Introduction
Predation and parasitism are important trophic interactions that shape ecological communities and food webs. The key differences between predators and parasites are their relative size compared with their victims (parasite < host and predator > prey), the number of victims made during a life-history stage (one for a parasite, but more than one for a predator; Lafferty and Kuris, Reference Lafferty and Kuris2002) and the duration of the interaction (very short in the case of predator-prey systems and much longer in the case of parasite-host relationships; Dubois et al. Reference Dubois, Savoye, Sauriau, Billy, Martinez and de Montaudouin2009). Additionally, in food webs, predators practically always have a higher trophic position than their prey, while the trophic position of parasites can be more complex. Firstly, parasites with complex life cycles involving multiple hosts may feed on different trophic levels across distinct life cycle stages, making it difficult to determine a single trophic level for all parasite life cycle stages (Lafferty et al. Reference Lafferty, Allesina, Arim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marcogliese, Martinez, Memmott, Marquet, McLaughlin, Mordecai, Pascual, Poulin and Thieltges2008). Secondly, some parasites feed on various host tissues and some may not feed directly on the host at all, but rather on the host's stomach contents or specific pre-digested biochemical compounds (Iken et al. Reference Iken, Brey, Wand, Voigt and Junghans2001; Lafferty et al. Reference Lafferty, Allesina, Arim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marcogliese, Martinez, Memmott, Marquet, McLaughlin, Mordecai, Pascual, Poulin and Thieltges2008). Hence, some endoparasites living inside a host's intestine may not necessarily be true parasites living strictly on host tissue, but may rather live in a (partially) commensal relationship with their host.
To address the latter problem, traditionally an analysis of parasite stomach contents was used to confirm a parasite-host relationship, but recently stable isotope analysis (SIA) has been proven to be a valuable method to determine the trophic position of parasites (e.g. Pinnegar et al. Reference Pinnegar, Campbell and Polunin2001; Deudero et al. Reference Deudero, Pinnegar and Polunin2002; Dubois et al. Reference Dubois, Savoye, Sauriau, Billy, Martinez and de Montaudouin2009) and other consumers (e.g. Inger et al. Reference Inger, Ruxton, Newton, Colhoun, Robinson, Jackson and Bearhop2006; Dubois et al. Reference Dubois, Blin, Bouchaud and Lefebvre2007). This method uses the differences (Δ) between isotopic ratios of naturally occurring stable isotopes of nitrogen (δ 15N) and carbon (δ 13C) between consumers and their diet to reconstruct trophic relationships (Post, Reference Post2002). The δ 13C discrimination factor (Δ13C) is used to determine the diet source of carbon (e.g. terrestrial vs marine primary producers; Hobson, Reference Hobson1986) with a standard discrimination factor of 1·0‰, while trophic enrichment (Δ15N) is used to estimate the trophic position (Vander Zanden et al. Reference Vander Zanden, Cabana and Rasmussen1997), in which a fixed value (also known as the trophic fractionation factor) of 3·4‰ is most commonly used to analyse relative species trophic levels (Minagawa and Wada, Reference Minagawa and Wada1984; Vander Zanden et al. Reference Vander Zanden, Cabana and Rasmussen1997; Post, Reference Post2002). However, studies which compare isotopic signatures of parasites with their hosts indicate that parasites do not always fit with the commonly accepted consumer-diet discrimination patterns seen in free-living species (e.g. Neilson and Brown, Reference Neilson and Brown1999; Iken et al. Reference Iken, Brey, Wand, Voigt and Junghans2001; Power and Klein, Reference Power and Klein2004; O'Grady and Dearing, Reference O'Grady and Dearing2006; Xu et al. Reference Xu, Zhang and Xie2007; Dubois et al. Reference Dubois, Savoye, Sauriau, Billy, Martinez and de Montaudouin2009; Navarro et al. Reference Navarro, Albo-Puigserver, Coll, Saez, Forero and Kutcha2014; Behrmann-Godel and Yohannes, Reference Behrmann-Godel and Yohannes2015).
In this study, we analyse the trophic relationship between the invasive endoparasite Mytilicola orientalis and its new host in European seas, the native blue mussel Mytilus edulis in the Dutch Wadden Sea. This parasitic copepod has been recently co-introduced with aquaculture imports of the invasive Pacific oyster (Magallana (previously Crassostrea) gigas) (Elsner et al. Reference Elsner, Jacobsen, Thieltges and Reise2011) and is known to spill over to native bivalves such as blue mussels and to a lesser extent to common cockles (Cerastoderma edulis) and Baltic tellins (Limecola (formerly Macoma) balthica; Goedknegt et al. Reference Goedknegt, Schuster, Buschbaum, Gergs, Jung, Luttikhuizen, van der Meer, Troost, Wegner and Thieltges2017a). Mytilicola orientalis was first described in the Sea of Japan (Mori, Reference Mori1935) and has a direct life cycle with a short non-feeding free-living stage, after which it lives in the intestines of its host. Here, the parasite is either feeding directly on the host tissue or indirectly on host gut content, resulting in a reduction in body condition of infected blue mussels (M. A. Goedknegt, unpublished results). As the exact diet source of the parasite is yet unknown, we performed a SIA to clarify the trophic relationship between the parasite M. orientalis and its new blue mussel host. Field samples of mussel hosts and parasites were analysed as well as the two principal food sources of mussels, being particulate organic matter (POM) and microphytobenthos (MPB, Dubois et al. Reference Dubois, Blin, Bouchaud and Lefebvre2007). This approach allowed us to determine the relative contributions of host tissue and host food to the diet of the invasive copepod and to identify the trophic relationship of this new parasite-host association that has resulted from the recent co-introduction of the copepods with their oyster hosts.
Material and methods
Collection of samples
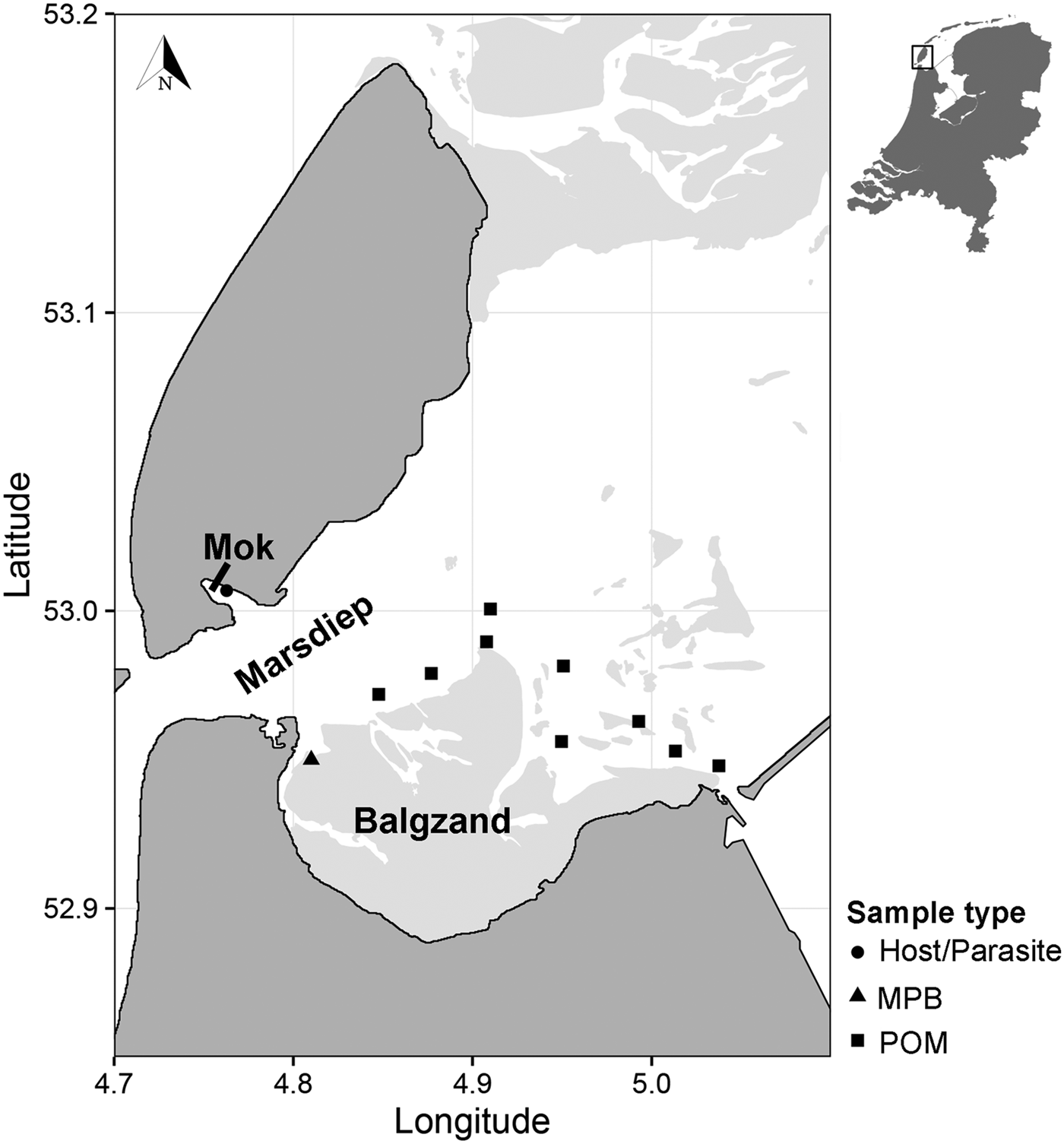
Suspended POM samples (n = 17) were collected on 2 and 4 July 2013 at nine locations in the subtidal Marsdiep channel (Wadden Sea, The Netherlands, Fig. 1). At high tide, water from this channel feeds a small intertidal bay in the south of the island of Texel (Mok, The Netherlands) and therefore we assumed that POM originating from this channel is a major food source for blue mussels (Mytilus edulis) living in the bay where we sourced the mussels and parasites (Mytilicola orientalis) for the SIA (Fig. 1; see below). At each sampling point, water samples were collected with a Niskin bottle from approximately 1 m below the water surface. Samples were then sieved through a 200 µm mesh to exclude larger zooplankton from the sample and subsequently filtered onto pre-combusted 25 mm GF/F filters using a 25 mm filter cartridge mounted on a 60 mL syringe. Between 80 and 250 mL of water was filtered depending on the amount of suspended matter in the water column. Filters were then stored at −20 °C until further analysis.
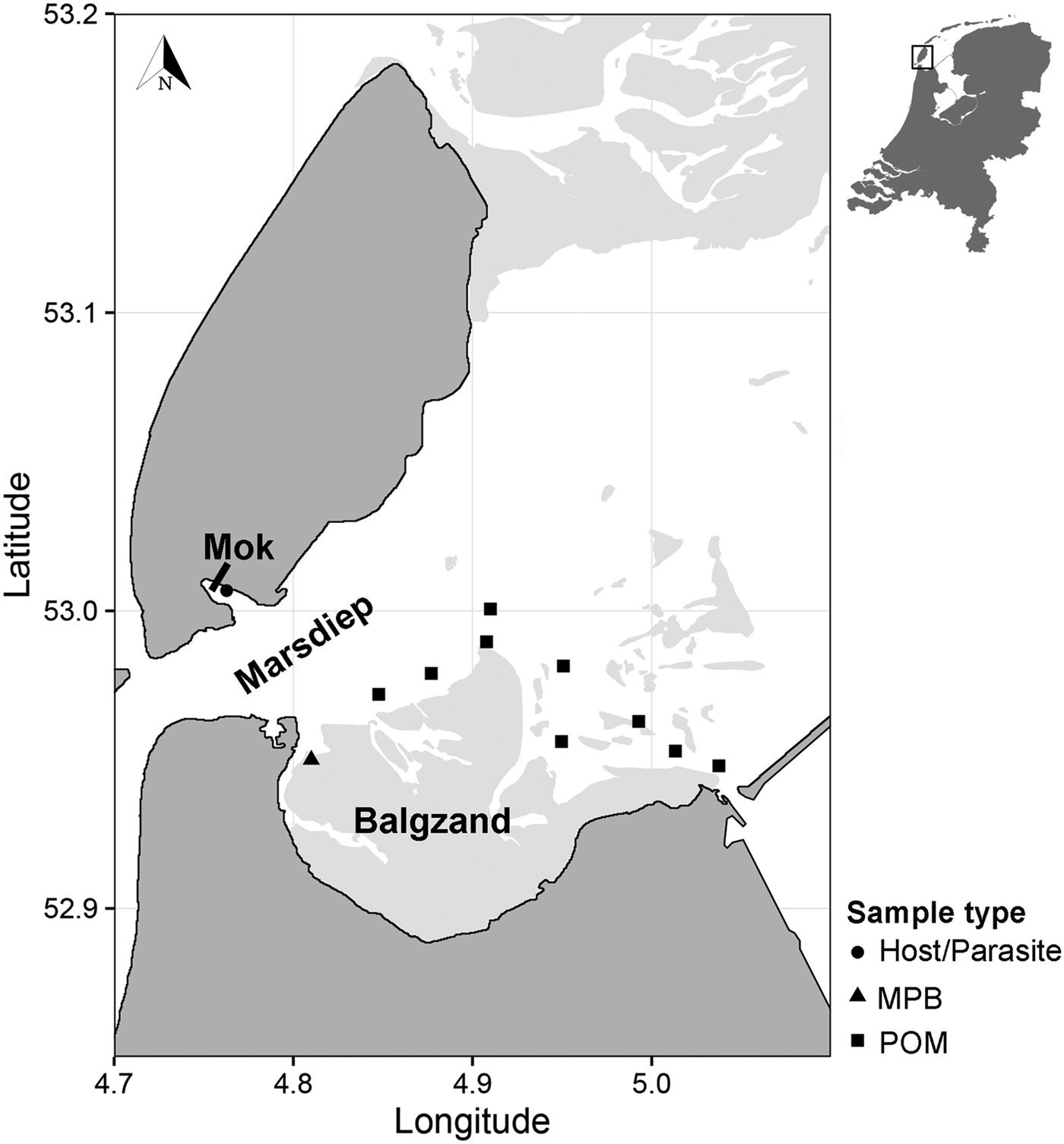
Fig. 1. Sampling locations of suspended POM, MPB, hosts (Mytilus edulis) and parasites (Mytilicola orientalis). On the MPB location, four samples were taken in an area of approximately 50 m2. POM locations were sampled once (2 locations), twice (6 locations) or three times (1 location), adding to a total of 17 samples. MPB = microphytobenthos; POM = particulate organic matter.
Microphytobenthos (MPB; n = 4 samples within an area of 50 m2; Fig. 1) was sampled in the beginning of July 2013 at an intertidal area south of the Marsdiep (Balgzand, Wadden Sea, The Netherlands, Fig. 1) by collecting sediment from diatom mats into plastic bottles that were put on ice and brought to the research facility. Extraction of microphytobenthic diatoms in the laboratory was done by following the method of Riera and Richard (Reference Riera and Richard1996), slightly modified by Herlory et al. (Reference Herlory, Richard and Blanchard2007). The sediment was spread in a tray, covered by three layers of nylon mesh (2 × 100 µm, 1 × 50 µm) that was kept moist by repeatedly spraying filtered seawater on top. The samples were then left in a temperature-regulated room overnight at 20 °C. The next morning, the algae were washed into a beaker with filtered seawater. This solution was centrifuged (10 min at 103 G) and the remaining pellet was collected and stored at −20 °C.
Blue mussel and parasite samples were collected about 3 months later than the POM and MPB samples (26 September 2013), to cover the minimum time it takes for the diet to be incorporated into consumer tissue (Dubois et al. Reference Dubois, Blin, Bouchaud and Lefebvre2007; Phillips et al. Reference Phillips, Inger, Bearhop, Jackson, Moore, Parnell, Semmens and Ward2014). Mussels (n = 150) were collected from a mixed oyster and mussel bed located in the Mok (Fig. 1) and checked for presence of M. orientalis parasites under a magnification glass (magnification 3 – 8×). Mussels infected with at least two female parasites (n = 28 mussels), which can be more than twice as large as males (Mori, Reference Mori1935), were selected for the analysis, as a minimum of 0·4 mg dry weight of each pooled parasite and corresponding mussel sample (the adductor muscle of the mussel) were required for the SIA. In these selected mussels, the mean M. orientalis intensity (±s.d.) was 3·6 ± 1·8 and ranged between 2 and 9 copepods, with an average (±s.d.) ratio of 0·78 ± 0·21 females per infected mussel. Both parasite and matched mussel samples (each n = 28) were then stored at −20 °C.
Stable isotope analysis
Prior to the SIA, all samples were freeze-dried for 48 h at −60 °C to remove water content. Additionally, as M. orientalis is a crustacean, parasite samples were treated with 1 m HCl to remove inorganic carbonate and dried for another 24 h at 60 °C. Isotope ratios of δ 15N and δ 13C in all samples were determined with a Thermo Scientific Delta V Advantage Isotope Ratio Mass Spectrometer equipped with a Flash 2000 Organic Element Analyser at the Royal Netherlands Institute for Sea Research, Texel, The Netherlands. In addition, mean total organic carbon (TOC) and mean total nitrogen (TN) content and the carbon-to-nitrogen ratio (C:N) were determined for hosts and parasites, but due to logistical constraints this was not possible for the POM and MPB samples.
The standard reference materials acetanilide (s.d.: δ 15N 0·3‰, δ 13C 0·1‰) and urea (δ 15N 0·2‰, δ 13C 0·1‰) were respectively used as a correction and control of the isotope ratios found in the samples. Isotope ratios of δ 15N and δ 13C were then expressed as permille (‰) differences from a standard reference material using the formula X = ((R sample/R standard) − 1) × 1000, with R being the ratio between the heavy and light isotopes of nitrogen (15N:14N) and carbon (13C:12C). The reference material used for 15N was atmospheric nitrogen N2 and for 13C Vienna Peedee-Belemnite Limestone (vPDB).
Statistical analysis
Normality and homoscedasticity of the data were checked with histograms, qqplots and boxplots (Zuur et al. Reference Zuur, Ieno and Elphick2010). Subsequently, differences in isotope ratios (δ 13C and δ 15N) among the trophic groups (POM, MPB, hosts, parasites) were analysed with analysis of variance (ANOVA) and post-hoc Tukey tests. Furthermore, comparisons and relationships between stable isotope data of parasites and corresponding hosts (Δ13C and Δ15N) and parasite intensity within the host were made using paired Student's t-tests and Pearson correlations, respectively. All statistical analyses were performed in the statistical software environment R (R Development Core Team, 2015).
Isotope mixing models
The relative contribution of diet sources in the consumers’ diet can be determined by the use of stable isotope mixing models (i.e. Phillips and Gregg, Reference Phillips and Gregg2003; Inger et al. Reference Inger, Ruxton, Newton, Colhoun, Robinson, Jackson and Bearhop2006). In this study, we used an isotope mixing model to determine the relative contributions of host tissue (blue mussel) and host gut content (represented by POM and MPB) to the diet of the parasitic copepod M. orientalis. The package simmr (Parnell, Reference Parnell2016) was used to solve mixing equations for stable isotopic data within a Bayesian framework in R (R Development Core Team, 2015). This package allows the use of multiple diet sources with adjustable source specific trophic fractionation factors. In the mixing model, individual δ 15N and δ 13C values of the parasite samples were used as the consumer data. Diet source data included the mean (±s.d.) δ 15N and δ 13C values of the sources POM, MPB and blue mussel, and were corrected for trophic fractionation. This correction for trophic fractionation was done in two different ways: first, we used the standard trophic fractionation factors of 3·4‰ for δ 15N and 1·0‰ for δ 13C for all diet sources (Minagawa and Wada, Reference Minagawa and Wada1984; Vander Zanden et al. Reference Vander Zanden, Cabana and Rasmussen1997; Post, Reference Post2002), as controlled diet studies and thus taxon-specific fractionation factors are not (yet) available for the parasite. Second, we varied the trophic fractionation values used for δ 15N between 1 and 4‰ to determine how much the estimated relative contribution of all diet sources changed with the fractionation factor. This second approach served as a sensitivity analysis to account for the unknown ‘real’ trophic fractionation factor of the parasites (see Discussion for more details). Finally, we ran a third mixing model approach where we used mussel, POM and MPB data from four seasons from the long-term monitoring at our sampling site to identify whether seasonal changes of mussel, POM and MPB isotope signals would change our results.
Results
All trophic groups
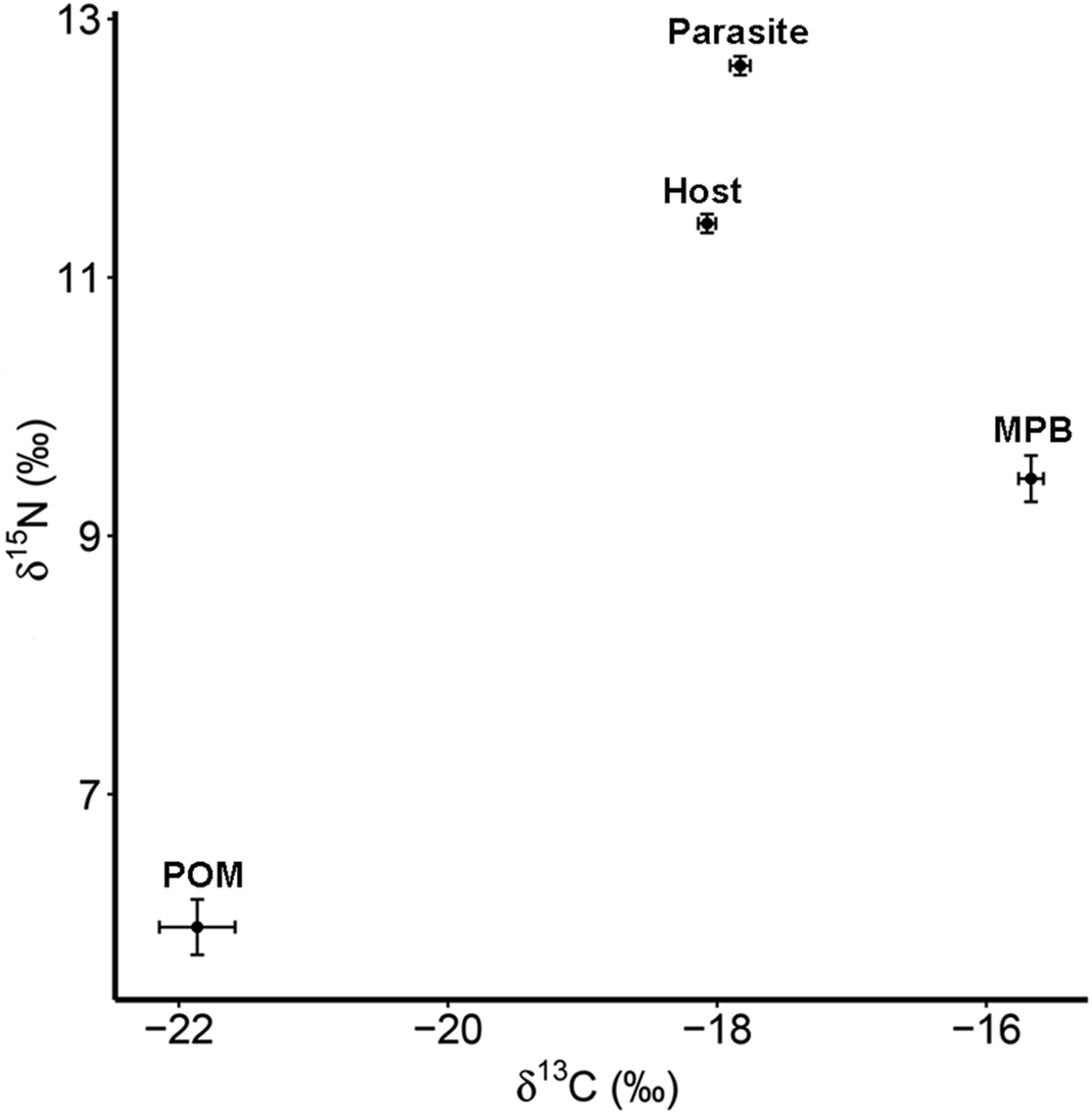
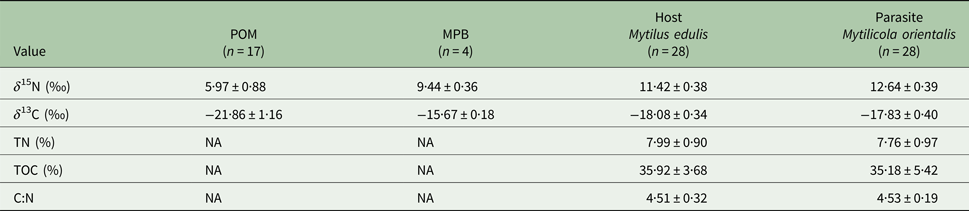
The four trophic groups (POM, MPB, mussel, parasite) differed significantly in δ 15N (ANOVA; F 3,73 = 588·16, P < 0·001) and δ 13C (F 3,73 = 200·41, P < 0·001). Values of δ 15N were highest for the parasitic copepod and lowest for POM, while for δ 13C MPB and POM had the highest and lowest values, respectively (Table 1; Fig. 2).
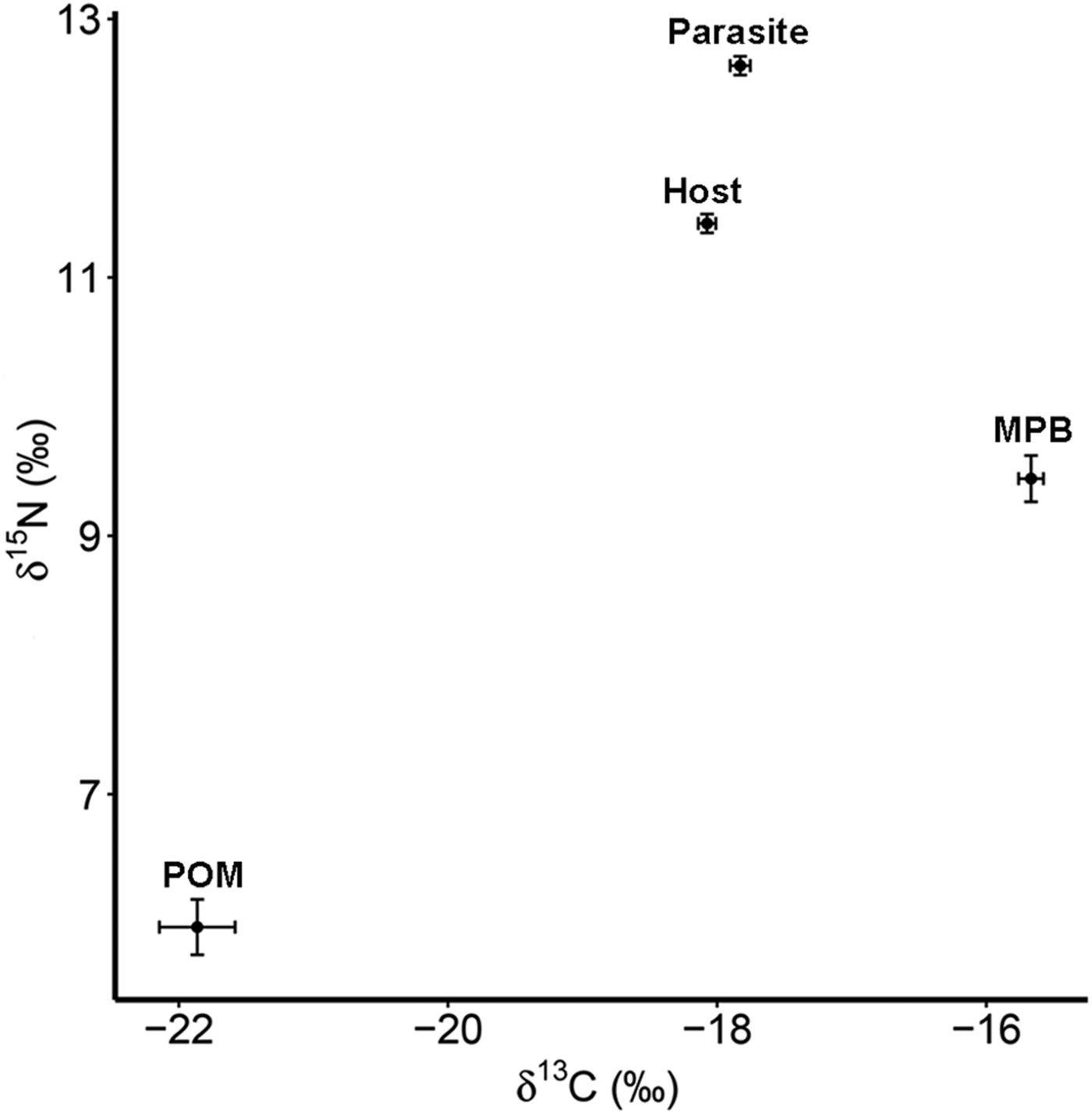
Fig. 2. Isotope values of δ 15N and δ 13C with standard errors for suspended POM, MPB, the blue mussel host (Mytilus edulis) and its intestinal parasite Mytilicola orientalis. MPB = microphytobenthos; POM = particulate organic matter.
Table 1. Mean values (±s.d.) of δ 15N (‰) and δ 13C (‰) for suspended particulate organic matter (POM), microphytobenthos (MPB), the blue mussel host (Mytilus edulis) and its intestinal parasite Mytilicola orientalis

Total nitrogen content (TN %), total carbon content (TOC %) and C:N ratios are only given for parasite and host.
Parasites and hosts
Parasitic copepods were significantly enriched in δ 15N and δ 13C with respect to their host, the blue mussel (Student's paired t-test; δ 15N: t = 11·178, df = 27, P < 0·001; δ 13C: t = 4·071, df = 27, P < 0·001; for means see Table 1; for raw data see Goedknegt et al. Reference Goedknegt, Shoesmith, Jung, Luttikhuizen, van der Meer, Philippart, van der Veer and Thieltges2017b). However, the levels of enrichment were relatively small (mean ± s.d.; 1·22 ± 0·58‰ for δ 15N and 0·25 ± 0·32‰ for δ 13C; Fig. 2). This minor enrichment of the parasite in relation to its host was not reflected in the differences in mean TN and TOC content in both tissues (Student's t-test; TN (%): t = −1·361, df = 27, P = 0·185; TOC (%): t = −0·741, df = 27, P = 0·465; for means see Table 1).
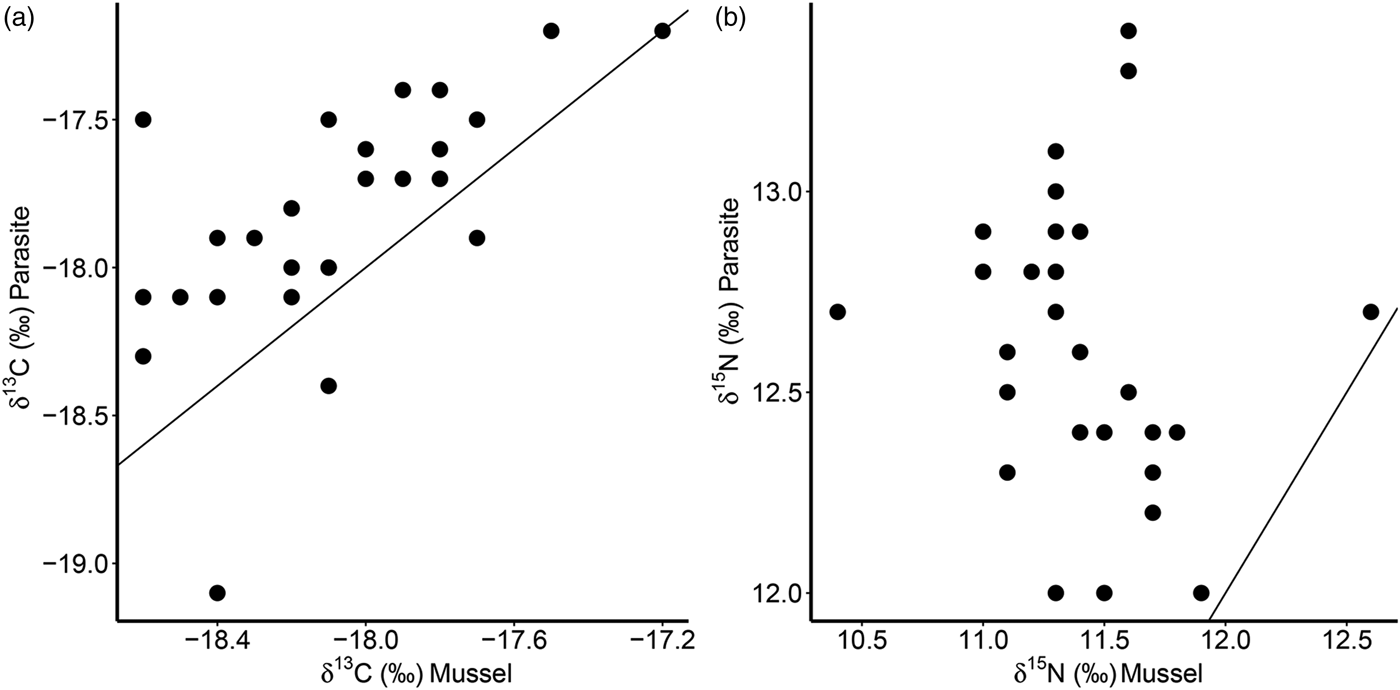
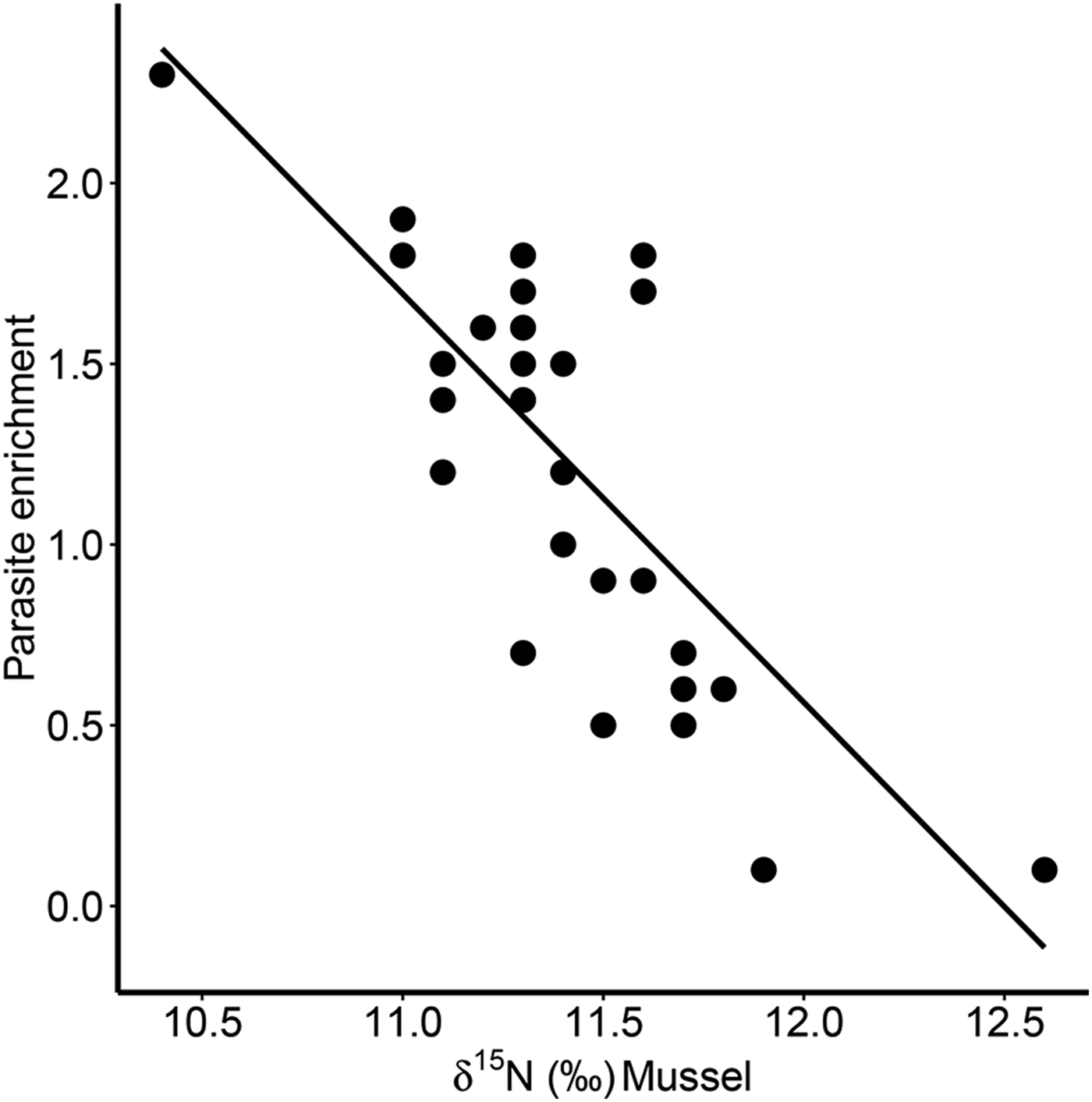
Furthermore, there was a significant positive correlation for δ 13C between host and parasite (Pearson correlation, r = 0·63, P < 0·001; Fig. 3a), but this relationship did not exist for δ 15N (r = −0·13, P = 0·509; Fig. 3b). Consequently, parasite enrichment (Δ15N: parasite δ 15N – mussel δ 15N) scaled negatively with mussel δ 15N mussel (Pearson correlation, r = −0·75, P < 0·001; Fig. 4), while this relationship was not significant for Δ13C enrichment (r = −0·29, P = 0·130). In addition, there was no relationship between the enrichment of the parasite (Δ15N: parasite δ 15N – mussel δ 15N) and the C:N ratio of the mussel (Pearson correlation, r = 0·06, P = 0·743). Finally, in our dataset parasite intensity in infected hosts did not affect the δ 15N (r = 0·35, P = 0·064) nor δ 13C (r = 0·07, P = 0·708) signals of the parasites and neither those of the hosts (δ 15N: r = −0·07, P = 0·719, δ 13C: r = −0·19, P = 0·334).
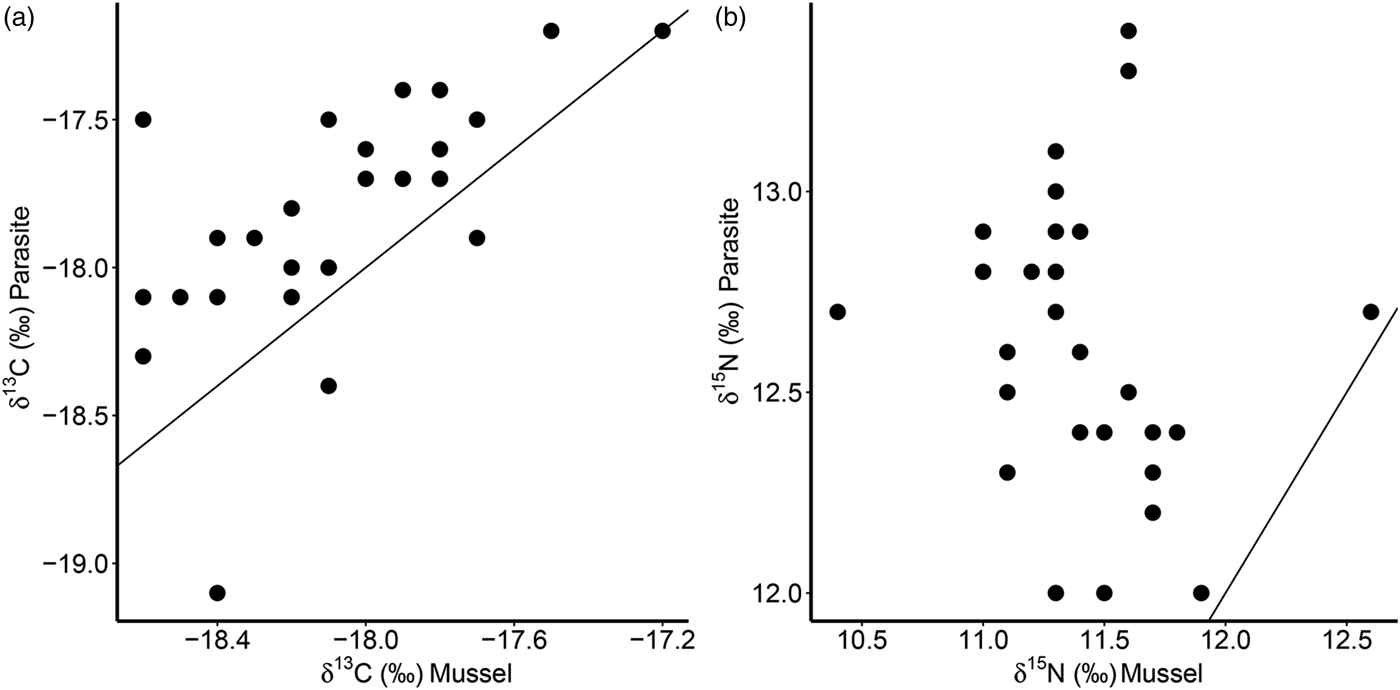
Fig. 3. Relationships of δ 13C (a) and δ 15N (b) for the native mussel host Mytilus edulis and the invasive parasite Mytilicola orientalis. Each dot represents values for one individual host-parasite pair. Black lines indicate the 1:1 relationship.
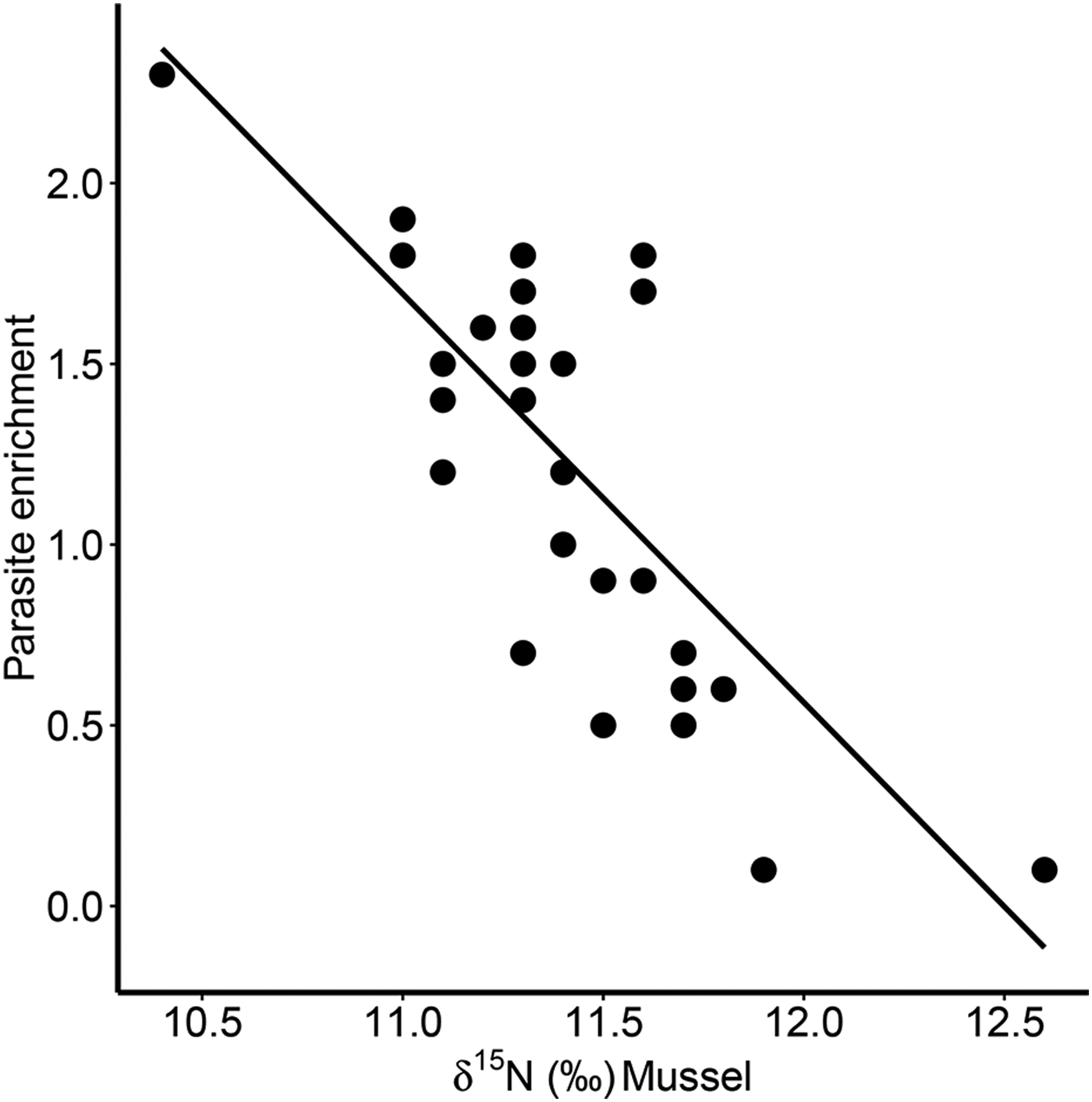
Fig. 4. Relationship between parasite enrichment (Δ15N = parasite δ 15N – mussel δ 15N) of the invasive parasite Mytilicola orientalis and the δ 15N of the blue mussel host Mytilus edulis. Each dot represents values for one individual host-parasite pair.
Isotope mixing models
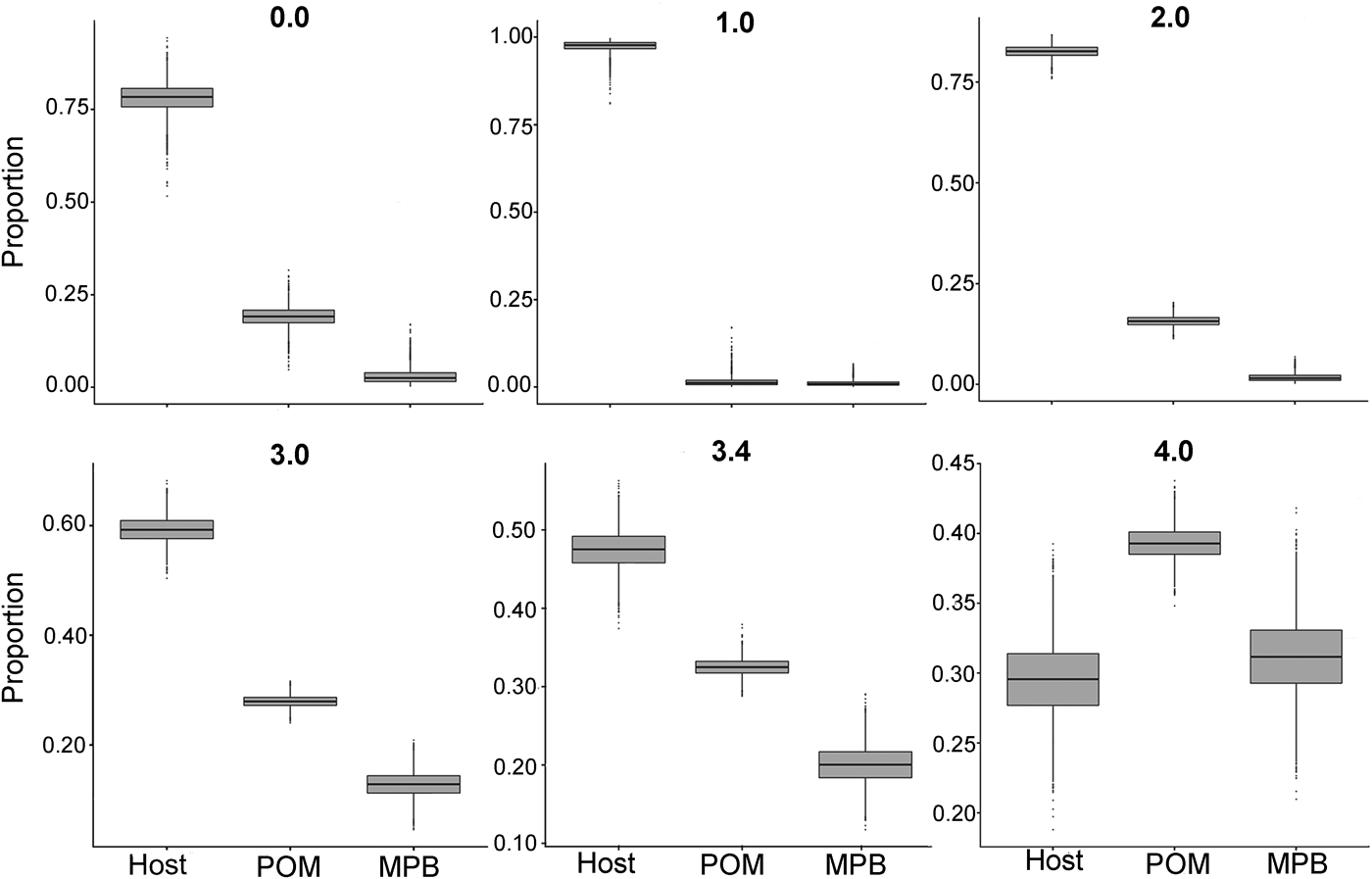
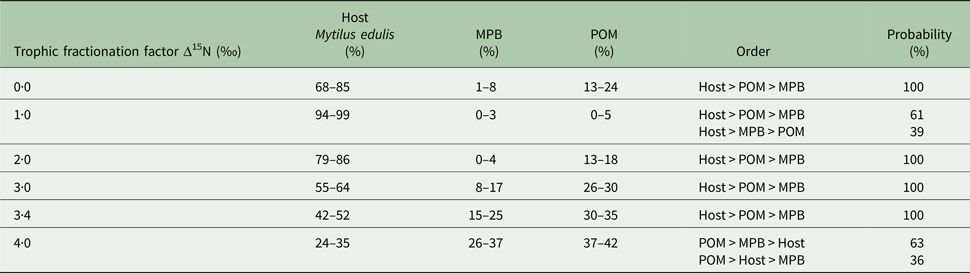
In the first mixing model, we used the standard trophic fractionation factor of 3·4‰ for δ 15N and the discrimination factor of 1·0‰ for δ 13C for all diet sources (POM, MPB, mussel host). Results of this model showed that mussel tissue was the main contributor to the parasites’ diet (95% confidence interval; 42–52%), with lower contributions by POM (30–35%) and MPB (15–25%). When we varied the δ 15N trophic fractionation factors in the second run of the mixing models, the relative contributions of all diet sources changed (Table 2; Fig. 5) but for fractionation factors between 0 and 3·4‰ for δ 15N this did not affect the dominance of mussel host tissue in the parasites’ diet. Only for a fractionation factor of 4‰ for δ 15N, the model showed higher proportions of POM (37–42%) and MPB (26–37%) in the diet of the parasite relative to blue mussel tissue (24–35%; Table 2; Fig. 5). Finally, using isotope values of mussels, POM and MPB from four seasons/months at our sampling site (March, June, September and December 2014) in the main mixing model (3·4‰ for δ 15N and 1·0‰ for δ 13C) did not change the results qualitatively (Supplementary Table S1).
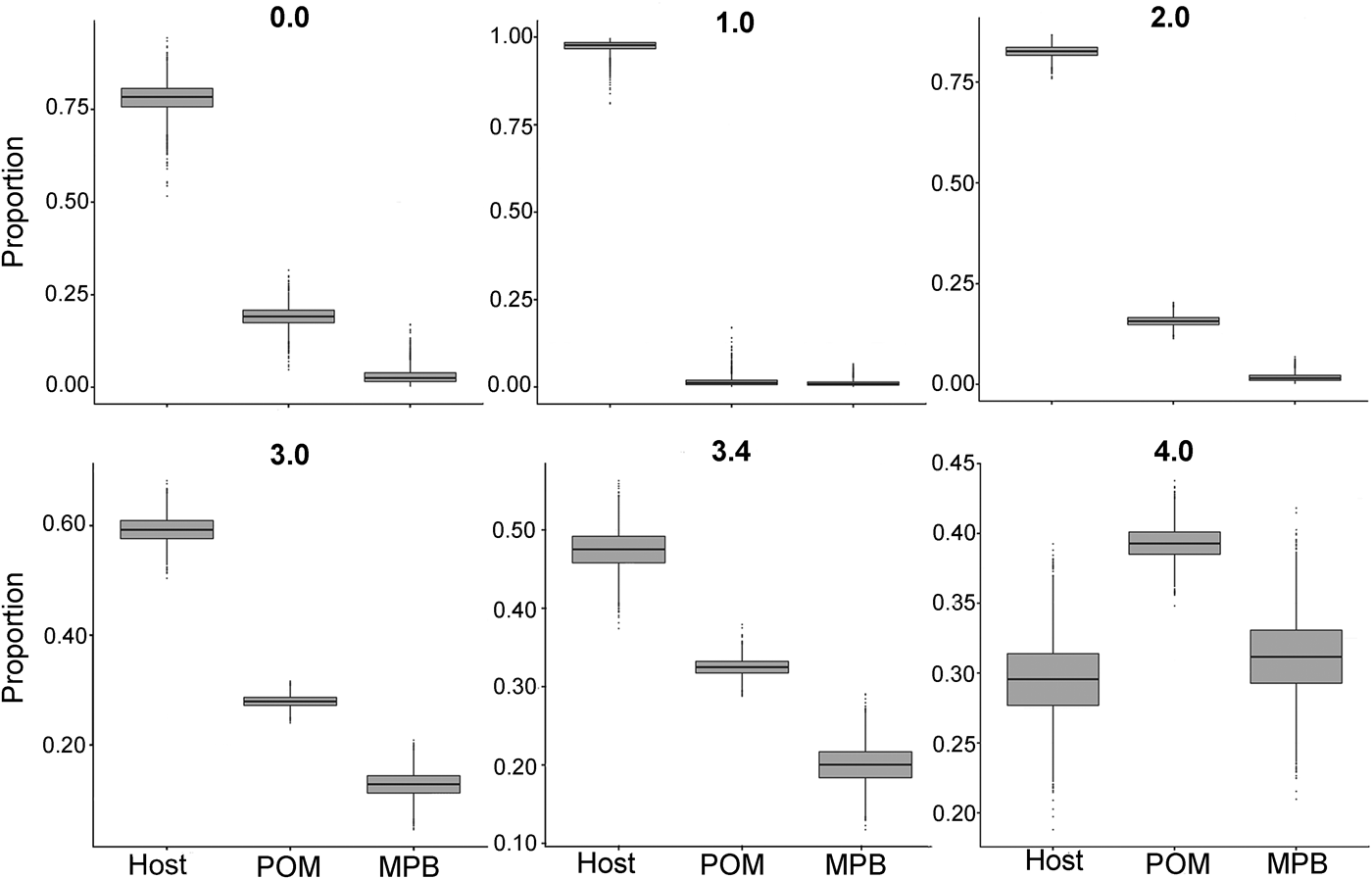
Fig. 5. Comparison of proportions of diet sources in the intestinal parasite Mytilicola orientalis by using different mixing models with a varying fractionation factor Δ15N (0·0, 1·0, 2·0, 3·0, 3·4 and 4·0‰) for the blue mussel Mytilus edulis host, MPB and POM. In every model, the discrimination factor of Δ13C was set at 1·0‰. MPB = microphytobenthos; POM = particulate organic matter.
Table 2. Results of the sensitivity analysis of the mixing model
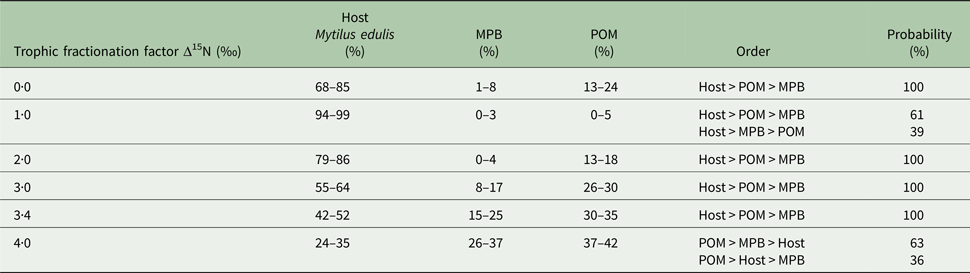
The trophic fractionation factor Δ15N was changed manually between 0·0–4·0‰ for all the three diet sources: host Mytilus edulis, microphytobenthos (MPB), particulate organic matter (POM). The discrimination fractionation factor Δ13C was in all scenarios set at 1·0 ± 0‰. The table shows the diet proportions (95% confidence interval) of each of the three diet sources, the hierarchical order of diet proportions in the parasites’ (Mytilicola orientalis) diet and the probability of this ordering.
Discussion
Our SIA showed that the intestinal parasitic copepod Mytilicola orientalis is enriched in δ 15N and δ 13C with respect to its blue mussel (Mytilus edulis) host. Yet, for both isotopes, the observed enrichment of the parasite compared with its host (1·2‰ for Δ15N and 0·25‰ for Δ13C) was considerably lower than the standard trophic fractionation factor of 3·4‰ for Δ15N and the standard discrimination factor of about 1‰ for Δ13C, which are commonly used to distinguish between trophic levels (e.g. Minagawa and Wada, Reference Minagawa and Wada1984; Vander Zanden et al. Reference Vander Zanden, Cabana and Rasmussen1997; Post, Reference Post2002). Given that these values are also appropriate for the parasites, this would indicate that this intestinal parasite does not only feed on host tissue, but also on host gut content, suggesting a complex mix of a parasitic and commensal relationship in this new parasite-host association. Such a mixed diet was also indicated by the results of the stable isotope mixing modelling, a statistical method that is increasingly used by ecologists (reviewed by Phillips et al. Reference Phillips, Inger, Bearhop, Jackson, Moore, Parnell, Semmens and Ward2014). Generally, the results of our mixing models (using standard fractionation factors) demonstrated that host tissue dominated with suspended POM and microphytobenthos contributing to the parasites’ diet. However, alternatively, the relatively small signals of enrichment may not result from mixed diet contributions but could also suggest parasite specific lower trophic fractionation patterns. Indeed, smaller than standard enrichment patterns have previously been found in other parasite-host systems (O'Grady and Dearing, Reference O'Grady and Dearing2006; Dubois et al. Reference Dubois, Savoye, Sauriau, Billy, Martinez and de Montaudouin2009; Yurlova et al. Reference Yurlova, Shikano, Kanaya, Rastyazhenko and Vodyanitskaya2014; Behrmann-Godel and Yohannes, Reference Behrmann-Godel and Yohannes2015; Demopoulos and Sikkel, Reference Demopoulos and Sikkel2015), including parasites with a strict parasitic way of life such as trematodes (Dubois et al. Reference Dubois, Savoye, Sauriau, Billy, Martinez and de Montaudouin2009). To investigate the effect of potentially lower and higher than usual trophic fractionation factors, we conducted a second run of mixing models using a variation of trophic fractionation values (0–4‰). These models showed that mussels were still the dominant food source in all but the highest trophic fractionation value (4‰). In addition, the positive correlation in carbon signatures between parasite and host suggests that the host represents a major carbon source for the parasitic copepod. All these results confirm that the parasite has, at least to a large extent, a parasitic trophic relationship with its host. This would also explain the negative effect of the parasite on host body condition, which has been previously observed in controlled laboratory experiments (M. A. Goedknegt, unpublished results). However, in all the scenarios of the mixing models developed in our study, host tissue (M. edulis; proportions of 24–99%) was never the only resource of M. orientalis but host gut content, represented by suspended POM (0 – 42%) and microphytobenthos (MPB: 0–37%), consistently contributed to the parasite's diet. This suggests that the trophic relationship of the parasite with its new host is also partly commensalistic. The exact contributions of the diet under different environmental conditions as well as the resulting diet specific trophic fractionation factors remain to be experimentally studied (although this will be logistically challenging, see below).
Our findings differ from the results of a SIA of a congeneric species of M. orientalis, the copepod M. intestinalis, which also lives in the intestine of M. edulis. Gresty and Quarmby (Reference Gresty and Quarmby1991) found δ 15N values of the parasite that were, on average, 2·8‰ higher than for the blue mussel and suggested a parasitic trophic relationship between the parasite and its host. In their study, infected mussels (collection season unknown) were kept in aquaria that were filled with estuarine water and mussels were fed with the diatom Phaeodactilym trycornutum 2–3 weeks prior to dissection, after which the mussel intestine was used in the SIA analysis. Methodological differences may underlie the diverging trophic fractionation factors in the two parasite species but it is also possible that the feeding behaviour of both congeneric copepods is different. Although M. intestinalis may not directly feed on host tissue but rather on sloughed-off cells of the intestine or on mucus produced by the host (Gresty and Quarmby, Reference Gresty and Quarmby1991), it may still mainly feed (indirectly) on its mussel host. In contrast, the much lower trophic enrichment (Δ15N) of 1·2‰ observed in M. orientalis in our study might suggest a more complex mix of a parasitic and commensal relationship between this parasite and its new host. A direct comparison of the two parasites in future experimental stable isotope studies would be interesting and could help to identify potential differences in diet composition of the two related parasite species.
In the present study, mussel diet sources were sampled at other sites (Marsdiep and Balgzand) than mussels and parasites (Mok). However, during flood the three areas are tightly connected, when water from the North Sea is feeding the intertidal areas of Balgzand and Mok via the same deep channel, the Marsdiep (Postma, Reference Postma1954; Duran-Matute et al. Reference Duran-Matute, Gerkema, de Boer, Nauw and Gräwe2014). Therefore, we assume that POM originating from this channel is incorporated in the mussel and parasite tissue 2–3 months later (Dubois et al. Reference Dubois, Blin, Bouchaud and Lefebvre2007; Phillips et al. Reference Phillips, Inger, Bearhop, Jackson, Moore, Parnell, Semmens and Ward2014). MPB samples were collected at the same time as the POM samples, but on a sampling site from a seasonal isotope monitoring study located on the tidal flats of Balgzand, on the opposite side of the channel feeding the Mok, where hosts with parasites were sampled. For the stable isotope mixing models, we considered the samples from Balgzand to be representative for the MPB available to the mussels. However, MPB is known to occur in higher abundances in the Mok than at Balgzand (4 g C m−2; Borsje, Reference Borsje2006) and both areas are under the influence of different freshwater sources. The exact impact of these discharges on the mussels’ diet is yet unknown. Potential differences in the isotopic composition of MPB between the areas may introduce bias in our analyses, but given the relatively small range of isotope signals observed in MPB on local scales as observed in a recent large-scale isotope study along the entire Dutch Wadden Sea (Christianen et al. Reference Christianen, Middelberg, Holthuijsen, Jouta, Compton, van der Heide, Piersma, Sinninghe Damsté, Van der Veer, Schouten and Olff2017; M. J. A. Christianen, pers. communications), we are confident that the spatial mismatch in sampling location is not adding a severe bias in MPB measurements. Besides spatial differences, seasonality may be another potential factor known to affect isotope signals over a wide range of trophic levels (Kang et al. Reference Kang, Lee, Choy, Shin, Seo and Hong2006; Cabanellas-Reboredo et al. Reference Cabanellas-Reboredo, Deudero and Blanco2009; Ezgeta-Balić et al. Reference Ezgeta-Balić, Lojen, Dolenec, Žvab Rožič, Dolenec, Najdek and Peharda2014; de la Vega et al. Reference de la Vega, Lebreton, Siebert, Guillou, Das, Asmus and Asmus2016), and expected to affect δ 15N and δ 13C ratios of mussel diet sources, potentially confounding our mixing models. However, data from a seasonal isotope investigation in our study area from which the POM/MPB data originated, suggest a limited effect of seasonality on our results. Preliminary results of this seasonal study showed only small differences in isotope values between June and September for POM (Δδ 15N = 0·01; Δδ 13C = 0·9) and some larger differences for MPB (Δδ 15N = 1·7; Δδ 13C = 1·6; A. S. Jung, pers. communications). These results demonstrate that the growing season of various phytoplankton species did not lead to strong changes in the isotopic signals of POM during the summer. For MPB on the other hand, the changes are larger and here a switch in microphytobenthos species composition may have caused a change of isotopic values during the summer. However, the seasonal change in isotopic values of these diet sources only affected the isotopic signal of the host Mytilus edulis to a small extent between June and September (Δδ 15N = 0·1; Δδ 13C = 0·9; A. S. Jung, pers. communications). As especially the δ 15N values of mussels barely changed during the summer, we do not believe that seasonality effects are confounding our analyses, in particular with respect to our main focus of investigation, the trophic relationship of the parasite M. orientalis with its mussel host. Our inferences are further supported by stable isotope mixing models in which we used original data for POM and MPB from the isotope study of four different seasons to investigate how this would change the results. These analyses showed that mussels remain the main food source for the parasites, independent of the season (see electronic appendix Table S1). However, over the course of a year, the relative contributions of POM, MPB and mussel to the parasites’ diet may of course change with season and/or salinity and to what extend this actually happens should be a topic of future studies.
For isotope mixing models, the use of appropriate fractionation factors is essential (Phillips et al. Reference Phillips, Inger, Bearhop, Jackson, Moore, Parnell, Semmens and Ward2014) but, as mentioned above, parasites may show enrichments patterns different from free-living species (O'Grady and Dearing, Reference O'Grady and Dearing2006; Dubois et al. Reference Dubois, Savoye, Sauriau, Billy, Martinez and de Montaudouin2009; Yurlova et al. Reference Yurlova, Shikano, Kanaya, Rastyazhenko and Vodyanitskaya2014; Demopoulos and Sikkel, Reference Demopoulos and Sikkel2015) and we accounted for this by using mixing models with different fractionation factors (see above). However, further diversion from standard enrichment patterns may arise from the universal pattern that trophic fractionation factors of consumers are known to scale negatively with the isotope ratio of their resource (Caut et al. Reference Caut, Angulo and Courchamp2009; Hussey et al. Reference Hussey, MacNeil, McMeans, Olin, Dudley, Cliff, Wintner, Fennessy and Fisk2014). The same negative scaling was observed in our data with the trophic enrichment in M. orientalis decreasing with host δ 15N. Such a negative scaling relationship between resource δ 15N and consumer trophic enrichment has also been observed within individual predators and their prey (Caut et al. Reference Caut, Angulo and Courchamp2009; Dennis et al. Reference Dennis, MacNeil, Rosati, Pitcher and Fisk2010) besides the general negative scaling relationship among species observed in comparative studies (Caut et al. Reference Caut, Angulo and Courchamp2009; Hussey et al. Reference Hussey, MacNeil, McMeans, Olin, Dudley, Cliff, Wintner, Fennessy and Fisk2014). However, the underlying mechanisms of both scaling relationships are not well understood (Caut et al. Reference Caut, Angulo and Courchamp2009; Hussey et al. Reference Hussey, MacNeil, McMeans, Olin, Dudley, Cliff, Wintner, Fennessy and Fisk2014). In the case of M. orientalis, the issue is further complicated by the fact that δ 13C values of the parasite correlated positively, as expected for a trophic relationship, with those of their hosts, but that, surprisingly, this relationship did not exist for δ 15N. Here, the variation in δ 15N values among individual M. orientalis samples was larger than the variation among individual mussels, resulting inevitably in a negative scaling relationship between parasite trophic enrichment (Δ15N) and δ 15N values of hosts. This suggests that M. orientalis might be relatively decoupled from its host nitrogen sources. However, why this is the case we can only speculate. Ratios of stable isotopes may change between parasite and host due to differential digestion or fractionation during assimilation and metabolic processes. For example, the parasite could selectively use alternative or depleted nitrogen compounds stored within the mussels (Barrett, Reference Barrett1981), bacteria in the gut of the mussel could cause substantial changes in the nitrogen cycle within the host or specialized nitrogen turnover processes within the parasite could cause potential decoupling between host and parasite. Alternatively, our sample choice of selecting mostly larger females (which was necessary to obtain sufficient parasite tissue for the SIA analysis), could also have affected the relatively large variation in δ 15N we have observed in parasite samples. Possibly, females exhibit different stable isotope composition than males due to differences in body size (growth rates) and feeding rates, as well as due to egg production by females. In addition, the natural variation in M. orientalis intensities in the selected hosts could have influenced the variation in nitrogen among parasites, but this correlation was not significant in our analyses. Controlled laboratory experiments may be needed to explore the exact mechanisms behind the stable isotope patterns observed in the new M. orientalis-mussel association. However, such experimental approaches with parasites are logistically challenging. In particular in the case of the parasitic copepod in our study, it will be difficult to experimentally disentangle the relative contributions of host mussel gut content (POM and MPB) and host tissue to the parasite's diet, as the parasite inhabits the gut of the mussel host and has access to both resources at the same time. Hence, if one feeds mussels with a different dietary source, the parasite will have access to the mussel diet and at the same time feed on mussel tissue. Thus, the parasite acquires isotopes that are a mix of both food sources. Also conducting the usual diet switch studies with parasites is difficult, as the parasites cannot live without their hosts. Hence, letting parasites feed on algae without a host is impossible and due to host specificity changing hosts is also an issue. Such logistical complications due the natural history of parasitic organisms are probably the reason why most isotope studies of parasites so far have not used any experimental approaches. However, these studies still show that also samples from the field can give some insight into how isotopes reflect trophic relationships of parasites and their hosts. In our case, the combination of careful interpretation of the data and sensitivity analyses using stable isotope mixing models allow for valid inferences in absence of experimental data.
In conclusion, our study indicates that the invasive parasite M. orientalis mainly feeds on tissue of its new mussel host, but, to a lesser extent, also on the gut content of mussels (represented by suspended particulate organic matter and microphytobenthos). This conclusion was also supported by stable isotope mixing models, which used various trophic fractionation values to account for potentially different isotope enrichment patterns in parasitic compared with free-livings species. We propose that SIA combined with additional stable isotope mixing models promises to provide a useful tool to explore the trophic relationships of new parasite-host associations that result from the increasing co-introductions of parasites with their hosts into new ecosystems.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017001779.
Acknowledgements
We are grateful to Kevin Donkers, Karsten Dekker, Jort Ossebaar and, in particular, Stefan Schouten for giving valuable advice and making the stable isotope analyses possible. Furthermore, we like to thank the students of the NIOZ marine master course (2013) for providing and preparing POM and MPB samples. Finally, we thank three anonymous reviewers for the valuable feedback on a previous version of our manuscript.
Financial support
This study was supported by the Netherlands Organization for Scientific Research (NWO) and the German Bundesministerium für Bildung und Forschung in a bilaterally funded project (BMBF, NWO-ZKO project 839·11·002).