Introduction
Lichens are not easy to cultivate under controlled laboratory conditions (e.g. Scott Reference Scott1960; Kershaw & Millbank Reference Kershaw and Millbank1969). An ability to maintain lichens under such conditions would be of great importance for experimental lichen biology. In earlier laboratory experiments, relative growth rate was measured in discs cut from lichens (Scott Reference Scott1956, Reference Scott1960; Pearson Reference Pearson1970), while others dealt with whole lichen thalli (Kershaw & Millbank Reference Kershaw and Millbank1969; Pearson & Benson Reference Pearson and Benson1977). In recent years, growth experiments have increasingly been used to investigate the effects of environmental impacts on entire lichens in field (Hyvärinen & Crittenden Reference Hyvärinen and Crittenden1998; Cooper & Wookey Reference Cooper and Wookey2001; Gauslaa et al. Reference Gauslaa, Lie, Solhaug and Ohlson2006, Reference Gauslaa, Palmqvist, Solhaug, Holien, Hilmo, Nybakken, Myhre and Ohlson2007; McCune & Caldwell Reference McCune and Caldwell2009; Yemets et al. Reference Yemets, Solhaug and Gauslaa2014; Marks et al. Reference Marks, Pett-Ridge, Perakis, Allen and McCune2015) and laboratory studies (Cooper et al. Reference Cooper, Smith and Wookey2001; Bidussi et al. Reference Bidussi, Gauslaa and Solhaug2013; Alam et al. Reference Alam, Gauslaa and Solhaug2015). By integrating important processes, lichen growth is among the most robust measures of lichen performance and viability. However, because lichens are poikilohydric and repeatedly undergo hydration-desiccation cycles under natural conditions (Green et al. Reference Green, Sancho and Pintado2011), they need special water delivery in growth chamber experiments to maintain, for example, a viable balance between involved symbiotic partners (MacFarlane & Kershaw Reference MacFarlane and Kershaw1982). Although very high growth rates have been measured in lichens transplanted to wet filter paper in short-term growth chamber experiments, damage such as local browning has often occurred in the last days of previous experiments (Bidussi et al. Reference Bidussi, Gauslaa and Solhaug2013; Alam et al. Reference Alam, Gauslaa and Solhaug2015). Our main objective is to improve short-term growth cabinet protocols in order to reduce such damage. To do so, we compare growth rates in lichens on well-drained nets and on wet filter papers.
Nocturnal hydration boosts lichen growth in growth cabinets (Bidussi et al. Reference Bidussi, Gauslaa and Solhaug2013; Alam et al. Reference Alam, Gauslaa and Solhaug2015), but how various transitions between day and night affect lichen growth has not been studied. Many lichens are susceptible to high light in hydrated (Demmig-Adams et al. Reference Demmig-Adams, Adams, Green, Czygan and Lange1990; Gauslaa & Solhaug Reference Gauslaa and Solhaug1996) and desiccated states (Gauslaa et al. Reference Gauslaa, Coxson and Solhaug2012; Färber et al. Reference Färber, Solhaug, Esseen, Bilger and Gauslaa2014; Carniel et al. Reference Carniel, Zanelli, Bertuzzi and Tretiach2015). Because temporal acclimation may occur (MacKenzie et al. Reference MacKenzie, MacDonald, Dubois and Campbell2001, Reference MacKenzie, Johnson and Campbell2004; Heber Reference Heber2008; Štepigová et al. Reference Štepigová, Gauslaa, Cempírková-Vrábliková and Solhaug2008; Fernández-Marín et al. Reference Fernández-Marín, Becerril and García-Plazaola2010; Heber et al. Reference Heber, Bilger, Türk and Lange2010), effects of gradual versus abrupt transitions between light and darkness should be studied. Thus, our last objective was to test the hypothesis that gradual increases and decreases in light mimicking natural sunrise and sunset, respectively, support higher growth rates than the common practice of switching light on/off.
Methods
The foliose lichen Lobaria pulmonaria (L.) Hoffm. was collected in Langangen, SE Norway (59·112–59·113°N, 9·832–9·835°E, 130–160 m a.s.l.), cleaned of adhering debris, and stored air-dry at −18°C. Young and healthy thalli (n=160) without or with just a few reproductive structures (soralia) were randomly selected for each treatment. Before and after the experiment, thalli were dried at 20°C for 24 h before their air-dried mass was recorded. Five thalli were additionally oven-dried for 24h at 70°C to measure DM. We used the oven-dried/air-dried mass ratio for these thalli to calculate the DM for all experimental thalli. Before and after cultivation, we sprayed thalli with excess deionized water and kept them in low light for 24 h, and measured the maximal quantum yield of photosystem II (F V /F M) after 15 min dark-adaptation.
The growth experiment (14 days) was run in Sanyo MLR-351 growth cabinets (Sanyo Electric, Osaka, Japan). The temperature was 18/12°C (day/night; 12h photoperiod; close to the optimal temperature for growth according to Bidussi et al. Reference Bidussi, Gauslaa and Solhaug2013), and relative humidity was kept high (70±5% during the light period and 90±5% during the night) by placing repeatedly wetted newspapers on the top and bottom shelves. In the on/off light treatment, light was constant (200±10 μmol photons m−2 s−1) during the day. In the gradual treatment, light was increased in five steps from 0 through 15, 30, 50, and 120 to 200 μmol photons m−2 s−1 for 1 h in the morning, and the protocol was reversed during the last daylight hour. 200 μmol photons m−2 s−1 (from Mitsubishi/Osram FL 40SS W/37) was used because it approximates light saturation in L. pulmonaria (Solhaug et al. Reference Solhaug, Xie and Gauslaa2014). In both light regimes, lichens experienced the same daily light dose, but thalli with gradual transitions had 1 h longer days. In each light regime, we let 40 thalli grow on filter papers, and 40 on nets. For the paper treatment, we turned open Petri dishes upside down and placed five layers of paper and two lichen thalli on each of them. For the net treatment, we used wet filter papers inside open Petri dishes, but glued a circular net to the edge of the top of each Petri dish to prevent direct contact between lichens and the wet filter paper underneath. All thalli were kept wet most of the day by spraying before the onset of light and shortly before darkness. The spraying regime allowed thalli to desiccate for 3–4 h at the end of the light period, whereas the evening spraying kept them hydrated until the morning. Generally, lichens on nets appeared not to be as wet as lichens on papers.
Afterwards, we measured chlorophyll in 20 randomly selected thalli from each treatment. Two discs from each thallus were removed by a 5 mm cork borer and put in an Eppendorf tube. We added 1·5 ml of DMSO with MgCO3. After incubating tubes at 60°C for 30 min in an ultrasonic water bath (model USC 200 TH, VWR, Leuven, Belgium), the absorbance of the extract was measured at 649, 665 and 750 nm and chlorophyll a and b were calculated using equations from Wellburn (Reference Wellburn1994).
A general linear model for log-transformed RGR was run with the fixed factors substratum and light regime, using F V /F M at start, and change in F V /F M, as covariates (Minitab v.17, Minitab Ltd, Coventry, UK). To compare parameters across treatments, we used the Kruskal-Wallis ANOVA of Variance on Ranks with all pairwise multiple comparison procedures (Dunn’s method; SigmaPlot v. 11, Systat Software Inc). Unless standard deviation (SD) is explicitly specified, means are given with±1 standard error.
Results
Growth in L. pulmonaria was high (Fig. 1). The highest individual growth was 37·1% (RGR=22·5 mg g−1 d−1) for a thallus on net with on/off light. On average, lichens grew 1·6 times faster on nets (21·0±0·7%, RGR=13·5±0·4 mg g−1 d−1, n=80) than on paper (12·6±0·5%, RGR=8·5±0·3 mg g−1 d−1, n=80). However, the light regime had no significant effect on growth rates, neither alone, nor in interaction with substratum type (Table 1). Growth was as fast with on/off transitions between day and night as with gradually increasing/dampened light mimicking natural sunrise and sunset. F V /F M at the start (0·710±0·017, mean±1SD, n=159, total range 0·654–0·744) did not vary between thalli selected for each of the treatments. Nevertheless this parameter, as well as the percent change in F V /F M (100·8±2·4, mean±1SD, n=159, total range 83·0–110·4, Fig. 1), were positively related to growth rate (Table 1).
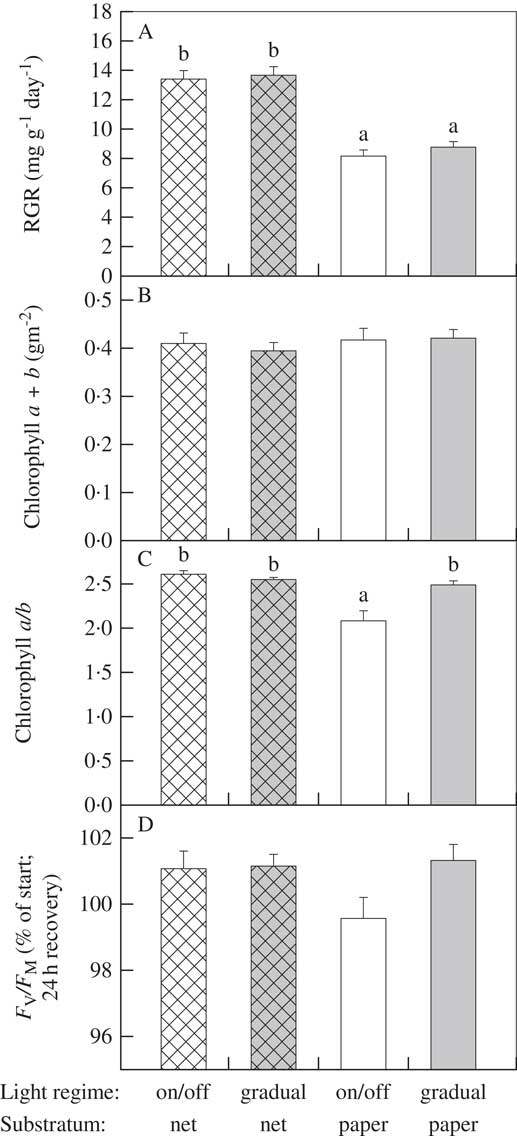
Fig. 1 A, Relative Growth Rates; B, chlorophyll a+b content; C, chlorophyll a/b-ratio; D, F V /F M in % of start values in the foliose lichen Lobaria pulmonaria after a 14 day growth experiment in growth cabinets across two light regimes (day-night on/off transitions versus gradual transitions) and two growth substrata (self-drained net versus wet filter paper). Means±1SE are given; n=40 for RGR and F V /F M; n=20 for chlorophyll a+b and the a/b-ratio. For each species, bars with different letters are significantly different (P<0·05) as determined by Kruskal-Wallis ANOVA with all pairwise multiple comparison procedures (Dunn’s method).
Table 1 General Linear Model for RGR with light regime (on/off or gradual) and substratum (paper or net) as fixed factors, and F V /F M at start and change in F V /F M (in % of start values) as covariates. The table analyzes the RGR-data shown in Figure 1

RGR was log-transformed. Coefficients for both F V /F M-parameters were positive. The most highly deviating thallus (standardized residual=3·71) was excluded before the analysis; its RGR was normal, but it had unusual F V /F V values.
Slowly, and gradually, large brown patches formed on most thalli placed on paper, whereas no visible colour changes occurred on those on nets (Fig. 2). These brown patches sometimes stained the paper (Fig. 2). Total chlorophylls did not vary between treatments (0·41±0·09 g m−2, mean±1SD, n=80, total range 0·21–0·62 g m−2), whereas the chlorophyll a/b-ratio (2·43±0·36, mean±1SD, n=80, total range 0·80–2·87) was significantly lower in thalli exposed to the on/off light regime on wet paper (Fig. 1). Thereby, thalli subjected to partial browning did not experience loss of chlorophylls.

Fig. 2 Lobaria pulmonaria after cultivation for 2 weeks on wet filter paper (upper series) and on nets without contact with wet paper (lower series). In colour online.
Discussion
The growth rates of L. pulmonaria on paper (Fig. 1) were as high as the unusually high rates reported in an earlier growth chamber study (Bidussi et al. Reference Bidussi, Gauslaa and Solhaug2013). Thus, the 60% increase in RGR for L. pulmonaria grown on nets compared to those on filter paper (Fig. 1) implies a substantial advancement in at least short-term growth chamber protocols. Furthermore, by growing lichens on self-drained nets, we eliminated the formation of brown patches that often occurs in thalli grown on paper. The observed browning was neither cortical melanins, because this browning does not occur in the upper cortex (Gauslaa & Solhaug Reference Gauslaa and Solhaug2001), nor was it a result of chlorophyll degradation (Fig. 1). The observed browning is probably some kind of damage because F V /F M in many thalli on paper was slightly reduced after 24 h recovery at low light, and the chlorophyll a/b-ratio was also significantly reduced (although only for the on/off light regime; see Fig. 1). At high hydration levels, MacFarlane & Kershaw (Reference MacFarlane and Kershaw1982) showed rapid transfer of carbohydrates from the photobiont to the mycobiont, suggesting some breakdown of the symbiosis due to starvation of photobionts. Unknown leached compounds staining the paper (Fig. 2) suggests that the browning is a kind of damage. Nevertheless, growth was high in thalli with browning.
During the experiment, we saw more surface water on lichens that were on papers than on nets. The combination of surplus water and reduced growth rates is consistent with more frequent suprasaturation depression of photosynthesis (Lange & Matthes Reference Lange and Matthes1981; Lange et al. Reference Lange, Büdel, Heber, Meyer, Zellner and Green1993) in thalli hydrated on paper than on nets. Depression of photosynthesis due to blocked CO2 pathways during light periods probably leads to increased oxidative stress and photoinhibition, evidenced by the reduced F V /F M in many thalli on paper. However, we cannot exclude other damaging factors associated with, for example, the localized browning in thalli on paper. Consistent with our results, Scott (Reference Scott1960) found the highest growth in Peltigera discs was with an intermediate supply of moisture, and dieback of lichens has been recorded in long-lasting, continuous rainy periods in the field (Gauslaa Reference Gauslaa2002).
By testing the effect of light conditions during transitions between day and night, we did not find any support for the hypothesis inferring lower growth at abrupt versus gradual transitions between darkness and light periods. This means that lichens handle well sudden shifts between darkness and at least moderate high light.
In conclusion, by placing lichens on nets rather than on paper in growth chambers, RGR increased by 60% from levels that were already considered high for lichens. By this improved protocol, growth chamber cultivation of lichens becomes a more powerful tool to assess effects of environmental factors on lichens during short-term experiments.
We thank William B. Sanders for constructive comments on the manuscript.





