INTRODUCTION
Methadone is one of several opiates used in the management of chronic cancer and noncancer pain; others include oxycodone, morphine, fentanyl, hydromorphone, oxymorphone, levorphanol, codeine, and hydrocodone. However, methadone has been hitherto underused in chronic pain management—relegated to a third- or fourth-line therapy—for several reasons, including the stigma associated with methadone use, physician lack of a skill set or knowledge in prescribing the drug, and the recent “black box” warnings by the United States Food and Drug Administration (FDA). Many lay people associate methadone with heroin addiction and therefore find the consideration of methadone for their chronic pain off-putting or even insulting. It is not uncommon for patients to respond with comments such as “I'm not an addict” and patients with a history of chemical dependence may refer to it as “junk.” Other common misconceptions about methadone include the fear that this drug will rot or decay their teeth and bones and negatively affect the liver. Many patients believe the drug to be highly addictive and are therefore reluctant to take it for their pain. Therefore, considerable patient and family education is usually necessary when using methadone in a patient with chronic pain.
Physicians also have been reluctant to prescribe methadone, mostly due to discomfort or lack of familiarity with its use as previously stated, and, in addition, because of the misconception that only physicians in addiction medicine or working in Methadone Maintenance Treatment Programs (MMTPs) can write prescriptions for it. In fact, any licensed physician can write a prescription for methadone to treat pain.
Clinicians treating patients with chronic pain are recognizing its important role in both chronic pain management and in the setting of palliative care and hospice. For many reasons, methadone is increasingly utilized for patients with refractory or difficult-to-manage pain syndromes. The goal of these guidelines are to assist physicians in treating patients with chronic pain in nonhospital settings (e.g., in nursing homes or in the community) as well as in palliative care settings (e.g., home hospice, hospice facilities, inpatient units). These guidelines will provide both an overview of and specific recommendations for the use of parenteral methadone in pain and palliative care, with attention to the transition from hospital to home/hospice care. The guidelines were developed from a roundtable discussion held on March 3, 2007, in New York City. The 10 panel members included pain and palliative care specialists, an oncologist, pharmacologist, cardiologist, and medical director of a hospice program.
Intravenous Methadone Profile and Delivery Systems
Indications
Methadone is indicated for the treatment of moderate to severe pain incompletely responsive to nonopioid analgesics [Dolophine prescribing information]. In palliative care, these patient populations include those with pain due to cancer, HIV, sickle cell disease, and other life-threatening or chronic illnesses. Other populations, in which methadone use may have particular benefit over other opiates, although the evidence is insufficient, are in patients with a history of opioid addiction, significant opioid tolerance, or in patients with neuropathic pain. In addition, patients who are poorly responsive to other opiates may have improved analgesia with rotation to methadone (Manfredi et al., Reference Manfredi, Borsook and Chandler1997).
Pharmacology
When considering an opiate for managing chronic pain, many pharmacologic and patient factors are considered. Among the pharmacologic factors are half-life, bioavailability, presence of active metabolites, possible nonopioid receptor-mediated effects, and incomplete cross-tolerance (i.e., the partial tolerance to other drugs in the same structural and mechanistic category). Factors intrinsic to the patient include the severity and type of pain, extent of tolerance (and cross-tolerance), age, organ function, unusual or altered half-life, or a genetic polymorphism of opioid receptor genes.
Methadone is a mu receptor agonist with a long half-life (~24 h, ranging from 8 to 90 h), compared with other opioid analgesics, such as morphine (t1/2 2–4 h), hydromorphone (t1/2 2–3 h), or fentanyl (t1/2 4 h); the time required to reach steady-state levels can therefore be much longer than for other opiates. There is enormous interindividual variation in half-life. Onset of analgesia occurs 10–20 min after parenteral administration, and its duration of action is 4–8 h in single-dose studies, which is shorter than its elimination half-life (Payne & Inturrisi, Reference Payne and Inturrisi1985). Moreover, methadone is a lipophilic drug, so it accumulates in tissues with repeated administration. IV methadone has a large volume of distribution (the steady-state volume of distribution ranges between 1.0 to 8.0 l/kg) and is extensively metabolized by N-demethylation and CYP3A4, CYP2B6, and CYP2C19. As a result, coadministration with inducers of these enzymes may result in more rapid methadone metabolism, whereas coadministration with inhibitors of these enzymes may result in reduced methadone metabolism and therefore stronger and prolonged clinical effects of methadone. The inactive metabolites of methadone are excreted in the urine and feces; methadone is almost 90% protein bound.
Methadone's incomplete cross-tolerance with other opiates, as will be discussed below, requires a reduction in dose when rotating from another opiate.
Side Effects
The most frequently observed adverse reactions to methadone include constipation, lightheadedness, dizziness, sedation, nausea, vomiting, and diaphoresis. Side effects are usually dose dependent and not a problem or obstacle if patients are monitored carefully. Overdoses, including excessive sedation and respiratory depression, typically occur with excessively rapid dose escalations during the initial titration phase, prior to reaching steady state. In general, the side effect profile is more prominent when therapy is initiated; once a patient is on a stable opiate analgesic dose, side effects are less bothersome.
Delivery Systems
Methadone can be administered parenterally in the following delivery systems, which allow for patient-controlled analgesia (PCA), continuous and/or intermittent bolus infusion: a Mediport, peripheral intravenous (IV) line, a peripherally inserted central catheter (PICC line), a midline catheter, and a subcutaneous line.
The Mediport is probably the easiest delivery method for use by the nursing staff or family/caregiver managing the infusion. It can last for several months, and multiple lumens are available to administer other medications concomitantly.
A PICC line is another reliable, long-term option, as it may last for several months. In general, most patients do better with a central access IV or a PICC line, as they are safer and last longer, with less chance of dislodging, infection, or infiltration.
An easily inserted peripheral IV line may be used for methadone infusion in an inpatient setting. However, home use is limited by its inherent instability.
The midline catheter is a hybrid of the PICC line and an IV peripheral line. It is 4–in. long and is inserted at the antecubital fossa. It can be inserted at home and is more durable than a peripheral IV line. Because of its high risk of accidental dislodgment, it requires a weekly dressing change by a skilled nurse.
Subcutaneous route of administration is commonly used for medication and fluids in patients with advanced illness. Frequent reasons for using the subcutaneous route include lack of IV access or when a single-lumen central line is being used for an incompatible medication or total parenteral nutrition. Although morphine and hydromorphone subcutaneous administrations have been shown to be tolerated similarly to IV, the use of subcutaneous methadone infusion may be uniquely limited by local erythema and induration in some patients (Morley & Makin, Reference Morley and Makin1998; Mathew & Storey, Reference Mathew and Storey1999; Makin, Reference Makin2000). The exact nature of this local toxicity is unknown. Several small inpatient observational studies successfully used a few strategies to reduce local toxicity of methadone. Rotation of infusion site every 1–2 days or using intermittent boluses versus continuous infusion have kept the local reaction tolerable, except in 2/10 patients with higher intermittent doses, probably by limiting the cumulative amount of methadone per site (Morley & Makin, Reference Morley and Makin1998; Centeno & Vara, Reference Centeno and Vara2005). Addition of dexamethasone 1–2 mg per day to the infusion solution has shown to extend the use of the same site from 2.6 to 4.9 days (Mathew & Storey, Reference Mathew and Storey1999). Possible solution instability with this method may make it unacceptable for home infusion agencies; in this case, a separate dexamethasone injection might be an alternative.
Another reported alternative is injecting hyaluronidase into the infusion site, at a dose of 150 IU single injection (Mathew & Storey, Reference Mathew and Storey1999) or 1500 IU per 20 ml of solution (daily dose not specified; Morley & Makin, Reference Morley and Makin1998). For a given patient, some of these methods may need to be used in conjunction. If the subcutaneous infusion route is chosen for a home-care patient, frequent monitoring of the site by a nurse may be necessary.
The subcutaneous infusion rate should not exceed 2–3 cc per hour, so the concentration may need to be altered to accommodate this rate. The rationale for infusing at the rate of no more than 2–3 cc per hour is that higher rates can cause infiltration of the methadone and painful local edema without adequate analgesia due to poor systemic uptake.
Use of Methadone in Palliative Care
Converting to Methadone
Conversion from other opiates to methadone is one of the most challenging aspects of methadone use. However, there are safe ways to convert patients to IV methadone, when the indication exists. The most common conversion is from morphine. The morphine-to-methadone ratio is typically reported as 1:1 (morphine:methadone), in single-dose studies (Derby et al., Reference Derby, Chin and Portenoy1998). However, the one-to-one conversion does not apply to continuous dosing. The pharmacologic properties of methadone (i.e., extensive bioavailability, long half-life, lipophilicity, and incomplete cross-tolerance) suggest that higher dose ratios are usually necessary (Shaiova, Reference Shaiova2006). Safe rotation to methadone is best practiced when there is close monitoring initially to ensure adequate analgesia and minimal side effects.
The few small reports of conversion from other opiates to IV methadone in the literature bear this out. For example, in a small study of 13 patients with terminally ill cancer switching from morphine to IV methadone, the mean morphine-to-methadone conversion ratio was 5.2, but wide interpatient variability (1.3 to 11) was observed (Auret et al., Reference Auret, Roger Goucke and Ilett2006) In another small report on four patients with cancer converting from IV morphine and hydromorphone to IV methadone, the equianalgesic methadone dose was 3% of the hydromorphone dose. All four patients were able to convert to IV methadone at this low dose with “excellent pain relief without significant side effects” (Manfredi et al., Reference Manfredi, Borsook and Chandler1997). A case report of conversion to IV methadone PCA from IV morphine indicates that the patient was successfully converted when the PCA demand dose was reduced by 33% and the initial infusion was reduced by 50% (Fitzgibbon & Ready, Reference Fitzgibbon and Ready1997). With regard to conversion from IV fentanyl, a small, prospective study of 18 cancer patients with uncontrolled pain using IV fentanyl suggests that a conversion ratio of 25 µg/h IV PCA fentanyl to 0.1 mg/h IV PCA methadone was efficacious to control pain with a reduction in side effects. In this study, mean pain scores decreased from 8.1 to 4.8 on Day 1 after the switch and to 3.22 on Day 4 after the switch. Mean sedation scores were reduced from 1.5 before the switch to 0.44 and 0.16 on Days 1 and 4, respectively. Moreover, of the six patients who experienced confusion while on fentanyl before the switch, five improved within 2 days of the switch (Santiago-Palma et al., Reference Santiago-Palma, Khojainova and Kornick2001). Unfortunately, these studies represent the totality of published literature on conversion to IV methadone from other opiates.
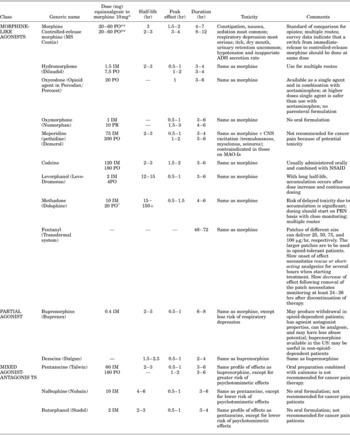
Other published studies and reports demonstrate that rotating from morphine to oral methadone can be successful despite the stylistic variability (Dyer & White, Reference Dyer and White1997; Lawlor et al., Reference Lawlor, Turner and Hanson1998; Ripamonti et al., Reference Ripamonti, De Conno and Groff1998; Mercadante et al., Reference Mercadante, Casuccio and Calderone1999). Most of the rotation methods involve short-acting opiates for breakthrough pain. We offer an equianalgesic dosing table in this guideline to help clinicians calculate the converting doses (Table 1).
Table 1. Equianalgesic dosing: opiates

* Dose that provides analgesia equivalent to 10mg intramuscular morphine. The equianalgesic dose should not be interpreted as the starting, standard, or maximum dose, but rather as a guide particularly useful in switching drugs or changing administration. Depending on patient characteristics and prior opiate exposure, the starting dose can be lower or higher, and dose titration – either upward or downward – is repeatedly necessary in virtually all patients.
** Extensive survey data suggest that the relative potency of IM: PO morphine of 1:6 changes to 1:2–3 with chronic use.
†The starting dose for opioid-naïve patients is 5 to 10mg PO
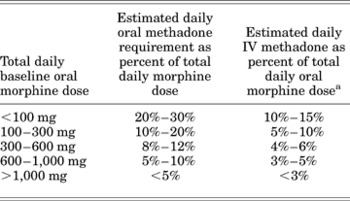
When converting oral methadone to IV methadone, the cumulative dose of oral methadone should be reduced by 50% and infused over 24 h or divided into intermittent doses administered every 6–8 h. A conversion table based on the specific oral morphine dose has also been published (Table 2; Drug Facts & Comparisons, 2007).
Table 2. Conversion table from oral morphine to intravenous methadone for chronic administration
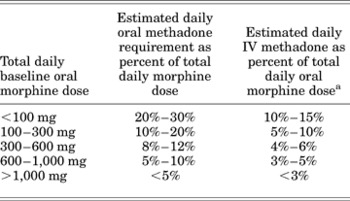
aThe total daily methadone dose derived from the table may then be divided to reflect the intended dosing schedule (i.e., for administration every 8 h, divide the daily methadone dose by 3).
Reproduced with permission from Drug Facts and Comparisons. (2007), p. 1082. St. Louis: Wolters-Kluwer Health.
During the titration stage, while methadone's plasma levels are rising, generous breakthrough dosing should be available, as analgesia from the scheduled dosing may be insufficient.
Safety and Risk Assessment
The most significant risk of IV methadone is the risk of prolongation of the QT interval that can lead to potentially fatal ventricular arrhythmias. The QT interval is recorded on a standard 12-lead electrocardiogram (ECG). It is dependent on heart rate, and after correction for the heart rate, the QT interval is referred to as the corrected QT or QTc. QTc prolongation can lead to a specific type of ventricular fibrillation called torsades de pointes (“twisting of the points” in reference to beat-to-beat change in the QRS axis). This can present as syncope (passing out) or sudden death if not recognized and treated promptly. Prolongation of the QT interval can be missed if measured on single or three-lead ECG. This interval should be recorded manually from the lead that shows the end of the T wave clearly and has the longest QT interval. It is measured from the onset of QRS to the end of the T wave (myocardial depolarization and repolarization), averaged over 3 to 5 beats, and adjusted for heart rate (Garson, Reference Garson1993). The ideal time to record the QT interval would be when peak concentration of the QT-prolonging drug is expected (Anderson et al., Reference Anderson, Al-Khatib and Roden2002). QT prolongation might occur as late as 1 week after initiation of methadone therapy; thus, close monitoring is needed for at least 1 week.
Drug-induced prolongation of the QTc interval is related to blockade of the human ether-a-go-go potassium channel. This blockade leads to inhibition of the outward potassium current during myocardial repolarization and thus longer repolarization time, which is represented on a surface ECG as a prolonged QT interval. Drug-induced QT prolongation is exaggerated in the presence of other causes of long QT interval, such as congenital long QT syndrome, female sex, low left ventricular ejection fraction, myocardial ischemia, slow heart rate, and electrolyte abnormalities including hypokalemia and hypomagnesemia (Roden, Reference Roden2004). Presence of any of these conditions is likely to increase QTc interval prolongation associated with methadone administration. The normal upper limit of QTc is 440 ms in males and 450 ms in females. If the baseline QTc is >450 ms in men and >460 ms in women, in the absence of interventricular conduction defects, all medication with potential of prolonging the QT interval should be avoided (Moss et al., Reference Moss, Zareba and Benhorin2001; Al-Khatib et al., Reference Al-Khatib, LaPointe and Kramer2003). In the case of methadone, as its use can be life saving, absolute QTc is not a contraindication for the use of IV methadone, but should be monitored closely. A more than 10% increase should prompt concern about torsades de pointes. Close monitoring is especially needed in the presence of other risk factors such as history of syncope, family history of unexplained syncope or sudden death, seizures or congenital deafness, history of abnormal potassium and magnesium levels, renal dysfunction, bradycardia, underlying cardiovascular disease, diabetes, old age, female gender, heart failure, hypotension, hypothermia, myocardial ischemia, and pituitary insufficiency. As mentioned earlier, because methadone is metabolized by the CYP3A4 enzyme, other drugs that inhibit this enzyme (Table 3) are likely to increase methadone blood levels and thus QTc prolongation with methadone.
Table 3. Examples of drugs that may provoke life-threatening arrhythmias in patients with prolonged QTc

The incidence of drug-induced torsades de pointes is variable with different groups of drugs, and few data are available about the exact frequency. A recent retrospective study of past and current injectable drug users, hospitalized at a tertiary care center, demonstrated that clinically significant QTc interval prolongation (>500 ms) occurred in more than 16% of patients receiving oral methadone. Among these patients receiving methadone, 3.6% presented with torsades de pointes (Ehret et al., Reference Ehret, Voide and Gex-Fabry2006). Differences in this study between patients on and off oral methadone were not discussed, and although this high incidence of arrhythmias may be important when treating this population, its significance is hard to extrapolate to most patients treated with methadone for pain. In addition, drug–drug interactions involving cytochrome P-450 3A4 inhibitors, hypokalemia, and altered liver function were all important predisposing factors in this cohort. Studies of IV methadone have shown a linear dose response with regard to QTc prolongation, with no floor effects: There was no dose below which QTc prolongation was not observed (Kornick et al., Reference Kornick, Kilborn and Santiago-Palma2003). This led to the U.S. FDA issuing a “black box” warning for methadone:
Deaths, cardiac and respiratory, have been reported during initiation and conversion of pain patients to methadone treatment from treatment with other opioid agonists. It is critical to understand the pharmacokinetics of methadone when converting patients from other opioids (see DOSAGE AND ADMINISTRATION). Particular vigilance is necessary during treatment initiation, during conversion from one opioid to another, and during dose titration.
Respiratory depression is the chief hazard associated with methadone hydrochloride administration. Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects, particularly in the early dosing period. These characteristics can contribute to cases of iatrogenic overdose, particularly during treatment initiation and dose titration.
In addition, cases of QT interval prolongation and serious arrhythmia (torsades de pointes) have been observed during treatment with methadone. Most cases involve patients being treated for pain with large, multiple daily doses of methadone, although cases have been reported in patients receiving doses commonly used for maintenance treatment of opioid addiction.
Methadone treatment for analgesic therapy in patients with acute or chronic pain should only be initiated if the potential analgesic or palliative care benefit of treatment with methadone is considered and outweighs the risks. [Dolophine prescribing information]
However, the U.S. FDA did not distinguish between oral and IV methadone preparations. IV methadone has a significantly greater risk of QT prolongation than oral methadone. This has been attributed to the preservative chlorobutanol in the IV preparation, which has been shown to independently prolong the QTc interval, or by their additive/synergistic effects (Kornick et al., Reference Kornick, Kilborn and Santiago-Palma2003).
Considering the known risk factors for arrhythmia, we propose the following recommendations for ECG monitoring of patients receiving methadone therapy. They are not meant to be exhaustive or binding, as no formula can substitute a judgment in each individual case.
• A screening ECG prior to initiation of therapy
• Repeat after 24 h of initiation of therapy
• When a steady state is achieved, after 4 days of therapy
• When the dose is significantly escalated
• When there is a change in patient's condition or therapy which may further increase the risk of the arrhythmia (i.e., electrolyte imbalance, congestive heart failure, new medications affecting QTc or impair methadone metabolism)
• Electrolytes may need to be monitored in high-risk patients
• In any given patient, a decision of ECG frequency should be adjusted based on the known risk for arrhythmia in that individual.
It is not clear how often the ECG should be performed in an outpatient. A decision to repeat the ECG, however, depends on the patient's condition and individual circumstances; risks versus benefits need to be evaluated in critically ill patients receiving medical care at home. Goals of care are paramount in the palliative care patient.
When conducting the ECG, the same lead should be used to measure the QTc interval each time, and manual measurement should be used. It is advisable to not rely on computerized readings. When prolongation of the QTc interval is observed, the presence of an additional cause (hypokalemia, hypomagnesemia, addition of a QT-prolonging drug, myocardial ischemia) should be excluded. Table 4 provides a summary of the precautions regarding use of IV methadone in palliative care patients.
Table 4. Precautions for use of intravenous methadone in palliative care patients

From Sekine, R., Eugenia, A.M.T., Coyle, N., et al. (Reference Sekine, Eugenia and Coyle2007). The successful use of parenteral methadone in a patient with a prolonged QTc interval. Journal of Pain and Symptom Management, 34, 566–569.
When discussing the risk–benefit ratio of methadone with the patient and family/healthcare proxy, it is important to convey a few key points. Each patient will need individual monitoring during the titration phase and any time deemed appropriate when the dose is escalated. Second, the risk of QTc prolongation and torsades de pointes is very small, but it does exist. However, the risk can be monitored with ECG. It is often reassuring to tell the patient he/she will be followed closely during the first week of treatment and until the dose is stabilized. Also, the risk of QTc prolongation can be put in the context of other methadone side effects, such as drowsiness, nausea, constipation, and dry mouth, which are more common. Family members of patients with cancer, in particular, should also be aware that autonomic failure and unexpected sudden death commonly occur in advanced cancer independent of methadone use. Many cancer patients die during methadone treatment for palliation of pain and the cause of death would never point to methadone (in fact, the patient's ECGs might have been normal prior to death without evidence of QTc prolongation). Ultimately, this risk has to be presented to the patient, and the final decision to begin methadone treatment should be based on a shared-decision model, although in the setting of poorly responsive or refractory cancer pain, there may be no other feasible therapeutic option. The key message from the clinician should be that the benefits of methadone can far outweigh its risks.
Use of Preservative-Free Methadone
In vitro studies showed that the preservative used in IV methadone (chlorobutanol) independently blocks potassium channels, suggesting that it might pose a higher risk for QTc prolongation (Kornick et al., Reference Kornick, Kilborn and Santiago-Palma2003). Thus, in patients receiving IV methadone and experiencing QTc prolongation, for whom methadone has been determined to be the cause, a trial of preservative-free methadone is warranted, provided it is available. Only in high-risk patients should preservative-free methadone be commenced. Some issues with preservative-free methadone are difficulty in obtaining the drug—it needs to be mixed in a “clean” pharmacy room without the presence of other drugs being mixed at the same site or under the same hood. It can easily be contaminated, and the intravenous bag of methadone is relatively unstable and needs to be changed frequently. Also, preservative-free methadone is more costly than methadone with the preservative present. There are no published trials comparing the effect of methadone with and without preservative on QTc. However, clinical experience with patients rotated to preservative-free methadone after QTc prolongation >500 ms was observed, showed a decrease in their QTc interval to an acceptable range.
Lastly, as mentioned earlier, home health agencies typically have strict guidelines regarding the use of preservative-free agents.
Strategy for Optimizing Analgesia with IV Methadone: Use of Patient-Controlled Analgesia
Patient-controlled analgesia (PCA) is a safe and convenient method of administration of methadone or other opioid for patients with severe cancer and noncancer pain. It is used in chronic pain management for severe pain that requires rapid dose escalation. Other reasons for the use of parenteral opioids are poor oral intake, nausea, vomiting, dysphagia, gastrointestinal malabsorption, intense breakthrough pain necessitating IV rescue, mucositis, bowel obstruction, pill burden (the inability to swallow large numbers of pills), and a need for doses too large to be accommodated by the oral route. Patient-controlled analgesia with IV methadone presents several challenges, including higher cost and limited availability of the IV solution in some areas, the required medical expertise for its administration, the existence of nursing guidelines to monitor the patient while he or she is receiving the infusion, and narrow or strict regulations by home health agencies regarding the use of IV methadone.
Although there are no standard guidelines for initiating IV Methadone PCA, the following suggestions may be helpful to ensure that both a sufficient dose is provided in the setting of severe or refractory pain and that a delayed overdose during the titration phase is avoided. A conservative initial continuous infusion (basal rate) should be calculated based on the patient's current opioid requirement. The continuous infusion rate should not be increased during the first 12 h after starting the methadone IV PCA, because both the analgesic and sedative properties have been observed to increase 12 h after initiating or increasing the infusion (Manfredi & Houde, Reference Manfredi and Houde2003). Liberal PCA boluses, roughly equivalent to the hourly infusion rate, can be offered during the titration phase and prior to achieving steady state. Some practitioners prefer to keep the lockout time at 20 or 30 min to avoid accumulation. Adjustment of the basal rate, as with other PCAs, should be based on the additional PCA doses taken over a period of time, usually over the last 8–24 h. Clinician activated bolus (CAB) doses can be given every hour and are typically twice the PCA dose. As previously mentioned, when converting patients from an oral methadone regimen to a methadone PCA, calculate the total daily dose of oral methadone, 50% of that dose is the IV equivalent, then divide by 24 h to establish an hourly infusion rate. When converting from another oral opioid regimen, convert the daily morphine equivalent to IV methadone (Table 2) and then follow the same steps for determining the continuous hourly infusion. Table 5 lists conservative parameters when converting IV infusions of morphine, hydromorphone, or fentanyl to a methadone PCA (Manfredi & Houde, Reference Manfredi and Houde2003). Careful monitoring and clinical assessments will provide the clinician with the necessary information to modify the PCA settings for the particular patient.
Table 5. Suggested safe and effective starting doses when rotating patients from other IV opioids to IV methadone with patient-controlled analgesia

aContinuous hourly infusion. Decrease the initial dose of methadone by 25–50% for high previous opiate doses (eg, 50mg/h of morphine) and increase the dose by 25–50% for low doses (eg, 5mg/h of morphine).
bDose available every 15 minutes by the patient pressing the demand button on the infusion pump.
cClinician-activated bolus: dose administered by nurse upon request if pain persists despite the self administration of demand doses.
Adapted with permission from Manfredi, P.L. & Houde, R.W. (2003) Prescribing methadone, a unique analgesic. Journal of Supportive Oncology, 1, 216–220.
Management and Follow-up
When the decision has been made with the patient, family, or health care proxy to begin IV methadone treatment, a follow-up strategy can be determined. This should take into consideration the previously established goals of care. Monitoring is recommended until a steady state is established and analgesia is obtained. Pain intensity, use of PRN rescue doses (PCA doses, loading doses, and clinician-activated bolus doses), analgesic efficacy, side effects, and level of sedation should be monitored per goals of care. There are patients for whom comfort is the primary goal of care, and thus such vigorous monitoring might be unwanted.
The consideration of IV methadone with or without the preservative chlorobutanol needs to be evaluated based on the disease and overall condition of the patient, with goals of care at the forefront of the decision. A patient with an imminently life-threatening disease with refractory pain may be a candidate for IV methadone, whereas a patient without cancer or life-threatening disease may warrant a trial of other opiates or adjuvant agents before commencing IV methadone. If this fails to provide analgesia and IV methadone is justified, ECGs, as aforementioned, should be ordered per recommendations above (safety and risk assessment section).
Prior to starting IV methadone, the effect of other medications on methadone's metabolism (inducers and inhibitors of the P450 system) should be noted, and coordination of care between providers should be established. One prescribing clinician will limit risk potential in patients at home on parenteral methadone. The patient and caregiver must have 24-h access to either a pain/palliative care service or a hospice team when parenteral methadone therapy is utilized.
Finally, documentation is an important component of successful parenteral methadone treatment for pain. Discussion of the goals of care and risks and benefits of parenteral methadone should be documented. The medical record should reflect analgesia, employing a numerical pain scale or any other validated pain scale. The extent of pain relief and adverse effects, if present, should also be documented. Several key points regarding patient and family education recommendations are listed in Table 6. If ECG and electrolyte monitoring is deemed too burdensome and a decision is made to forgo them, documentation should be explicit.
Table 6. Recommended key points in patient and family education regarding IV methadone

Summary of Recommendations
1. In palliative care, IV methadone should be considered in patients with cancer-related pain syndromes, HIV-related pain, pain in sickle cell disease, and for postoperative pain or acute pain in opiate-tolerant patients. Other populations, in which methadone use may have particular benefit over other opiates, although the evidence is insufficient, are in patients with a history of opioid addiction, significant opioid tolerance, or in patients with neuropathic pain. IV methadone may also be used in end-of-life care with terminally ill patients, with careful consideration to high-risk patients.
2. IV methadone should be administered via PCA with sufficient rescue dosing provided.
3. When converting from other opiates, methadone shows incomplete cross-tolerance, so the opiate dose should be reduced by 75% to 90% of the calculated morphine equivalents; then IV methadone can be titrated with careful monitoring over 24–48 h. This is the safest way to achieve conversion, although analgesia may not be achieved rapidly during this process.
4. The risk of QTc prolongation (and therefore torsades des pointes and sudden cardiac death) should be discussed openly with the patient, family, and health care proxy so that an informed decision can be made. However, it should be noted that the risk is small and close monitoring with ECG can diminish such risk.
5. Use of IV methadone requires frequent monitoring for response to therapy and emergence of any side effects.
6. Patient/family education and careful documentation are crucial.
7. Consideration of burden versus benefit is paramount in treating pain patients. In those with a life-threatening illness, the potential benefit of controlling otherwise refractory pain may far outweigh the risks, even when monitoring for arrhythmia is impractical.