) belonged to the age group of zero to five years. Among the 40 patients with microtia, unilateral deformity of the auricle was seen in 24 patients (60 per cent), while bilateral deformity was present in 16 (40 per cent); hence, the total number of ears in our study was 56. Among the unilaterally affected ears, congenital aural atresia was predominant on the right side (40 per cent).
Mean hearing loss was 62.47 dB in the minor microtia subgroup and 62.37 dB in the major microtia subgroup. No statistically significant association was found between microtia subgroup and mean hearing loss.
Auricular deformities were graded according to the Marx classification,Reference Marx, Henke and Lubarsh8 modified by RogersReference Rogers, Tanzer and Edgerton9 (Figure 2 and Table 1). Grade III microtia was the most commonly encountered auricular deformity in our study (39.2 per cent of ears). Thirty out of 56 ears (53.6 per cent) were in the minor microtia subgroup, while 26 out of 56 ears (46.4 per cent) were classified as having major microtia.

Fig. 2. Grades of microtia. (a) Grade I microtia – photograph of 18-year-old female demonstrating small right auricle; however, different parts of the auricle are recognisable. (b) Grade II microtia – photograph of 31-year-old female demonstrating underdeveloped upper half of left auricle (absence of upper half of helix, anti-helix and triangular fossa). (c) Grade III microtia – photograph of eight-year-old male showing a small remnant of peanut-shaped skin and cartilage instead of left auricle. (d) Grade IV microtia – photograph of same child as in part (c) showing complete absence of right auricle (i.e. anotia).
On high-resolution CT, all but one ear were found to have external auditory canal involvement. The external auditory canal anomalies were categorised according to Schuknecht's classificationReference Schuknecht7 (Table 2). Schuknecht type D was most common external auditory canal anomaly, seen in 37.5 per cent of ears (13.3 per cent in the minor microtia subgroup vs 65.9 per cent in the major subgroup), followed by type B, in 21.4 per cent of ears, all of which belonged to minor microtia subgroup. We found a significant association between type B and D external auditory canal anomalies and microtia subgroup (p < 0.01). External auditory canal cholesteatoma was noted in 20.8 per cent of ears with stenotic external auditory canals and all of these were Schuknecht type A external auditory canals (Figure 3). We found a significant association between external auditory canal cholesteatoma and Schuknecht type A external auditory canals (p = 0.03).

Fig. 3. Stenosis of bony external auditory canal, with dysplastic ossicles and external auditory canal cholesteatoma. (a) Axial high-resolution computed tomography (CT) image of right temporal bone shows dysplastic ossicle with malleo-incudal fusion (yellow arrow). (b) Axial and (c) coronal high-resolution CT images show stenosis of external auditory canal (white arrow), with soft tissue contents (*) filling the entire external auditory canal, causing its expansion in the medial part, suggestive of cholesteatoma. It is leading to erosion of the scutum (arrowhead) in the coronal image (c) and extending to the middle-ear cavity (red arrow).
Table 2. Distribution of ears according to Schuknecht's classificationReference Schuknecht7 of EAC stenosis and atresia

*Indicates a significant finding (p < 0.05). EAC = external auditory canal
The middle ear was involved in all the atretic ears (Table 3). Small sized middle-ear cavities were more commonly seen in the major microtia subgroup (80.8 per cent) than in the minor microtia subgroup (60.0 per cent); however, there was no statistically significant association between these variables. Of the ossicular deformities, the malleus was the most commonly dysplastic ossicle, observed in 76.8 per cent of ears, followed by a dysplastic incus (66 per cent of ears) and dysplastic stapes (17.9 per cent of ears). However, a reverse trend was noted in absent ossicles; specifically, the stapes was the most frequently absent ossicle, in 48.2 per cent of ears, followed by an absent incus (12.5 per cent of ears) and absent malleus (10.7 per cent of ears).
Table 3. Middle-ear findings on high-resolution CT

*Total n = 56. CT = computed tomography
The malleus bar,Reference Carfrae, Jahrsdoerfer and Kesser11 defined as the bony bar extending from the manubrium or neck of the malleus to the posterior wall of the tympanic cavity, ear canal or posterior atretic plate, was noted in 7.1 per cent of ears. If not recognised and drilled away carefully at the time of middle-ear exploration, this abnormality can cause persistent ossicular fixation and post-operative hearing loss.
The ‘boomerang’ complexReference Mukherjee, Kesser and Raghavan12 is a rare abnormality in which the malleus is hypoplastic, with an absent neck, manubrium and umbo. The incus is the dominant ossicle and is fused to a rudimentary, hypoplastic malleus head to form the boomerang. This abnormality is associated with the absence or hypoplasia of the stapes capitulum, and ossicular discontinuity, with a fibrous attachment of the dysplastic incus to the tympanic segment of the facial nerve canal. This deformity most often requires partial ossicular replacement prosthesis reconstruction because of incudostapedial joint discontinuity. Hence, presence of the boomerang complex mandates a careful search for these associated anomalies and communication to the referring surgeon to ensure appropriate management. In our study, the boomerang complex was found in two ears (Figure 4). Ossicular discontinuity and attachment of a dysplastic incus to the tympanic segment of facial nerve was noted in one ear. In the second ear with a boomerang complex, the stapes was normal and there was no ossicular discontinuity.
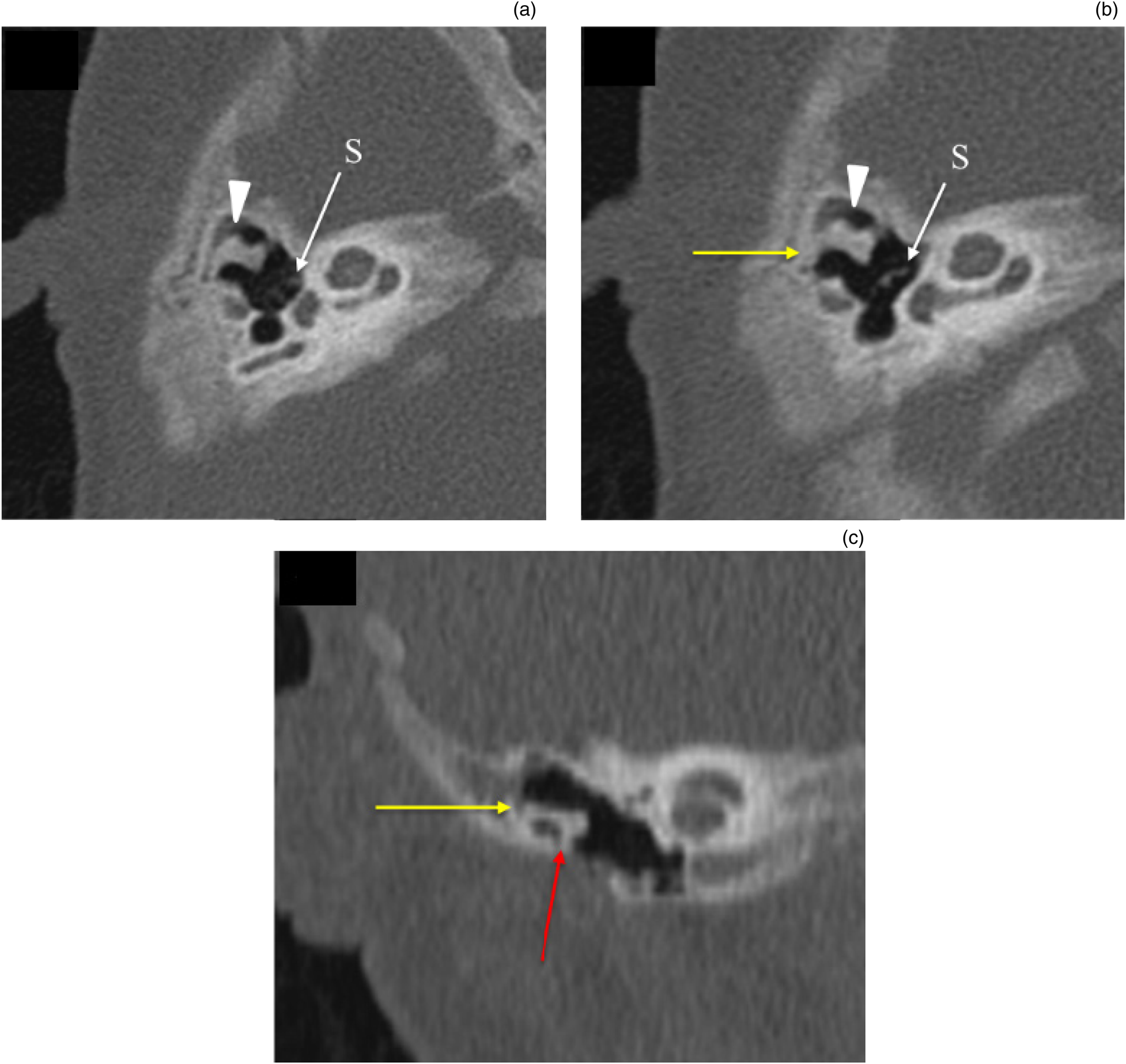
Fig. 4. ‘Boomerang’ complex with ossicular fixation. (a & b) Axial and (c) coronal high-resolution computed tomography images of the right temporal bone show dysplastic malleus and incus with malleo-incudal fusion giving the boomerang appearance (arrowhead in parts (a) and (b)). Stapes (S) appear normal. Body of incus (yellow arrow in parts (b) and (c)) and neck of malleus (red arrow in part (c)) are fixed to the epitympanic wall.
Anomalies of the facial nerve canal were very common in our study group (Table 4 and Figure 5), observed in 96.4 per cent of ears. The mastoid segment was the most commonly aberrant segment of the facial nerve, seen in 90.1 per cent of ears. The most common abnormality encountered was anterior displacement, seen in 60.7 per cent of ears. We found a significant association between anomalies of the mastoid segment of the facial nerve and microtia subgroup (p < 0.05). Other abnormalities found were: anterior displacement of the second genu, noted in 11 ears; duplication, in 4 ears; triplication, in 2 ears; dilatation of the facial nerve canal, in 4 ears; lateral displacement of the nerve canal, in 2 ears; and the exiting of the facial nerve canal through the atretic plate, in 3 ears.

Fig. 5. Duplication of tympanic and mastoid segments of facial nerve. (a & b) Axial high-resolution computed tomography (CT) images of right temporal bone show normal labyrinthine segment (Fl in part (a)) of facial nerve canal, with duplication of tympanic segment of facial nerve (Ft in part (b)). (c & d) Coronal high-resolution CT images (anterior to posterior) show duplication and anterior displacement of mastoid segment of facial nerve (Fm), with one of the duplicated mastoid segments exiting at the level of the oval window (O) in part (c), and the other exiting more posteriorly at the level of the round window (R) in part (d).
Table 4. Facial nerve canal, oval window and round window anomalies on high-resolution CT

*Total n = 56. †Indicates a significant finding (p < 0.05). CT = computed tomography
Abnormalities of the tympanic segment of the facial nerve included cases of the canal: overlying the oval window, in 14 ears; lying just lateral to the stapes, in 6 ears; foreshortened, in 12 ears; duplicated, in 5 ears; and passing through the middle-ear cavity, in 1 ear. A few ears showed a combination of these anomalies. Anomalies of the tympanic segment were more frequent in the major microtia subgroup (96.2 per cent) than in the minor subgroup (80.0 per cent); however, no significant association was found (Table 4).
The labyrinthine segment of the facial nerve canal was normal in the majority of ears (91.1 per cent). An aberrant number, size or course of the labyrinthine segment of the facial nerve canal was noted in only 8.9 per cent of ears, including an aberrant course of the labyrinthine segment in two ears, duplication in one ear, stenosis of the facial nerve canal in one ear and dilatation in one ear.
Anomalies of the oval window were more common (50 per cent) than those of the round window (25 per cent). Anomalies included stenosis and atresia of the oval and round windows (Table 4, and Figures 6 and 7).

Fig. 6. Stenotic and atretic oval windows. Coronal high-resolution computed tomography images of (a) right temporal bone showing stenotic (less than 1 mm) oval window (white arrow), and (b) left temporal bone in another patient showing complete bony obliteration of oval window suggestive of atretic oval window (yellow arrow).

Fig. 7. Stenotic and atretic round windows. (a) Axial and (b) oblique reconstructed (reconstructed along the plane parallel to the posterior semi-circular canal) high-resolution computed tomography (CT) images of the right temporal bone showing stenotic (less than 1 mm) round window (white arrow). (c) Axial and (d) oblique reconstructed high-resolution CT images of the left temporal bone in another patient showing atretic round window (yellow arrow), not opening in the middle ear (*).
Inner-ear anomalies were noted in 23.2 per cent of ears (Figure 8). The most common abnormality found was vestibule–lateral semi-circular canal dysplasia (10.7 per cent of ears), followed by cochlear hypoplasia, posterior semi-circular canal dysplasia, and duplication of the internal auditory canal with vestibule–lateral semi-circular canal dysplasia, noted in 3.6 per cent of ears each, and cochlear hypoplasia with vestibule–lateral semi-circular canal dysplasia, in 1.7 per cent of ears.

Fig. 8. Associated inner-ear anomalies (dysplastic vestibule–lateral semi-circular canal). (a & b) Axial high-resolution computed tomography images of the right temporal bone showing dysplastic vestibule fused with dysplastic lateral semi-circular canal (arrow in part (a)) and bony atresia of the external auditory canal (arrowhead in part (b)).
The diploic mastoid was the most common pattern of mastoid pneumatisation, found in 46.4 per cent of ears; this was followed by well-pneumatised mastoid air cells in 26.8 per cent of ears, sclerotic pneumatisation in 12.5 per cent of ears and a mixed pattern in 10.8 per cent of ears (Figure 9). No significant association was found between microtia subgroup and mastoid pneumatisation.
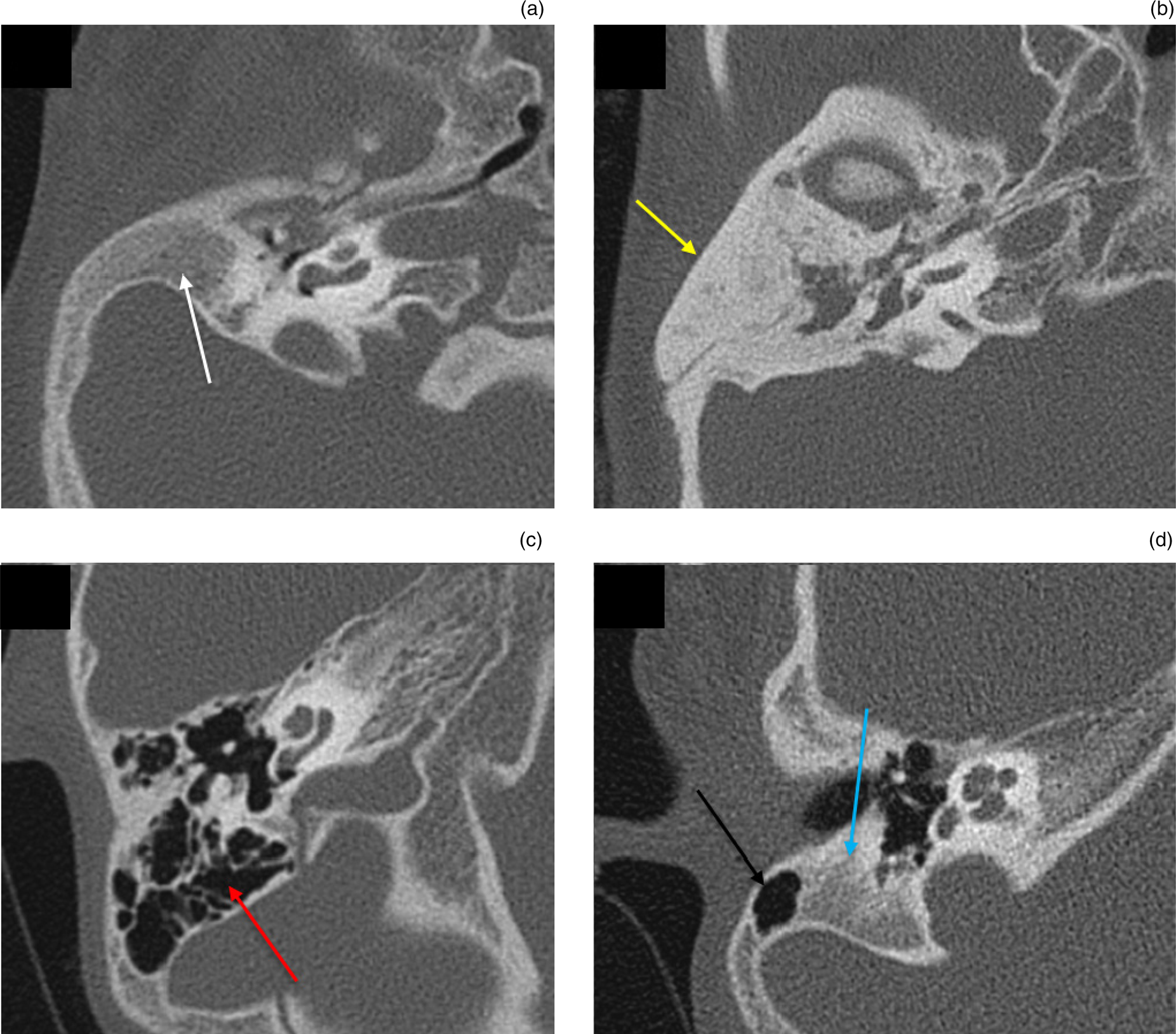
Fig. 9. Patterns of mastoid pneumatisation. (a) Diploic mastoid: axial high-resolution computed tomography (CT) image of the right temporal bone showing hypoplastic diploic mastoid (white arrow). (b) Sclerotic mastoid: axial high-resolution CT image of the right temporal bone showing sclerosis of mastoid air cells (yellow arrow). (c) Pneumatised mastoid: axial high-resolution CT image of the right temporal bone showing well-pneumatised mastoid air cells (red arrow). (d) Mixed mastoid: axial high-resolution CT image of the right temporal bone showing partially pneumatic (black arrow) and partially diploic (blue arrow) mastoid.
Anomalies of vascular structures, namely the carotid canal and jugular bulb, were observed in 28.6 per cent of ears, with the most common being a high-riding jugular bulb, noted in 23.2 per cent of ears. A dysplastic temporomandibular joint was noted in 23.2 per cent of ears (Figure 10).

Fig. 10. Associated dysplastic temporomandibular joint. (a) Coronal high-resolution computed tomography and (b) volume-rendered images of the temporal bone in a patient with right-sided microtia show a dysplastic condylar head (white arrow) and mandibular fossa (yellow arrow) on the right side.
The ears with congenital aural atresia were evaluated using the Jahrsdoerfer scoring system, which evaluates the candidacy for surgery. The majority of ears (60.7 per cent) were poor candidates for surgery, scoring 5 points or less. Of the ears, 10.7 per cent fell into the very good category, scoring 9 points, while 8.9 per cent were categorised as good for surgery, scoring 8 points; 12.5 per cent and 7.1 per cent of ears fell into the fair and marginal categories, scoring 7 and 6 points respectively.
There were other systemic findings in five patients with congenital aural atresia. Of these, four were syndromic cases, including two cases of hemifacial microsomia, and one case each of Goldenhar syndrome and Pearl syndrome.
The associations between mean hearing loss and microtia subgroup, external auditory canal anomalies (Schuknecht classification) and Jahrsdoerfer scoring system were also analysed. However, no statistically significant associations were found (Table 5).
Table 5. Association of mean hearing loss with microtia subgroup, Schuknecht classification and Jahrsdoerfer score

SD = standard deviation
We studied the association of hearing loss with the subtotal points for the five parameters of the Jahrsdoerfer CT scoring system. We found no significant associations of hearing loss with low and high scores in any of these parameters, in either the stenosis (including Schuknecht type A and B external auditory canals) or atresia (including Schuknecht type C and D external auditory canals) groups (Table 6).
Table 6. Association of hearing level with Jahrsdoerfer scoring system subtotals,Reference Jahrsdoerfer, Yeakley, Aquilar, Cole and Gray10 for EAC stenosis and atresia cases

EAC = external auditory canal; SD = standard deviation
Discussion
Congenital aural atresia is associated with a number of temporal bone abnormalities that can be explained on the basis of their common embryological development. The development of structures and insult at different embryological ages also indicates that not all the atretic ears are the same in terms of their deformity and severity of affliction.Reference De la Cruz, Teufert, Brackmann, Shelton and Arriaga1,Reference Tasar, Yetiser, Yildirim, Bozlar, Tasar and Saglam13
The management of microtia addresses hearing rehabilitation, for which available options include surgical management (atresiaplasty or canaloplasty), a bone-anchored hearing aid (BAHA) and a Vibrant® Soundbridge™ middle-ear implant system. The choice of management depends upon various factors, such as the degree and type of hearing loss, contralateral ear hearing, associated temporal bone abnormalities on high-resolution CT, and patient expectations. Proper counselling of the parents, special education for atresia patients and preferential seating at the front of school classrooms are some of the important aspects that should be addressed. Children with bilateral congenital aural atresia are managed with a BAHA as soon as possible, to expedite language and speech acquisition. In unilateral and bilateral cases, surgical management is prescribed only after six years of age, as auricular cartilage development and mastoid pneumatisation has taken place by age of six years. The patients, at times, also opt for auricular reconstruction, for cosmetic purposes, in cases of normal hearing and speech development.Reference De la Cruz, Teufert, Brackmann, Shelton and Arriaga1,Reference Gao, Wang, Fan, Ai, Zhang and Xue6,Reference Schuknecht7
Surgery for congenital aural atresia is considered challenging, as congenital aural atresia is associated with a number of temporal bone anomalies. The surgery includes reconstruction of the auricle using skin or cartilage grafts, the formation of a new external auditory canal, and/or reconstruction of the ossicles for hearing restoration, depending upon the status of the external auditory canal and ossicles. The candidacy for surgery is determined by the Jahrsdoerfer scoring system.Reference Jahrsdoerfer, Yeakley, Aquilar, Cole and Gray10 Specifically, a score of 8 or more suggests that the patient is a good candidate for surgery and will have a likely favourable post-operative hearing outcome. A score of 7 indicates a fair outcome, and a score of 6 suggests that the chances of hearing improvement are marginal. A score of 5 or less disqualifies the patients from surgery. It is essential to carry out high-resolution CT in these patients, in addition to clinical and audiological evaluation, in order to delineate various associated anomalies, decide management, and determine the appropriateness and approach for surgery if operative management is contemplated.Reference Gao, Wang, Fan, Ai, Zhang and Xue6,Reference Schuknecht7,Reference Jahrsdoerfer, Yeakley, Aquilar, Cole and Gray10
The auricle and external auditory canal share the same embryological origin, with the auricle developing from the first and second pharyngeal arches, and the external auditory canal arising from the first pharyngeal cleft at about six weeks of gestation. This accounts for the association between external auditory canal abnormalities and microtia patients.Reference Jacob, Gupta, Isaacson, Kutz, Roland and Xi14 External auditory canal atresia is more common than stenosis, and among atretic ears, bony atresia is more common than membranous atresia. Similar conclusions were made in studies by Takegoshi et al.Reference Takegoshi, Kaga, Kikuchi and Ito15 and Swartz and Faerber.Reference Swartz and Faerber16
External auditory canal cholesteatoma was found in 20.8 per cent of ears in our study. A previous study by Cole and JahrsdoerferReference Cole and Jahrsdoerfer17 reported external auditory canal cholesteatoma in 48 per cent of ears with congenital aural stenosis. A study by Casale et al.Reference Casale, Nicholas and Kesser18 reported that 19 per cent of ears with congenital aural stenosis had acquired ear canal cholesteatoma. We found a significant association between external auditory canal cholesteatoma formation and Schuknecht type A (stenotic type) external auditory canals (p = 0.03). Stenosis of the external auditory canal predisposes to entrapment and accumulation of keratinised epithelium in the stenotic external auditory canal, which precipitates the development of cholesteatoma.Reference Casale, Nicholas and Kesser18
A reduced middle-ear cavity size and cholesteatoma in middle-ear cavity are common in congenital aural atresia cases, and were found in 69.6 per cent and 60.7 per cent of ears, respectively, in our study. A reduced middle-ear capacity and the presence of soft tissue hinder the approach for ossiculoplasty, as they limit the space available for the surgeon to work on.Reference Patil, Bhalla, Gupta, Goyal, Vishnubhatla and Ramavat19 Takegoshi et al.Reference Takegoshi, Kaga, Kikuchi and Ito15 found that hypoplasia of the tympanic cavity was present in all patients (18 ears). Patil et al.Reference Patil, Bhalla, Gupta, Goyal, Vishnubhatla and Ramavat19 found that a small capacity mesotympanum was the most common middle-ear anomaly in 84.3 per cent of their patients, and cholesteatoma was reported in 38.4 per cent of patients. A higher occurrence of middle-ear cholesteatoma in our study could be attributed to the small sample size. The majority of the ossicles are derived from the first and second pharyngeal arches at 16–30 weeks, barring the stapes footplate which develops from the otic capsule.Reference Swartz and Faerber16
The course of the facial nerve is frequently altered in atretic ears. The mastoid segment was the most common anomalous facial nerve canal segment in our study (90.1 per cent), followed by the tympanic segment (87.5 per cent) and labyrinthine segment (8.9 per cent). The tympanic segment of the facial nerve often overlaid and concealed the oval window and stapes; this was noted in 25 per cent of ears in our study. The mastoid segment of the facial nerve is usually displaced anteriorly or antero-laterally, as seen in 60.7 per cent of ears in our study, which may hinder a lateral approach to the surgery making it prone to injury. Thus, the position of the facial nerve is a critical factor in determining candidacy for aural atresia repair. Mayer et al.Reference Mayer, Brueckmann, Siegert, Witt and Weerda20 found that labyrinthine segment abnormalities occurred rarely; labyrinthine segment aberration was found in 5 out of 113 patients and hypoplasia in 11 out of 113 patients. Dedhia et al.Reference Dedhia, Yellon, Branstetter and Egloff21 found that in 41 per cent of ears, the facial nerve position was low-lying and would possibly obstruct surgical access to the oval window. Swartz and FaerberReference Swartz and Faerber16 found that the most common facial nerve canal variation was an anteriorly located mastoid segment (53.33 per cent of ears).
The embryological development of the oval window hinges on the development of the facial nerve. The oval window develops from the otic capsule. It has been theorised that migration of the stapes and the impetus of contact by the stapes (at the fourth month of gestation) are necessary steps for oval window development. Alteration in the course of the facial nerve anteriorly and inferiorly causes it to lie between the stapes blastema and oval window, which impedes the normal migration of the stapes and hampers oval window development.Reference Booth, Vezina, Karcher and Dubovsky22 The oval window was found to be atretic in 39.3 per cent of ears in our study and was stenosed in 10.7 per cent. These findings are in agreement with observations by Mayer et al.,Reference Mayer, Brueckmann, Siegert, Witt and Weerda20 who found the oval window to be absent in more than 33 per cent of microtic ears.
Anomalies of the round window were less common; the round window was atretic in 17.9 per cent of ears in our study and stenotic in 7.1 per cent. Mayer et al.Reference Mayer, Brueckmann, Siegert, Witt and Weerda20 reported an absence of the round window in 6 per cent of the microtic ears.
We noted a significant incidence (71.4 per cent) of absent or reduced mastoid air cell pneumatisation in our study. A diploic mastoid was the most common type of pneumatisation, found in 46.4 per cent of ears. Tekes et al.Reference Tekes, Ishman, Baugher, Brown, Lin and Tunkel23 reported mastoid hypo-pneumatisation or underdevelopment in 57.1 per cent of microtic ears.
We found inner-ear anomalies in 23.2 per cent of ears in our study. This incidence is similar to that reported by Vrabec and Lin;Reference Vrabec and Lin24 they noted inner-ear anomalies in 22 per cent of patients with congenital aural atresia. Swartz and FaerberReference Swartz and Faerber16 stated that associated inner-ear abnormalities should not occur in congenital aural atresia; however, in reality they do occur, with frequencies of 11–30 per cent. Although the inner ear develops from the otic capsule at four weeks and not from pharyngeal arches, complete differentiation of the inner ear is influenced by the pharyngeal arches, which could explain these findings.Reference Mayer, Brueckmann, Siegert, Witt and Weerda20
Another finding associated with congenital aural atresia is a dysplastic temporomandibular joint, which can be explained by the embryological development of the mandible from the first branchial arch. This was seen in 23.2 per cent of ears in our study. Mayer et al.Reference Mayer, Brueckmann, Siegert, Witt and Weerda20 found that dysplasia of the mandibular condyle was frequently associated with microtia, more so in the major microtia subgroup (p < 0.001).
• Congenital aural atresia is not restricted to external ear anomalies; it is also associated with various middle-ear and sometimes inner-ear anomalies
• Microtia grade does not always correlate with the severity of associated temporal bone anomalies
• Management strategy requires detailed information on associated temporal bone anomalies, in addition to hearing degree and disease laterality
• High-resolution computed tomography is therefore essential investigation in each congenital aural atresia case
We did not find any statistically significant associations between mean hearing loss and microtia subgroup or Schuknecht classification.
Contrary to previous studies,Reference Takegoshi, Kaga, Kikuchi and Ito15,Reference Mayer, Brueckmann, Siegert, Witt and Weerda20 we found no significant link between the grade of auricular deformity and most associated temporal bone anomalies in our study, except for type B and D external auditory canal anomalies and anomalies of the mastoid segment of the facial nerve canal. Similar results were reported by Patil et al.;Reference Patil, Bhalla, Gupta, Goyal, Vishnubhatla and Ramavat19 those authors found no significant associations between microtia grade and external auditory canal or middle-ear anomalies. This implies that the grade of microtia cannot be used as an accurate criterion to anticipate the presence and severity of associated anomalies, and, hence, cannot be used to further guide the management of such patients. Therefore, high-resolution CT becomes a problem-solving and fundamental investigation, to describe the various anomalies and tailor the management accordingly, in each congenital aural atresia case.
Conclusion
Congenital aural atresia does not merely involve deformities of the auricle and external auditory canal; rather, it reflects a gamut of temporal bone malformations that vary in severity depending upon the time of insult during embryological development. Auricular and external auditory canal abnormalities found on clinical examination preclude otoscopic examination of the external auditory canal; furthermore, they hinder the ability to reliably predict the status and severity of middle- and inner-ear anomalies. Thus, high-resolution CT is a sine qua non for evaluation prior to management in these patients.
Competing interests
None declared


















