The use of winter cover crops in conservation tillage was initially aimed at reducing erosion control and improving soil health. However, over time cover crops have gained greater attention as a result of the physical weed suppression offered by the cover crop biomass (Reddy Reference Reddy2001; Teasdale and Mohler Reference Teasdale and Mohler2000). Cover crop residue can affect weed germination and growth by modification of the soil microenvironment. Light availability, soil moisture, and temperature are some of the attributes that can lead to suppressed weed seed germination (Masiunas et al. Reference Masiunas, Weston and Weller1995; Creamer et al. Reference Creamer, Bennett, Stinner, Cardina and Regnier1996).
Legume cover crops have been widely used to provide nitrogen (N) credits to the subsequent crop. The amount of available N in legume cover crop residues depends on crop species, residual soil N, and timing of cover crop termination (Sainju et al. Reference Sainju, Whitehead and Singh2005). Rochester et al. (Reference Rochester, Peoples, Hulugalle, Gault and Constable2001) reported that legume cover crops can provide considerable savings on N fertilizers required to optimize cotton lint yields and improve soil quality. The successful weed control achieved with legume cover crops is often attributed to the biomass production, which can suppress weed germination. However, legume cover crops generally have low persistence on the soil surface due to a low C:N ratio (Touchton et al. Reference Touchton, Rickerl, Walker and Snipes1984).
Cereal cover crops are known to produce high amounts of aboveground biomass, with cover crops like cereal rye producing 20% to 30% of the total biomass belowground (Meisinger et al. Reference Meisinger, Hargrove, Mikkelsen, Williams and Benson1991). This characteristic makes cereal cover crops an excellent option for N scavenging and for increasing water infiltration, aeration and soil aggregation, and soil protection (Dabney et al. Reference Dabney, Delgado and Reeves2001; Langdale et al. Reference Langdale, Blevins, Karlen, McCool, Nearing, Skidmore and Williams1991; Roberson and Firestone Reference Roberson and Firestone1991; Snapp et al. Reference Snapp, Swinton, Labarta, Mutch, Black, Leep and O’Neil2005). The high aboveground biomass production of cereal cover crops is also an excellent means of suppressing Palmer amaranth (Norsworthy et al. Reference Norsworthy, McClelland, Griffith, Bangarwa and Still2011). Another factor related to weed suppression provided by cereal cover crops is the release of allelochemicals produced by root exudation and plant residue decay that ultimately reduces seed germination (Chon and Kin Reference Chon and Kim2004). Brassica cover crops have the unique ability to produce glucosinolates, which are hydrolyzed to form a wide assortment of allelopathic isothiocyanates (Norsworthy and Meehan Reference Norsworthy and Meehan2005; Malik et al. Reference Malik, Norsworthy, Culpepper, Riley and Bridges2008). Norsworthy et al. (Reference Norsworthy, McClelland, Griffith, Bangarwa and Still2011) found that the production of 747 g m–2 and 677 g m–2 of biomass by turnip (Brassica rapa L.) and white mustard (Sinapis alba L.) provided 79% and 80% control of Palmer amaranth, respectively, when herbicides were not applied in cotton.
Cover crops often provide early-season weed suppression and protect cotton yield (Bauer and Roof Reference Bauer and Roof2004; Raper et al. Reference Raper, Reeves, Burmester and Schwab2000). The amount of biomass produced by the cover crop is a great tool to gauge the achievable level of weed control. However, growers have observed considerable variability in cover crop biomass production among years and locations––a situation that often makes weed suppression with cover crops less consistent than with herbicides. Hence, it is imperative that cover crop performance be evaluated over a wide range of conditions and locations. Although considerable data are available about the impact of cover crops on weed emergence and cotton yield, additional information is needed on the effect of cover crops on Palmer amaranth emergence throughout the season, so as to develop a more appropriate conservation tillage system for the sustainable management of this troublesome weed in cotton. Hence, the objective of this study was to evaluate the influence of cereal, legume, and brassica cover crops on Palmer amaranth emergence throughout the growing season and the resulting impact of the cover crops on seed cotton yield.
Materials and Methods
A field experiment was conducted in 2014 and 2015 at the University of Arkansas Research and Extension Center in Fayetteville, AR, to determine the efficacy of different cover crops for Palmer amaranth suppression in cotton. In 2014, the experiment was conducted in a Nixa gravelly silt loam soil (loamy-skeletal, siliceous, active, mesic Glossic Fragiudults), and in 2015 the soil series was a Leaf silt loam (fine, mixed, active, thermic Typic Albaquults). The experiment was conducted under dryland conditions, and amounts of rainfall are shown in Table 1. The experimental design was a randomized complete block with a strip plot, where the main plot factor was cover crop species and the strip-plot factor was the use or nonuse of residual herbicides (Table 2). Four replications were used with plot sizes of 3.6 by 9.9 m. The treatments consisted of seven cover crop species plus a no cover crop control. All cover crops were planted on October 8, 2013 and September 12, 2014 using a 10-row Almaco Light-Duty Grain Drill with a single-drop cone (Almaco headquarters, Nevada, IA). The drill was set to plant the cover crops at a 2-cm depth. Before cover crop sowing, the field was tilled to an approximate 10-cm depth using a disk followed by two passes of a field cultivator at a 5-cm depth to allow for a smooth seedbed. The seeding rate of each cover crop species is described in Table 3.
Table 1 Monthly rainfall data for 2014 and 2015.
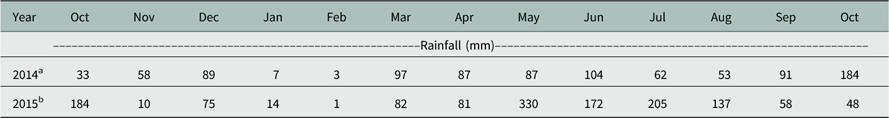
a Planting date: May 23.
b Planting date: June 3.
Table 2 Sources of herbicides used in the experiment.
a Abbreviations: EC, emulsifiable concentrate; FL, flowable liquid; SL, soluble liquid; WDG, water-dispersible granule.
Table 3 Influence of cover crop on Palmer amaranth emergence at 2, 4, 6, and 8 wk after planting (WAP) cotton and total Palmer amaranth emergence at Fayetteville, AR, averaged over 2014 and 2015.
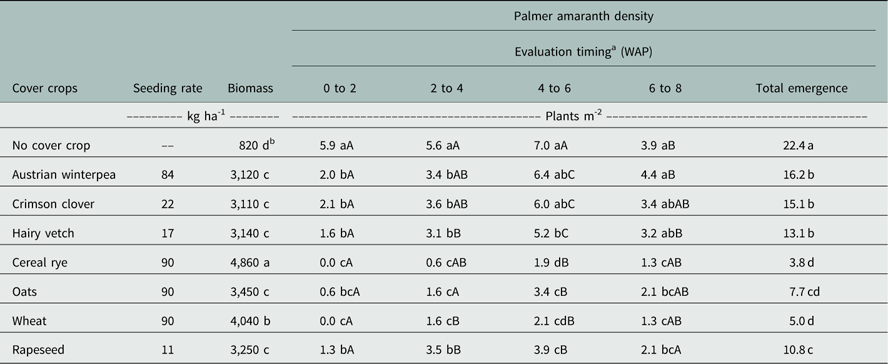
a Lowercase letters are used to compare cover crops within an evaluation timing, and uppercase letters are used to compare evaluation timing within a cover crop. Means followed by the same letter are not different according to Fisher’s protected LSD test at α≤0.05.
b The biomass in the no cover crop treatment was obtained by collecting the natural vegetation present at cotton planting.
Cover crops were terminated with glyphosate at 870 g ae ha-1 plus dicamba at 280 g ae ha-1 at 21 d before cotton planting, followed by a subsequent application of paraquat at 480 g ai ha-1 one day before cotton planting. Aboveground cover crop biomass was sampled from two random 1-m2 quadrats in each main plot at cotton planting. Biomass of the natural vegetation was collected in the no cover crop plots. ST 4946 GLB2 (Stoneville, Bayer Research Triangle Park, NC) cotton was planted with a four-row planter (John Deere 6403, Deere and Company, Moline, IL) equipped with a double-disk opener set to 91-cm-wide row spacing at a seeding rate of 123,000 seeds ha-1. Cotton was planted on May 22, 2014 and June 3, 2015. After cotton seedling emergence, crop density was assessed in 2 m of randomly selected row within each plot and converted to cotton plants per hectare. The residual portion of each main plot was treated with fluometuron at 1,120 g ai ha–1 immediately following cotton planting, followed by S-metolachlor at 1,070 g ai ha–1 plus glufosinate at 595 g ai ha–1 at 14, 28, and 42 d after planting (DAP), followed by flumioxazin at 71 g ai ha–1 plus MSMA at 2,240 g ai ha–1 at 56 DAP as a directed layby application. Glufosinate was applied at 594 g ha–1 to the nonresidual portion of each main plot at 14, 28, and 42 DAP (Table 2). The residual program was designed to prevent weed emergence throughout the season to accurately assess the impact of each cover crop on seed cotton yield. The nonresidual portion of each plot allowed assessment of temporal and total weed emergence in each cover crop. Herbicide treatments were applied using a CO2-pressurized backpack sprayer equipped with a handheld boom that contained four 110015 flat-fan nozzles (Teejet Technologies, Springfield, IL) spaced 48 cm apart and calibrated to deliver 143 L ha-1 at 276 kPa.
Palmer amaranth density was measured in two 0.5-m2 quadrats marked with flags randomly placed within the nonresidual portion of each plot after cotton planting. Palmer amaranth plants emerging were counted and plants removed from both quadrats in each plot every 2 wk until 8 wk after planting (WAP). Herbicide treatments were applied immediately after counts. Seed cotton was hand-harvested from 6 m of row from the residual and nonresidual portions of each plot.
Data were subjected to ANOVA in JMP 12 Pro (JMP, Version 12. SAS Institute Inc., Cary, NC) to test for the main effect of the cover crop. The responses of biomass production, cotton stand, and seed cotton yield were analyzed with an ANOVA to test for significance of the factor cover crop. Palmer amaranth emergence data were fit with a repeated measures model using the Fit Mixed procedure in JMP 12 Pro to describe the number of individuals emerging during an emergence event over time. A first-order autoregressive (AR[1]) covariance structure was assumed, because observations closer in time are expected to have a higher correlation than treatments further apart in time. Hence, Palmer amaranth emergence was analyzed by cover crop as well as by evaluation timing, and means were separated using Fisher’s protected LSD (α=0.05). A regression line was fit to describe the relationship between cotton density and cover crop biomass using SigmaPlot v. 10.0 (Systat Software Inc., San Jose, CA).
Results and Discussion
Cover Crop Biomass
Analysis across years resulted in a significant effect of cover crop species for total biomass production (P<0.0023). Averaged over the 2 years, the maximum biomass production at cotton planting was provided by cereal rye with 4,860 kg ha-1 (Table 3). Wheat provided the second highest biomass production with 4,040 kg ha-1, followed by oats (3,450 kg ha-1), rapeseed (3,250 kg ha-1), crimson clover (3,110 kg ha-1), Austrian winterpea (3,120 kg ha-1), and hairy vetch (3,140 kg ha-1). Winter fallow (no cover crop) produced the least amount of biomass (820 kg ha-1). Amount of total cover crop biomass production at spring planting is highly dependent on climate variables such as growing degree days and rainfall events. Management also plays an important role in biomass production, in that seeding rate, cultivar, and termination timing of cover crops can influence the total amount of biomass produced (Brennan and Boyd Reference Brennan and Boyd2012). The literature contains many reports showing diverse amounts of cover crop biomass production. The amount of biomass produced by each cover crop in this study was consistent with amounts reported by several researchers (Sainju et al. Reference Sainju, Whitehead and Singh2005; Davis Reference Davis2010; Mirsky et al. Reference Mirsky, Curran, Mortenseny, Ryany and Shumway2011).
Palmer Amaranth Emergence
No significant interaction was observed between cover crop species and year; hence, Palmer amaranth emergence was averaged over years. The number of Palmer amaranth plants emerging was influenced by the interaction of cover crop and evaluation timing (P<0.0423). All cover crops significantly suppressed Palmer amaranth emergence at 0 to 2 WAP compared to no cover crop, ranging from 65% to 100% emergence reduction (Table 3). No Palmer amaranth emergence occurred in the cereal rye and wheat plots by the first evaluation timing. It is likely that the amount of residue was the key factor in suppression, because cereal rye and wheat were the cover crops that provided the highest amounts of biomass, which probably affected light penetration to the soil surface and the extent of diurnal temperature fluctuations.
All cover crops continued to reduce Palmer amaranth emergence at 2 to 4 WAP. However, the level of suppression by cover crop residue started to dissipate after 4 WAP, especially among legume cover crops. During 4 to 6 WAP, the number of emerged Palmer amaranth plants in Austrian winterpea and crimson clover plots were not different from the no cover crop plots. During 6 to 8 WAP, similar results were observed, with hairy vetch also not providing any Palmer amaranth suppression. Reddy (Reference Reddy2001) reported similar results where the suppression provided by several cover crops on browntop millet [Urochloa ramosa (L.) T.Q.Nguyen] averaged 87% compared to the no cover crop at 3 WAP. By 6 WAP, the browntop millet suppression declined to 45% compared to no cover crop. That effect is probably linked to the biomass decomposition over time. As the amount of biomass over the top of the soil decreases as a result of the decomposition process, the weed suppression capacity also decreases (Teasdale and Mohler Reference Teasdale and Mohler2000). The rate of biomass decomposition cover depends on several factors such as chemical and physical composition of the soil and cover crop biomass, C:N ratio, microfaunal remains, and climate (Johnson et al. Reference Johnson, Barbour and Weyers2007). Residues with a high C:N ratio are often linked with a slow rate of decomposition, explaining why the cereal cover crops (high C:N ratio) persisted and provided Palmer amaranth suppression up to 8 WAP. The residue from these cover crops persisted on the soil surface longer than legume cover crops (low C:N ratio), as seen in other research (Creamer and Baldwin Reference Creamer and Baldwin2000). Hence, even though cover crops can suppress weeds during early spring, they do not provide acceptable full-season Palmer amaranth suppression (Burgos and Talbert Reference Burgos and Talbert1996; Reeves et al. Reference Reeves, Price and Patterson2005). Nevertheless, a highly productive cover crop system can be integrated with PRE herbicides to provide early-season control and flexibility in timing of POST herbicides. Teasdale et al. (Reference Teasdale, Pillai and Collins2005) reported a synergistic effect of hairy vetch residue and metolachlor on suppression of smooth pigweed (Amaranthus hybridus L.) emergence. Reeves et al. (Reference Reeves, Price and Patterson2005) also reported that weed control obtained with cereal rye in combination with PRE herbicides was similar to the no cover crop plot with PRE and POST herbicides at 60 DAP.
Cotton Density
Further analysis of the 2-year data revealed a significant interaction between cover crop species and year on cotton density (P<0.0445). Hence, the effect of cover crop on cotton density is presented separately. In 2014, all cover crops reduced cotton emergence compared to no cover crop (Table 4). Cereal rye, the highest biomass producer, reduced the cotton stand by 47% compared to the no cover crop plot. Oats were not statistically different from cereal rye, with a cotton stand loss of 42%. Among legume cover crops, crimson clover reduced cotton stand establishment 31% and Austrian winterpea 36%. Hairy vetch compared to no cover crop reduced cotton emergence by 38%; this was not different from Austrian winterpea, but was significantly lower than crimson clover. Among all the cover crop treatments, rapeseed was the least detrimental to cotton emergence; however, it still led to an 18% reduction, which was significantly lower than no cover crop.
Table 4 Cotton density in 2014 and 2015 growing season at Fayetteville, AR, as a function of cover crop.Footnote a
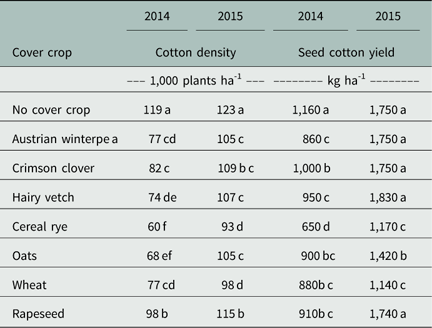
a Means followed by the same letter, either lowercase or uppercase, are not different according to Fisher’s protected LSD test at α≤0.05.
In 2015, a similar trend was observed in cotton emergence, where all cover crop residues decreased cotton emergence compared to the no cover crop treatment (Table 4). Wheat and cereal rye reduced cotton stand by 20% and 25%, respectively. Comparable results were reported by Boquet et al. (Reference Boquet, Hutchinson and Breitenbeck2004), where a reduction of 15% was observed for cotton stands planted into wheat (4,800 kg ha-1 of biomass) plots. Oats and the three legume cover crop species were not significantly different from each other, ranging from 11% to 15% cotton stand reduction. Rapeseed led to a 7% cotton stand reduction.
The negative impact of all cover crops on cotton stand establishment was partly attributed to conditions at time of planting in 2014. A light rainfall event started as the plots were being planted, which hampered the ability of the double-disk openers to effectively cut through the residue. Instead, the double-disk openers tended to cause a “hair pinning” effect, reducing the ability of the press wheels on the planter to cover the cotton seed, especially in plots having high amounts of residue. The result of this effect is a condition wherein the residue interferes with the achievement of adequate seed–soil contact, leading to a negative impact on crop emergence (Kornecki et al. 2009).
The causes of occasional cotton stand reductions as a function of cover crop biomass are not well understood but may be partially a result of the planter setup. The use of new (sharper) double-disk openers would probably have enhanced the ability to cut through cover crop residue, because this planting setup is routinely used by several large-acreage cotton farmers in Arkansas (J.K. Norsworthy, personal communication). Additionally, soil temperature and allelopathy, along with the hair-pinning effect discussed previously, might be considered as factors that contributed to the reduction in cotton emergence in cover crops. Teasdale and Mohler (Reference Teasdale and Mohler1993) reported that high-residue cover crops reduced light transmittance and soil temperature amplitude compared to a fallow field. Low soil root-zone temperatures early in the season can also negatively impact root and shoot growth, leading to reduced cotton stand establishment (Gosselin and Trudel Reference Gosselin and Trudel1985; Tachibana Reference Tachibana1982).
Previous research conducted by Hicks et al. (Reference Hicks, Wendt, Gannaway and Baker1989) showed that cotton emergence was reduced up to 21% when wheat stubble was incorporated into the seedbed. However, when the residues were left on the soil surface, no negative effects were observed. This result may lead to the conclusion that allelopathic effect of cover crop residue is likely to be more concerning when the residue is incorporated into the soil because of the greater soil contact in tilled systems (White et al. 1Reference White, Worsham and Blum989). However, some reports contradict this result. According to Boquet et al. (Reference Boquet, Hutchinson and Breitenbeck2004), cotton seedling establishment was higher when the biomass of wheat and hairy vetch was incorporated into the soil compared to non-incorporation. Stevens et al. (Reference Stevens, Johnson, Varco and Parkman1992) reported that cotton no-till planted into hairy vetch residue led to 30% cotton stand reduction compared to a conventional system planted to fallow. Hence, it is difficult to attribute the effect of allelopathic substances in cotton, as reports on this subject are inconsistent. The most likely reason for cotton stand reduction in this study was the physical interference of the cover crop biomass in the planting operation. Regression analysis performed between cotton density and amount of cover crop biomass at timing of planting shows that as the amount of residue increased, cotton emergence decreased (Figure 1).
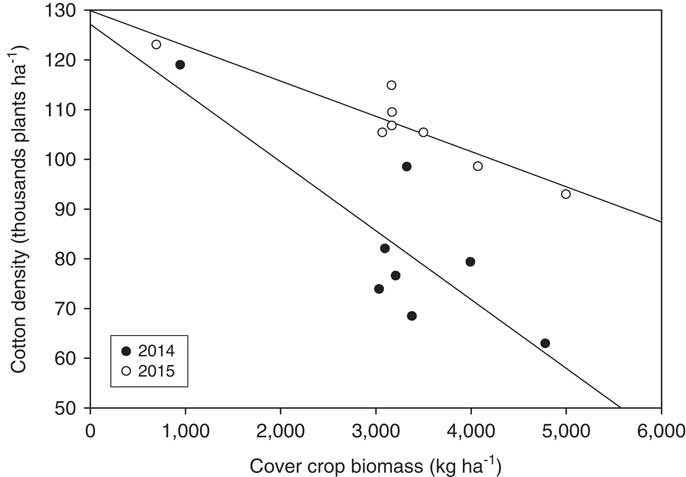
Figure 1 Relationship between cotton density and cover crop biomass at cotton planting for 2014 and 2015. 2014: y=–13.85x+127,186; r2=0.70. 2015: y=–7.09x+129,886; r2=0.87
Seed cotton Yield
In 2014, overall yields were extremely low, probably because of a cooler than normal summer and dry growing conditions (Table 4). The effect of cover crop on seed cotton yield was negative, as all the cover crops decreased seed cotton yield compared to no cover crop. The reduction in final cotton plant population resulting from planting into heavy cover crop biomass is probably the cause of yield reduction. Bridge et al. (Reference Bridge, Meredith and Chism1973) showed that cotton yield increased as the population increased up to 118,000 plants ha-1. As population further increased, yield started to decrease gradually. In addition, positive yield response to increased plant density varies depending upon soil fertility level. The occurrence of large skips and uneven stands are probably the cause of yield reduction observed in these studies.
In 2015, brassica and legume cover crops did not differ from the fallow treatment in seed cotton yield (Table 4). An extensive body of research shows that all brassica species can produce glucosinolates. These compounds have proven to be toxic to cotton (Norsworthy Reference Norsworthy2003) as well as other organisms (Boyd et al. Reference Boyd, Shaw and Martens1994; Zasada and Ferris Reference Zasada and Ferris2004). However, brassica cover crops differ in toxicity level to cotton. Norsworthy et al. (Reference Norsworthy, McClelland, Griffith, Bangarwa and Still2011) observed that cotton yield was not affected by mustard [Brassica juncea (L.) Czern.], but yields were lower in plots followed by turnip (Brassica napus L.), possibly as a result of allelopathy. Rapeseed appears to be safe to use in a cover cropping system prior to cotton planting.
A seed cotton yield decrease was observed for cereal cover crop plots. This result contrasts sharply with several other reports wherein there were no negative effects on cotton yield due to cover crops (Daniel et al. Reference Daniel, Abaye, Alley, Adcock and Maitland1999; Reeves et al. Reference Reeves, Price and Patterson2005). The negative effect in this specific case may be attributed to the stand loss observed in the cereal cover crops (Table 4).
Practical Implications
As discussed previously, it is well known that cover crops have the potential of providing numerous benefits to agricultural systems. However, it is important to set a goal whenever deciding to use cover crops. The species selected to serve as the cover crop will be crucial to achieve the goal desired. If the intention is to use a cover crop to achieve early-season weed suppression, it seems that cereal cover crops are the best option. Two years of data showed that no Palmer amaranth plants emerged in the cereal rye and wheat plots within the first 2 WAP. The suppression effect in the cereal cover crop plots was still significant up to 8 WAP. Brassica and legume cover crops also provided Palmer amaranth suppression early in the season; however, the level of suppression was drastically reduced after 2 WAP, mostly as a result of the higher decomposition rate of these cover crops compared to cereal cover crops (Kuo et al. Reference Kuo, Sainju and Jellum1997; Creamer and Baldwin Reference Creamer and Baldwin2000). Unfortunately, in the high biomass production plots there was greater difficulty in establishing a stand of cotton. Hence, further investigation may be needed to identify better methods to obtain an adequate crop stand into cover crop residues. The decreased cotton stand observed in this study was probably a result of the moist conditions that occurred at the time of planting, and proper equipment should alleviate this problem.
Acknowledgments
Funding for this research provided by the Arkansas Cotton Support Committee.







