Digoxin and propranolol are enteral medications commonly used for the treatment of supraventricular tachycardia in neonates and infants.Reference Binder, Boeche and Atkinson 1 – Reference Gillette, Garson, Eterovic, Neches, Mullins and McNamara 4 Previous reports have noted that the use of digoxin or propranolol is largely based on physician- and practice-related variables as opposed to clinical data.Reference Wong, Potts, Etheridge and Sanatani 3 This is largely owing to the paucity of information comparing efficacy outcomes of the two medications in a clinical setting.
A randomised controlled trial was recently published – the study of antiarrhythmic medications in infancy (SAMIS) trial – that evaluated the efficacy of digoxin as compared with propranolol for prophylaxis of recurrent supraventricular tachycardia in infants.Reference Sanatani, Potts and Reed 5 No differences were noted in the efficacy of digoxin as compared with propranolol. However, the trial, which included 61 infants, may have been underpowered to detect meaningful differences between the two groups, and there was no assessment of acute parameters of efficacy. Therefore, there continues to be a debate regarding the use of these medications. We sought to evaluate the efficacy of digoxin and propranolol as initial enteral therapy, and factors associated with lack of efficacy in the treatment of hospitalised infants with supraventricular tachycardia by reviewing data from a large, national database of freestanding paediatric hospitals. By using a large retrospective database, the issues concerning a small sample size can be overcome. In addition, we sought to determine hospital readmission rates and risk factors for the readmission for patients discharged on digoxin or propranolol for treatment of infant supraventricular tachycardia.
Materials and methods
A retrospective, cohort study was designed using the Pediatric Health Information System database. The Pediatric Health Information System is an administrative database that contains inpatient, emergency department, ambulatory surgery, and observation data from 43 not-for-profit, tertiary care paediatric hospitals in the United States. These hospitals are affiliated with the Child Health Corporation of America (Shawnee Mission, Kansas, United States of America), a business alliance of children’s hospitals. Data quality and reliability are assured through a joint effort between the Child Health Corporation of America and participating hospitals. The data warehouse function for the Pediatric Health Information System database is managed by Thomson Reuters (Ann Arbor, Michigan, United States of America). For the purposes of external benchmarking, participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures. A total of 42 of these hospitals also submit resource utilisation data – for example – pharmaceuticals, imaging, and laboratory – into Pediatric Health Information System. Data are de-identified at the time of data submission and are subjected to a number of reliability and validity checks before being included in the database. A total of 40 hospitals met the study criteria and were included in our analyses.
Patients were included if they had a diagnosis of supraventricular tachycardia (International Classfication of Diseases 9th edition code 427.0), were initiated on either propranolol or digoxin monotherapy as their first enteral antiarrhythmic drug, and were <1 year of age at therapy initiation. Patients were excluded if they received any other enteral antiarrhythmic medication before or on the same day as initiation of propranolol or digoxin therapy, if they received intravenous antiarrhythmic therapy (other than adenosine) during their inpatient stay, had a diagnosis of Wolff–Parkinson–White, or if they died before being discharged (Fig 1).

Figure 1 Flow chart for study.
The Pediatric Health Information System database was queried for patients meeting the study criteria who were discharged from the hospital from 1 January, 2001 to 31 December, 2010. The first admission during the study period was used in the data set. Readmission for any cause and readmission for supraventricular tachycardia for 1 year after discharge were also queried for all patients discharged on either digoxin or propranolol as sole antiarrhythmic therapy.
Patients were considered to have had successful therapy with digoxin or propranolol if they were initiated on either drug as monotherapy and were discharged on the same drug, as they were initiated without the addition of another enteral antiarrhythmic drug or change in drug therapy.
The following patient data were collected: age, gender, race, ethnicity, preterm (<37 weeks on admission) status, length of stay, hospital, presence of a congenital heart defect, type of congenital heart defect, cardiac surgical procedure, ICU admission, use of extracorporeal membrane oxygenation (extracorporeal membrane oxygenation), mechanical ventilation, discharge year, cardiologist as the primary attending physician, and days to all-cause readmission and readmission for supraventricular tachycardia.
Descriptive statistics were used to characterise the population. Student’s t-test and Fisher’s exact test were used to determine differences in patient populations and efficacy of digoxin and propranolol. The correlation between patient volume per hospital and number of successful outcomes was determined using Pearson’s correlation coefficient. A univariate logistic regression analysis was performed, and variables with a p-value of <0.2 were included in a multivariable model to determine factors associated with the success of digoxin, as opposed to propranolol, therapy. Kaplan–Meier analysis was performed to determine differences in time to all-cause readmission, and a stepwise Cox regression analysis was performed in a similar manner as the logistic regression model to determine incidence of readmission for supraventricular tachycardia based on digoxin or propranolol at discharge. A p-value of <0.05 was considered significant a priori.
Results
A total of 374 patients met the study criteria. The population was 59.6% male and the majority of patients were Caucasian (54.8%), followed by Hispanic (17.1%), African-American (14.4%), other (6.9%), and Asian (1.9%). The median age at admission was 33 days (interquartile range of 4–121 days), and 8.9% of the patients were premature, <37 weeks gestation at birth. CHD was present in 53.2 and 32.9% of the patients who underwent a cardiovascular surgical procedure during the admission. The most common congenital heart lesions present included: atrial septal defect (67.8%), patent ductus arteriosus (42.7%), ventricular septal defect (30.7%), endocardial cushion defects (15.1%), and tetralogy of Fallot (11.6%). The most common surgical procedures included: surgical occlusion of the thoracic vessels (53.7%), atrial septal defect repair (32.5%), ventricular septal defect repair (26.8%), repair of tetralogy of Fallot (16.3%), systemic-to-pulmonary artery shunt (14.6%), complete atrio-ventricular canal repair (13.8%), arterial switch operation (11.4%), valvuloplasty (11.4%), and caval pulmonary anastomosis (8.1%). ICU admission occurred in 63.6%, mechanical ventilation in 39.0% of the patients, and extracorporeal membrane oxygenation was used in 0.8% of the population, and hospital length of stay was a median of 7 days (interquartile range of 3–16 days). A paediatric cardiologist was noted as the primary attending physician for 42.3% of the patients.
Digoxin was initiated as the first enteral antiarrhythmic therapy in 47.3% of the patients, and propranolol in 52.7% of the patients. The median age at initiation of therapy was 37 days (interquartile range of 12–127 days), and 45.2% of the patients were ⩽30 days of age. Differences in baseline patient demographic and hospital-related factors for patients initiated on digoxin or propranolol are presented in Table 1. No statistical differences were noted in race or ethnicity in patients started on digoxin or propranolol. Patients receiving digoxin were older at the time of initiation of therapy and more likely to have had CHD and undergone cardiac surgery (Table 1).
Table 1 Patient and hospital variables for use of digoxin or propranolol.

The inpatient efficacy of digoxin and propranol were 73.1 and 73.5%, respectively (Fig 1). Other enteral medications, in combination or monotherapy, used at discharge in patients who did not have monotherapy success (n=100) included: flecainide (13%), amiodarone (11%), sotalol (9%), propafenone (2%), and atenolol (1%). Digoxin and propranolol were used in combination in 28% of the patients who had monotherapy failure. Propranolol was switched to digoxin in 21% and digoxin switched to propranolol in 22% of the patients. All of the variables listed in Table 1 were evaluated with regard to the success of digoxin therapy in a univariate logistic regression model to determine entry into a final multivariable logistic regression model. On univariate analysis, initial therapy with digoxin versus propranolol was not associated with an increased odds of success (odds ratio 1.01, 95% CI 0.64–1.61, p=0.93). In the multivariable logistic regression model, involvement of a paediatric cardiologist (odds ratio 0.46, 95% CI 0.29–0.75, p<0.01) was associated with monotherapy failure (Table 2). Male gender (odds ratio 1.66, 95% CI 1.03–2.67) was associated with successful monotherapy (Table 2). Variability in the use of digoxin and propranolol was noted among paediatric hospitals, and no significant correlation was noted in the number of patients treated per hospital and overall hospital success of therapy (r2=0.03; Figs 2 and 3).
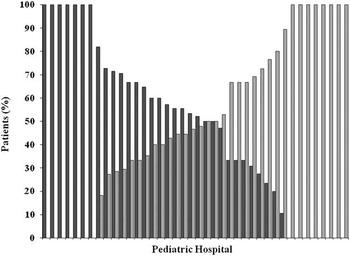
Figure 2 Patients receiving digoxin or propranolol by paediatric hospital. Dark grey box=per cent of patients who received digoxin; light grey box=per cent of patients who received propranolol.

Figure 3 Hospital per cent successful therapy by number of patients treated.
Table 2 Multivariate logistic regression model for success of monotherapy.

Patients discharged on either digoxin or propranolol alone (n=274) were evaluated for readmission for supraventricular tachycardia. On univariate analysis, patients discharged on propranolol were more likely to be readmitted for supraventricular tachycardia (3.1 versus 9.7%, p=0.03). Median time to supraventricular tachycardia readmission for propranolol (53 days, interquartile range of 22–176 days) or digoxin (38 days, interquartile range of 10–67.5 days) was not statistically significantly different (p=0.24; Fig 4). On multivariable analysis, no variables were identified as statistically significant for increasing the risk for readmission for supraventricular tachycardia (p>0.05 for all).

Figure 4 Time to supraventricular tachycardia readmission for patients discharged on propranolol or digoxin.
Discussion
This is the largest evaluation of propranolol and digoxin therapy for the treatment of infant supraventricular tachycardia to date. The benefit of using a large database is the ability to utilise a large sample, and therefore overcome the common issue of lack of statistical power associated with many paediatric studies. It does not appear that digoxin or propranolol exhibit a significant benefit over one another for inpatient treatment of supraventricular tachycardia in infants. Whereas readmission for supraventricular tachycardia after discharge was greater in the propranolol group on univariate analysis, multivariable analyses did not demonstrate a significant difference in digoxin and propranolol. This supports the data from the previous study by Sanatani et alReference Sanatani, Potts and Reed 5 (SAMIS trial), who also did not find a significant difference in propranolol or digoxin therapy when treating infants on an outpatient basis for supraventricular tachycardia.
Dosing strategies of the medications can play a significant role in efficacy. The dosing of digoxin or propranolol was unable to be evaluated from this data source, and may be a significant factor in the efficacy of pharmacotherapy. The dosing of propranolol is variable, with the dose ranging from 1 to 4 mg/kg/day divided every 6–8 hours. High doses (13 mg/kg/day) of propranolol have been used when serum propranolol concentrations have been evaluated.Reference Pickoff, Zies and Ferrer 6 Anecdotally, institutions have adopted propranolol doses on the lower end of this range to avoid adverse events. In contrast, the dosing for digoxin is fairly consistent (10 μg/kg/day divided every 12 hours). It is possible that those patients who failed propranolol therapy were being administered a lower dose and would have better control at higher dosing regimens. Our anecdotal experience suggests that dosing of propranolol at the higher end of recommended dosing (4 mg/kg/day) may provide better outcomes. Adult literature has noted that β-blockers are often less effective in the treatment of various cardiac disease states in different races and ethnicities owing to β-receptor polymorphisms.Reference Shin and Johnson 7 We were unable to assess the effect of dosing of propranolol or the effect of race on propranolol therapy in this study, but both of these areas warrant future investigations when evaluating the efficacy of β-blocker therapy.
The time to readmission after discharge gives some insights into the length of therapy for supraventricular tachycardia in infants. It has been suggested that patients may not need therapy longer than 4 months after initial diagnosis.Reference Sanatani, Potts and Reed 5 The SAMIS trial, which evaluated breakthrough arrhythmia in patients with Holter monitoring, noted that no patient had a first recurrence of supraventricular tachycardia while receiving digoxin or propranolol after 6 months of therapy.Reference Sanatani, Potts and Reed 5 Our data support this suggestion, as the median time to readmission for supraventricular tachycardia in our data set was <3 months. Practice is often to let children “grow out of” their dose while weaning patients from medication on an outpatient basis. There are no data recommending an appropriate weaning schedule, and one would surmise that some patients may have broken through when their dose was weaned as an outpatient. This would likely be different between institutions and practitioners and even from patient to patient, as growth rates would be different. Timing for discontinuation of therapy in infants is an important issue that is not addressed by these data.
Confounding by indication is likely present in the choice of digoxin or propranolol in this data set. A cardiologist may have been more likely to have been consulted in the care of a patient with a difficult to manage arrhythmia. Survey data have suggested that paediatric cardiologists are more likely to use propranolol as compared with digoxin.Reference Wong, Potts, Etheridge and Sanatani 3 Conversely, digoxin was used more frequently in older, critically ill patients, such as those who underwent cardiac surgery, were intubated, had CHD, were admitted to the ICU, or had an increased length of stay. The substrate for arrhythmias in a patient after cardiac surgery is often different and would have different resolution rates as compared with those patients who have an arrhythmia but have not undergone a cardiac surgical procedure. We attempted to overcome these biases by using multivariable statistical analyses, although intrinsic discrepancies may persist. Choice of antiarrhythmic agent will be influenced by patient comorbidities and management of adverse event profiles of each of the medications.
The limitations to this study are those common to retrospective reviews of large databases populated with billing and International Classfication of Diseases 9th edition code information. The International Classfication of Diseases 9th edition code we used is 427.0, which may include re-entrant as well as ectopic focus tachyarrhythmias, which is a limitation to our study. There is a loss of granularity that can be associated with increased sample size, including lack of electrocardiogram data. This limitation cannot be stressed enough when applying the results from this study to a particular patient or patient population. Knowledge of electrophysiologic mechanism for arrhythmia is essential when selecting antiarrhythmic therapy, and the strategies for selection of antiarrhythmic therapy can potentially affect outcomes. Owing to the limitations of the database, we were unable to assess adverse events associated with each of the medications. As we have already mentioned, we were unable to evaluate the effect of dosing of medications on outcomes that may be critical. The broad dosing range of propranolol may have resulted in some institutions using a lower dose and potentially having more breakthroughs. Similarly, the strategy used to maintain doses of drug as patients gained weight may have varied among institutions. The effect of other patient comorbidities, such as diminished kidney or liver function or gastrointestinal conditions that may cause variability in absorption, were not able to be evaluated and would likely impact patient outcomes as well. ICU admission may not have been directly related to the patient arrhythmia. Our endpoint for outpatient efficacy was readmission for supraventricular tachycardia. This may not be the most clinically sensitive endpoint to demonstrate control of supraventricular tachycardia; the SAMIS trial used Holter monitoring to detect breakthrough arrhythmia, and demonstrated a higher incidence of breakthrough (12–18%) as compared with our readmission incidence (3–9%).Reference Sanatani, Potts and Reed 5 Overall, the data from this study demonstrate and reinforce, in a broad manner, the lack of superiority of digoxin or propranolol in a cohort of supraventricular arrhythmias. Despite these limitations, this study does provide the largest data set regarding the potential efficacy of propranolol and digoxin in the treatment of inpatient infant supraventricular tachycardia.
Digoxin or propranolol may have similar efficacy in the inpatient treatment of infant supraventricular tachycardia. The effects of medication dosing strategies and patient pathophysiology may need to be considered for antiarrhythmic selection to attain best possible patient outcomes.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sector.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional review board of Baylor College of Medicine.








