INTRODUCTION
Gastrointestinal parasites are responsible for significant morbidity in lambs during their first grazing season and in grazing sheep (Mulcahy et al. Reference Mulcahy, O'Neill, Donnelly and Dalton2004). The value of an anthelmintic-based approach to parasite control is threatened by the emergence of anthelmintic resistance; thus other control measures are needed. There is substantial evidence for within- and between-breed differences in resistance to nematode infection in sheep (inter alia, Miller et al. Reference Miller, Bahirathan, Lemarie, Hembry, Kearney and Barras1998; Hanrahan and Crowley, Reference Hanrahan and Crowley1999; Matika et al. Reference Matika, Nyoni, van Wyk, Erasmus and Baker2003; Gruner et al. Reference Gruner, Aumont, Getachew, Brunel, Pery, Cognie and Guerin2003; Amarante et al. Reference Amarante, Bricarello, Rocha and Gennari2004). A key aim of research on resistance to nematodes is to identify the physiological and genetic basis for natural resistance. This would aid in the development of sustainable methods of nematode control, including breeding programmes for increased resistance. The majority of loci associated with resistance are involved in immunological processes, for example MHC genes (Schwaiger et al. Reference Schwaiger, Gostomski, Stear, Duncan, McKellar, Epplen and Buitkamp1995; Stear et al. Reference Stear, Bairden, Bishop, Buitkamp, Epplen, Gostomski, McKellar, Schwaiger and Wallace1996; Sayers et al. Reference Sayers, Hanrahan, Good, Ryan, Angles and Sweeney2005a), the interferon gamma gene (Coltman et al. Reference Coltman, Wilson, Pilkington, Stear and Pemberton2001; Sayers et al. Reference Sayers, Good, Hanrahan, Ryan and Sweeney2005b) and the IgE gene (Clark et al. Reference Clarke, Burn, Lenane, Windon and Beh2001), suggesting that the nature and level of an immune response to nematode infection is an important facet of natural resistance.
In efforts to characterize the immune response to gastrointestinal nematode invasion in sheep, studies have principally focused on cytokine, humoral and cellular responses to infection. Consensus in the literature indicates a T helper cell type 2 (TH2) response predominate (Lacroux et al. Reference Lacroux, Nguyen, Andreoleiti, Prevot, Grisez, Bergeaud, Gruner, Brunel, Francois, Dorchies and Jacquiet2006), proliferation characterized by increased expression of interleukin-5 (IL-5), IL-13 and tumor necrosis factor alpha (TNF-α) (Gill et al. Reference Gill, Altmann, Cross and Husband2000; Pernthaner et al. Reference Pernthaner, Cole, Morrison and Hein2005a, Reference Pernthaner, Cole, Morrison, Green, Shaw and Hein2006), increased IL-4 and IL-13 (Finkelman et al. Reference Finkelman, Shea-Donohue, Morris, Gildea, Strait, Madden, Schopf and Urban2004) and increased mRNA expression for IL-6 following a nematode infection (Shen et al. Reference Shen, Bao, McClure, Emery and Husband2000). Analysis of humoral responses to infection have highlighted elevated levels, and potential roles, for IgG1 (Pernthaner et al. Reference Pernthaner, Cole, Morrison, Green, Shaw and Hein2006), IgA (Stear et al. Reference Stear, Bishop, Doligalska, Duncan, Holmes, Irvine, McCrire, McKellar, Sinski and Murray1995, Reference Stear, Bairden, Bishop, Buitkamp, Duncan, Gettinby, McKellar, Park, Parkins, Reid, Strain and Murray1997; Martinez-Valladares et al. Reference Martinez-Valladares, Vara-Del Rio, Cruz-Rojo and Rojo-Vazquez2005; Henderson and Stear, Reference Henderson and Stear2006) and IgE (Prichard et al. Reference Prichard, Hewitt and Moqbel1997; Shaw et al. Reference Shaw, Gatehouse and McNeill1998; Huntley et al. Reference Huntley, Redmond, Welfare, Brennan, Jackson, Kooyman and Vervelde2001; Pernthaner et al. Reference Pernthaner, Shaw, McNeill, Morrison and Hein2005b; Pettit et al. Reference Pettit, Jackson, Rocchi and Huntley2005). Research on the cellular response to infection includes eosinophils regulating worm growth (Stear et al. Reference Stear, Henderson, Kerr, McKellar, Mitchell, Seeley and Bishop2002; Balic et al. Reference Balic, Cunningham and Meeusen2006; Henderson and Stear, Reference Henderson and Stear2006), elevated mucosal mast cells (Pfeffer et al. Reference Pfeffer, Douch, Shaw, Gatehouse, Rabel, Green, Shirer, Jonas and Bisset1996), eosinophilia, mastocytosis and IgE production (Mulcahy et al. Reference Mulcahy, O'Neill, Donnelly and Dalton2004). In addition it is suggested that non-resistant lambs may be characterized by comparatively weak serum IgE and eosinophil responses (Schallig, Reference Schallig2000).
Studies in Ireland have shown that the Texel breed is naturally more resistant to nematode infection than the Suffolk breed, based on faecal egg counts (FEC) and worm burden following natural infection (Hanrahan and Crowley, Reference Hanrahan and Crowley1999; Good et al. Reference Good, Hanrahan, Crowley and Mulcahy2006). As part of a programme aimed at identifying the immunogenomic basis for the difference in resistance between these breeds, the present study was undertaken to investigate the mucosal and systemic humoral and haematological responses to a natural gastrointestinal nematode infection in the Suffolk and Texel breeds. The animals used in the present study were a subset of those described in an earlier paper that described the differences in FEC and worm burden between Suffolks and Texels (Good et al. Reference Good, Hanrahan, Crowley and Mulcahy2006).
MATERIALS AND METHODS
Animals and tissue samples
A total of 57 Suffolk and 85 Texel pure-bred lambs were used, representing 2 lamb crops (2001 and 2002). The cohort of animals used had at least 4 sires represented for each breed, in each year. Animal management and standard parasitological measurements have been described previously by Good et al. (Reference Good, Hanrahan, Crowley and Mulcahy2006). Briefly, all lambs were born indoors in early March each year and were put to pasture with their dam within 1 to 3 days of birth. Both breeds were co-grazed on pasture until weaning at 17 weeks of age. Blood samples were taken in vacutainers with EDTA, lithium-heparin or no anticoagulant, respectively (Becton Dickinson Company, UK) from all lambs by jugular venepuncture at 11, 14 and 17 weeks for subsequent haematological, blood biochemistry and serum antibody measurements. Blood collected for haematological analysis was refrigerated prior to analysis and blood collected for blood biochemistry measurements was centrifuged at 2000 g for 5 min at 5°C, plasma extracted and stored at −20°C. The blood collected for serum antibody measurements was stored overnight in a refrigerator to facilitate clotting. Samples were centrifuged at 2000 g for 5 min, serum extracted and stored at −20°C.
At weaning, lambs were chosen at random within sire groups (2001, n=6 per breed; 2002, n=4 per breed), for measurements that would describe the mucosal cellular and humoral response to infection. At necropsy, surface and mucus epithelial layers of the abomasum and intestine were scraped off using a glass slide, placed in cryovials and snap frozen using dry ice and stored at −80°C. Data on the FEC at slaughter and worm burdens in the abomasum and small intestine were available from a previous study (Good et al. Reference Good, Hanrahan, Crowley and Mulcahy2006).
Blood biochemistry
Total serum protein concentration (g/dl) was determined by refractometry and albumin (g/dl) by colourimetry, the difference calculated between these two measurements being the serum globulin concentration. Plasma pepsinogen concentration was determined using a standard method (Ross et al. Reference Ross, Purcell, Dow and Todd1967).
Haematology
Blood smears were made, air dried and fixed in ethanol within 2 h of sample collection. Smears were subsequently stained with DiffQuik® (Dade Behring). White blood cells (n=100) were subsequently differentiated using a binocular microscope (×100). The numbers of lymphocytes, neutrophils, monocytes, basophils and eosinophils were calculated from the total white blood cell count determined as described below.
In 2001, haematological variables, which included total white blood cell count (WBCC×109/l), red blood cell count (RBCC×1012/l), packed cell volume (PCV l/l), mean corpuscular volume (MCV fl), haemoglobin concentration ([Hb] g/dl) thrombocyte count (×109/l), mean corpuscular haemoglobin concentration (MCHC×109/L), mean platelet volume (MPV) and platelet count (PCT), were determined using an automatic analyser (Cobas Minos Vet, ABX).
In 2002, 50 μl of whole blood were added to 950 μl of Turks solution and WBCC determined using a haemocytometer.
Mucosal antibody recovery
Tissue preparation for antibody recovery was carried out in accordance with Sinski et al. (Reference Sinski, Bairden, Duncan, Eisler, Holmes, McKellar, Murray and Stear1995). Briefly, thawed samples were homogenized using an electric homogenizer (PT-MR 2100, Kinematica, Switzerland) in 3 volumes of PBS (phosphate-buffered saline) containing a protease inhibitor cocktail (broad specificity for the inhibition of serine, cysteine, aspartic proteases and aminopeptidases) at 5 μg/ml (Sigma, UK). The homogenate was centrifuged at 12 000 g for 30 min and the supernatant removed and stored at −20°C prior to analysis. Prior to freezing, an aliquot of the supernatant was removed and protein concentration determined using the BCA protein assay reagent kit (Pierce, IL, USA).
Antigen preparation for ELISA
Antigen from third-stage larvae (L3) was prepared according to the method of Sinski et al. (Reference Sinski, Bairden, Duncan, Eisler, Holmes, McKellar, Murray and Stear1995). Teladorsagia L3 larvae (1×106) obtained from faecal cultures were cleaned extensively by centrifugation, filtered through saturated sucrose solution (MAFF, 1986) and washed in PBS. Larvae were homogenized by vortexing with 180 μm (diameter) glass beads (Sigma, UK) for 60 min. The homogenate was repeatedly frozen and thawed 10 times and stored at 4°C overnight after the addition of 5 mg/ml of a protease inhibitor cocktail (Sigma, UK). The homogenate was centrifuged at 14 000 g for 60 min and the supernatant sterilized by passing through a 0·22 mm diameter filter. Protein concentration was determined using the BCA protein assay kit (Pierce, IL, USA), adjusted to 250 μg and then stored at −20°C.
Nematode-specific antibody measurement: ELISA procedure
The wells of polystyrene microtitre plates (96-well, flat bottomed; F96 Maxisorb, Nunc, Denmark) were coated with 100 μl of L3 antigen (5 μg/ml) in carbonate buffer at pH 9·6, and incubated overnight at 4°C. Plates were washed 4 times in PBS-T (PBS containing 1% Tween). An aliquot (100 μl) of either mucosal sample (adjusted to 500 μg/ml in PBS-T/ 3% bovine serum albumin (BSA)) or serum (diluted 1/1000 for IgG1, 1/100 for IgG2, and diluted to 1/100 for IgE, and 1/50 for IgA) was added to each well and incubated at 37°C for 30 min. For IgE analysis, the mucosal sample and serum were pre-heated to 56°C (Kooyman et al. Reference Kooyman, VanKooten, Huntley, MacKellar, Cornelissen and Schallig1997). Plates were then washed 4 times in PBS-T. For each antibody assay, 100 μl of the relevant monoclonal anti-sheep antibody was added to each well and incubated at 37°C for 30 min. The monoclonal antibodies used were as follows: mouse anti-sheep-IgG1 (CSIRO Livestock Industries, Australia, diluted 1/1000 in PBS-T 3% BSA), mouse monoclonal anti-sheep-IgG2 (CSIRO, Sydney, Australia, diluted 1/1000 in PBS-T 3% BSA), rat monoclonal anti-sheep-IgA (received from Professor John Hopkins, Edinburgh University, UK, un-purified cell culture supernatant diluted 1/20 in PBS-T 3% BSA), mouse monoclonal anti-sheep-IgE (received from Professor Frans Kooyman, Utrecht University, The Netherlands, diluted 1/1000 in PBS-T 3% BSA). Following the incubation step, plates were washed 4 times with PBS-T, and 100 μl of either a secondary horseradish peroxidase (HRP)-labelled rabbit anti-mouse (DakoCytomation, Denmark) (used for IgG1, IgG2 and IgE based wells) or a HRP-labelled rabbit anti-rat (DakoCytomation, Denmark) (used for IgA based wells) were added and incubated at 37°C for 30 min. Following 4 washes with PBS-T, 100 μl of the chromogen tetramethylbenzidine (TMB) (DakoCytomation, Denmark) were added to each well and incubated at room temperature for 15 min. The reaction was stopped using 100 μl of 10% 1 m HCl. The optical density of each well was measured at 450 nm using a microwell plate reader (Expert 96 with Kim software v5.2, Asys Hitech, Austria). All samples were assayed in duplicate and each plate included a blank (PBS replacing serum), a negative control (a single serum sample yielding low levels of nematode-specific antibodies for all isotypes) and a positive control (a single serum sample yielding high levels of nematode-specific antibodies for all isotypes). Antibody isotype activity was interpreted from the optical density (OD) minus the OD of the blank. The inter-assay variation was estimated using the positive control which was run on all plates. This variation, estimated at 24%, was accounted for in statistical models by including ELISA plate number as a factor.
Statistical analyses
Haematological data were analysed using Proc MIXED (SAS, 2000) with animal within breed as a random effect. Prior to analysis, the haematological variables were transformed using square root transformation to stabilize the variance. The initial model included breed, rearing type (single or twin), sex, lamb age (weeks), year (where data were collected over the 2 years i.e. white blood cell data) and all 2-way interactions between these effects. With the exception of breed-by-age interaction, all other terms were dropped from the model (P>0·25 in each case).
Blood biochemistry data
Pepsinogen, albumin, globulin and protein data were analysed using Proc MIXED (SAS, 2000) with animal within breed as a random effect. Prior to analysis, the pepsinogen was transformed to logarithms (ln (x+1)) to normalise the distribution. No transformation was applied to the albumin, globulin or protein data. The initial model had fixed effects for year, breed, rearing type (single or twin), sex, age of lamb (weeks) and 2-way interactions representing year-by-age, breed-by-age and year-by-breed interaction. Year-by-breed interaction was not significant for any variable and was dropped from the final model.
Antibody data
Data on the antibody measurements were analysed using Proc MIXED (SAS, 2000) with individual animal within breed declared as random. The initial model used for serum antibody concentration had effects for breed, rearing type (single or twin), sex, lamb age (weeks) and ELISA plate number (which was confounded with year) and all 2-way interactions between these effects. With the exception of the fixed effects of breed, lamb age and ELISA plate number, all other terms were dropped from the final model (P>0·25 in each case). The initial model used for mucosal antibody responses had effects for breed, site (abomasum or small intestine), year (=ELISA plate number) and all 2-way interactions between these effects. Interaction terms were dropped from the final model as P>0·25.
Correlation of mucosal antibody response with FEC and worm burdens
Analysis of mucosal antibodies and their association with FEC and worm burden at post-mortem was examined by breed, in 10 Suffolks and 10 Texels. FEC and worm burden were transformed by ln (FEC+25) and ln (worm burden+1), respectively. Residual values from the least square models above for mucosal antibody responses were matched with corresponding residual values for FEC at slaughter, worm burden in the abomasum and worm burden in the small intestine. These sets of data were used to calculate separate correlation estimates for each combination of breed (n=2) by antibody isotype (n=4) by tissue site (n=2). This yielded 3 sets of 16 correlation coefficients. These correlations were transformed (Fisher z) to give normally distributed values which were subjected to analysis of variance to allow an examination for evidence of breed and isotype effects on the relationships. The model used had fixed effects for breed, antibody isotypes and their interactions.
RESULTS
Faecal egg count and worm burden data
A summary of the mean and range for FEC and worm burden measurements of lambs is presented in Table 1. Texels had a significantly lower FEC and worm burden than Suffolks. As these lambs are a subset of those previously described, the reader is referred to Good et al. (Reference Good, Hanrahan, Crowley and Mulcahy2006) for a detailed comparative analysis and discussion of the nematode burden and FEC data, in Suffolks and Texels.
Table 1. Mean worm burden and faecal egg count (epg) for Suffolks and Texels under a natural infection at post-mortem

† The range values are presented in parenthesis.
Blood biochemistry
Pepsinogen concentration, albumin and globulin concentrations and the albumin:globulin ratio are shown in Figs 1, 2A and B, respectively. Suffolks had a higher concentration of pepsinogen than Texels at all time-points (as shown in Fig. 1) and the overall breed effect was significant (P<0·001). Pepsinogen concentrations were also significantly (P<0·001) affected by age, and breed-by-age interaction.

Fig. 1. Least squares means (back transformed) for pepsinogen concentration in Suffolk and Texel lambs at 11, 14 and 17 weeks of age (effects of breed, age and breed by age interaction were all highly significant (P<0·001)).

Fig. 2. Albumin and globulin levels (A) and albumin:globulin ratio (B) in Suffolk and Texel lambs at 11, 14 and 17 weeks of age (effects of age were significant (P<0·001) for all variables; breed-by-age interaction was significant for globulin (P<0·001) and for the albumin:globulin ratio (P<0·05)).
The overall breed effect for albumin and globulin concentrations and the albumin:globulin ratio were not significant; however, concentrations significantly changed over time as indicated by a significant (P<0·001) age effect. The significant breed-by-age interaction for globulin reflected the fact that there was no apparent difference between breeds at 11 weeks and that while Suffolks had a higher globulin concentration than the Texel at 14 weeks this pattern was reversed at 17 weeks, as shown in Fig. 2A. The breed and age effects for albumin:globulin ratio largely reflects the opposite age trends for these variables and the breed differences in globulin at 14 weeks.
Haematology
The mean values for haematological measurements are presented in Fig. 3. Haematological measurements were within the reference values for both breeds over time. Breed and age had significant effects on most red blood cell variables. Where there were significant breed-by-age interactions, the direction for the difference between breeds was consistent across ages. Suffolks had higher RBC count (P<0·001), PCV (P<0·05) and [Hb], MCV was greater in Texels (P<0·001). While there were significant effects associated with sex and interactions involving sex, these differences were generally small and did not display any consistent patterns.

Fig. 3. Least squares means (back transformed values) for haematological measurements in Suffolk and Texel lambs at 11, 14 and 17 weeks of age (breed differences were highly significant (P<0·001) for all variables except packed cell volume (P<0·05) and haemoglobin concentration (P<0·10); age effects were highly significant (P<0·001) for all variables except haemoglobin concentration (P<0·01); breed-by-age interaction was significant for red blood cell number (P<0·01) and mean corpuscular volume (P<0·05)).
The results for white blood cells show that Suffolks had a significantly (P<0·001) higher numbers of total leukocytes at all ages and that this was reflected in the corresponding differences in lymphocytes and neutrophils. Eosinophil numbers were low at 11 and 14 weeks for both breeds but had increased by 17 weeks, especially in Texels, although the breed-by-age effect was not significant.
Nematode-specific serum antibody response
The mean OD values for the 4 antibody isotypes at each age are presented in Fig. 4. Nematode-specific serum antibody activities were significantly greater in Texels for all the isotypes and the highly significant breed-by-age interaction reflected the increasing divergence between the breeds as age increased.

Fig. 4. Least squares means for serum anti-nematode-IgG1, -IgG2, -IgA and -IgE antibodies in Suffolk and Texel lambs at 11, 14, 17 weeks of age (breed, age and breed-by-age effects were highly significant (P<0·001) for all variables. Background OD values (mean±s.e.) were as follows; IgG1: 0·23±0·018; IgG2: 0·13±0·015; IgA: 0·09±0·007; IgE: 0·12±0·009.
Nematode-specific mucosal antibody response
Mucosal antibody OD values at 17 weeks of age are presented in Fig. 5. While the Texel lambs had greater mucosal antibody activity in the abomasum for all isotypes than Suffolks, the differences were not significant. There was no evidence for any effects due to site, year or breed-by-year or breed-by-site interactions, with the exception of a breed-by-year interaction for IgG1 (P<0·05).

Fig. 5. Least squares means (with s.e. bars) for mucosal anti-nematode-IgG1, -IgG2, -IgA and -IgE antibodies (as optical density readings) in the abomasum (Ab) or small intestine (Si) of Suffolk and Texel lambs at 17 weeks of age (breed differences were not significant although the difference for IgE approached significance (P=0·11); there was no evidence for any breed-by-location interactions).
Correlation coefficients
Inspection of the correlation coefficients in Table 2 suggests a more consistent pattern of events in the Texel breed with the majority of mucosal antibody isotypes negatively correlated with FEC and worm burden. While Suffolks showed negative correlations with FEC, the correlation coefficients with worm burden were inconsistent and variable. A clear divergence between the breeds for the relationships involving IgE was observed; consistently negative and significant in Texels but near zero or positive in Suffolks. The least square analyses of variation for the correlation coefficients indicated significant breed-by-isotype effects for the set of correlations involving FEC and the set involving small intestine worm burden. Breed effects were not significant for abomasum worm burden.
Table 2. Least squares means (back transformed) for the correlation coefficients between parasitological variables and mucosal antibody measurements
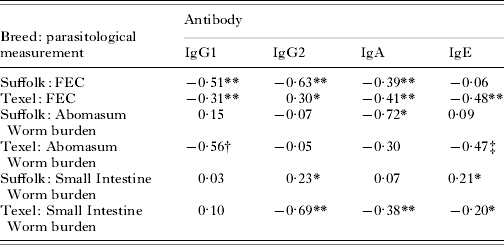
* P<0·05
** P<0·005
† P=0·07
‡ P=0·011.
DISCUSSION
The data presented here were based on a simple ELISA assay for the estimation of serum and mucosal antibody activity. The estimates used to characterize the humoral response were based on OD values. No attempt was made to estimate absolute antibody concentration, as such an analysis, using crude nematode homogenate as an antigen, would be of limited utility. In consideration of this fact and the potential for inherent variability in the monoclonal anti-ovine immunoglobulins used, the interpretation of antibody activity was confined to between-breed analysis within each isotype. The low serum IgA activity, and the high activity of serum IgE found in this study were unexpected, given previous reports (Huntley et al. Reference Huntley, Schallig, Kooyman, MacKellar, Jackson and Smith1998; Shaw et al. Reference Shaw, Gatehouse and McNeill1998) and likely to be an artefact of the in-house ELISA conditions. The IgA monoclonal used was un-purified supernatant, and thus required low serum dilution to achieve reasonable OD values. The high serum IgE activity values could be due to non-specific environmental factors. However, the inherent specificity of the ELISAs used is supported by the ability to detect changes in antibody activity over time and the low background OD observed throughout. Since the key comparisons are between breed and the same ELISA procedures were applied to both Suffolk and Texels animals, with both breeds represented on all assay plates, the breed differences in antibody isotype are considered to be valid.
A predominant feature from the haematological measurements was the significantly higher levels in Suffolks, over Texels for all but 3 of the variables. These differences were already evident at 11 weeks of age and suggest an innate breed difference with respect to these variables. However, the lack of a breed-by-age interaction demonstrated that levels did not change differentially over time and thus were not differentially affected by increasing nematode burden. The significantly higher pepsinogen concentration in Suffolks is likely to be a consequence of the greater tissue damage and clinical abnormalities caused by the higher worm burden in this breed.
Breed difference in the number of eosinophils was not significant; however, the pattern of change over the infection period may indicate an involvement in combating nematode infection. The level of increase in eosinophil numbers between 14 and 17 weeks in Texels was not matched in Suffolks and may indicate an enhanced eosinophil recruiting capability in Texels, in response to increasing nematode burden. The role of eosinophils in combating nematode infection in terms of degranulation or collaboration with other effectors (for example IgA (Henderson and Stear, Reference Henderson and Stear2006) or IL-4/mast cells (Balic et al. Reference Balic, Cunningham and Meeusen2006)) would seem, prima facia, an important facet in resistance to infection.
The nematode-specific humoral response to infection was noticeably different between breeds; the resistant Texels had significantly higher serum antibody OD activity than the Suffolk for all isotypes measured. Such differences were evident at 14 weeks of age and had increased further by 17 weeks. This observation is consistent with Gill et al. (Reference Gill, Gray, Watson and Husband1993) where a similar comparative study of resistant and random bred lambs, to Haemonchus contortus infection, showed significantly higher serum IgG1 and IgA levels in resistant lambs.
As documented in an earlier study comparing local and systemic immune response (Stear et al. Reference Stear, Bairden, Innocent, Mitchell, Strain and Bishop2004), it might be proposed that the lower level of serum antibody activity observed in Suffolks may be a consequence of the larger nematode burden accommodated in this breed, which would maintain antibodies at a mucosal level, preventing transfer to the plasma. If such an effect was evident in this study, then one would expect the Suffolk breed to have a higher mucosal antibody activity, relative to Texels. However, the evidence here suggested otherwise, with an overall trend for a higher level of mucosal antibody activity for all isotypes in the Texel breed.
Analysis of the correlation coefficients between mucosal antibody isotype and nematode burden parameters highlighted a more consistent pattern of events in Texels, with a greater proportion of mucosal antibodies negatively correlated with worm burden and FEC, in comparison to Suffolks. This differential pattern between breeds is most notable in the case of IgE. The correlations between this isotype and all parasitological measurements were significant and negative for Texels, while insignificant and variable for Suffolks, despite equivalent levels of mucosal IgE in both breeds. This suggests an important role for mucosal IgE or associated mechanisms in controlling worm establishment in the Texel breed. The fact that such mechanisms of resistance are less pronounced in the Suffolk breed may highlight either a reduced ability to create IgE antibodies of high affinity/avidity or a deficiency in related activities, such as mast cell production, recruitment or activation. An inadequacy in either of these mechanisms would increase susceptibility to infection. The binding of antigens to surface-bound IgE antibodies rapidly activates mast cells causing granular release, including eosinophil chemotatic factor, leading to recruitment of eosinophils and basophils. Mast cells can stimulate muscular contraction, which contributes to physical expulsion of worms from the gut and increased lymph flow. Recruited eosinophils can degranulate causing harm and possibly death to the invading parasite (Balic et al. Reference Balic, Cunningham and Meeusen2006). Perhaps the affinity/avidity of IgE, and/or activity of mast cell, is more enhanced in Texels, which primes the immune system leading to rapid expulsion of the parasite. If this is the case, triggered Fc receptors on mast cells and eosinophils may ultimately be responsible for helminth destruction.
An important role for nematode specific IgA has been previously described (Stear et al. Reference Stear, Bairden, Bishop, Buitkamp, Duncan, Gettinby, McKellar, Park, Parkins, Reid, Strain and Murray1997; Amarante et al. Reference Amarante, Bricarello, Hyuntley, Mazzolin and Gomes2005) and was also evident in this study. A negative correlative pattern for IgA was identified for both Suffolks and Texels; however, the pattern did not reflect the breed differences in resistance to infection.
The results of this study suggest that IgE related mechanisms are the potential basis for the greater susceptibility to nematode infection in Suffolks. The close functional association between IgE, mast cells and other mechanisms prevents definitive identification of the source of susceptibility. However, the fact that MHC DRB1 alleles have been associated with variations in resistance to infection in Suffolks (Sayers et al. Reference Sayers, Hanrahan, Good, Ryan, Angles and Sweeney2005a), may indicate that inadequate antigen presentation, affecting IgE affinity and avidity, may account for the increased susceptibility of Suffolk lambs to nematode infection.
We are most grateful to Peter McWaters, CSIRO Livestock Industries, Geelong, Australia, to Professor John Hopkins, University of Edinburgh, UK and to Professor Frans Kooyman, Utrecht University, The Netherlands for supply of monoclonal antibodies. We also wish to thank P. J. Dwyer, C. Joyce and P. Duignan from the Department of Agriculture Regional Veterinary Laboratory, Athlone, Co. Westmeath, Ireland for haematological measurements. This work was supported by a grant from the Irish Department of Agriculture, Food and Rural Development (RSF16). The Wellcome Trust is acknowledged for an equipment grant (Ref. 061354).









