Introduction
Bell's palsy, also known as idiopathic facial palsy, is generally of viral origin and has a good overall prognosis.Reference McCormick1 Electromyography (EMG) is undertaken in cases of severe palsy, and can indicate the degree of nerve degeneration possible as well as giving vital prognostic information.
This retrospective study was undertaken in the ENT and cervico-facial surgical unit of the Pitié Salpetrière Hospital, Paris, between January 1997 and March 2007. It involved 13 patients with Bell's palsies estimated as grade V or VI on the House–Brackmann scale, after medical treatment. The clinical data correlated with the electromyographic and radiological data, leading the authors to propose nerve decompression. This facial nerve decompression was performed within four months of the onset of symptoms. We describe our diagnostic and therapeutic procedures, and compare these with previously published reports.
Materials and methods
Material
This retrospective study involved 13 patients with severe idiopathic Bell's palsy diagnosed between January 1997 and March 2007. The group comprised eight men and five women, aged between 23 and 69 years (average age: 43 years). All patients had unilateral palsies: seven cases were right-sided and six left-sided.
Method
A retrospective analysis of patients' files was undertaken. The following clinical data were recorded: House–Brackmann grading of palsy, external ear examination findings (for skin lesions), parotid examination findings, otoscopy results, and vestibular and neurological test results. We also recorded the results of: tonal and vocal audiometry with stapedial reflex assessment, auditory brainstem evoked potentials and caloric tests. The results of the following serological tests were likewise documented: varicella-zoster virus, herpes simplex virus, cytomegalovirus, Epstein–Barr virus, human immunodeficiency viruses one and two, treponema pallidum haemagglutination assay–venereal disease research laboratory, hepatitis B and C viruses, and Lyme syndrome. Results for fasting blood sugar were also noted. No localisation test apart from the stapedial reflex was undertaken.
We also recorded the results of EMG, magnetic resonance imaging (MRI) and computed tomography (CT) investigations, and noted the medical treatment administered in the out-patients clinic or during hospitalisation.
Electromyography (detection and stimulation data, with blink reflex testing) was performed systematically, if possible before the 14th day of symptoms. Surface electrodes were placed on the corrugator, orbicularis oculi (pars inferior), orbicularis ori and mentalis muscles.
Magnetic resonance imaging (with injection of gadolinium contrast) focussed on the facial nerve trajectory. Pre-operative CT scanning of the petrous bone was also undertaken.
Finally, we recorded the surgical route initially chosen and the peri-operative findings, any post-operative complications, and the clinical and post-operative evolution of EMG evidence of Bell's palsy.
Ethical considerations
All the patients were informed of the possible side effects of surgical treatment, and were given a choice between conservative treatment (i.e. re-education) and surgical treatment.
Results
The results of patients' clinical examinations on admission are summarised in Table I.
Table I Patients' clinical data on admission

*n = 13. †Before medical treatment. ‡Homolateral labyrinthine and/or eruption in Ramsay Hunt zone. H–B = House–Brackmann; HIV = human immunodeficiency virus; FP = facial palsy
Medical treatment consisted of intravenous corticosteroid (Solumedrol® 2 mg/kg/day) together with intravenous aciclovir (Zovirax® 30 mg/kg/day) for 7 days. Following medical treatment, all patients had persistent Bell's palsy (House–Brackmann grade V or VI).
Patients' EMG results are summarised in Table II.
Table II Electromyography results

*n = 13. FN = facial nerve
All patients underwent MRI scanning with gadolinium contrast, focussing on the facial nerve trajectory. In 10 cases, a greater amount of gadolinium was observed on the affected side in the geniculate ganglion, the petrosal nerves, and the first and second portions and intrameatal portion of the facial nerve, compared with the healthy side (Figure 1). In the remaining three cases, the MRI scan with contrast was interpreted as normal, without any pathological increase in contrast medium. No neoplastic pathology was suspected.

Fig. 1 Axial, T1 ponderation magnetic resonance imaging scan with gadolinium contrast in patient with left-sided Bell's palsy, showing contrast medium highlighting the geniculate ganglion, the petrosal nerves, and the first and second segments and intrameatal segment of the facial nerve.
Pre-operative CT scanning of the petrous bone was undertaken in nine patients. No abnormality was observed in the facial canal or its connections.
On completion of assessment, all patients were diagnosed with severe idiopathic Bell's palsy, and were offered nerve decompression.
Nerve decompression procedures took place a mean of five weeks after onset of Bell's palsy; procedures took place in the first month for seven patients, in the second month for five and in the fourth month for one. All procedures used the subpetrous approach. After location of the petrosal nerves, decompression consisted of opening the geniculate bone cavity, the initial segment of the tympanic segment, and then the labyrinthine segment up to the meatal foramen. At least 50 per cent of the surface of the facial canal was exposed. Oedema of the nerve in its sheath, together with an inflamed appearance, was observed in all cases. Careful, nontraumatic opening of the nerve sheath at the geniculate ganglion and tympanic segment revealed significant inflammatory oedema of the nerve fibres, creating a hernia outside the epineurium.
Early post-operative complications are summarised in Table III. All patients improved to House–Brackmann grade III (i.e. good tone and facial symmetry at rest, eye closure possible, and synkinesis and/or moderate hemifacial spasm present) at one year post-operatively; three patients reached this grade in the fifth post-operative month. Some patients had very good facial mobility, but also had hemifacial spasm and thus could not be described as grade II. Seven patients were still evaluated as grade III two years post-operatively. Only one case of ‘crocodile tears’ syndrome was observed.
Table III Early post-operative complications

*n = 13. †Non-surgical.
Eleven patients agreed to undergo a post-operative EMG to assess axonal regrowth. In one case, we observed the possible reappearance of nervous excitability – weak but present – one month after decompression. The blink reflex reappeared in all cases, at least by the 10th post-operative month, and as early as the fifth post-operative month in three cases.
Discussion
Histopathological and physiological findings in idiopathic Bell's palsy
In the past few decades, numerous and sometimes contradictory hypotheses have been proposed to explain the mechanism responsible for Bell's palsy; these may be categorised as ‘vascular versus viral’. Each theory has been backed by the presence of histological neural lesions to justify surgical decompression in cases of severe palsy.
In the 1960s, KettelReference Kettel2 considered that nerve ischaemia, mainly at the stylomastoid foramen, was the main cause of Bell's palsy. Such ischaemia was thought to cause intraneural oedema, which diminished vascularisation and thus caused further ischaemia.
However, the majority of authors now appear to have rejected the vascular theory in favour of a viral aetiology of Bell's palsy. The most frequently discussed viruses are herpes simplex virus one and varicella-zoster virus.Reference McCormick1, Reference Linder, Bossart and Bodmer3–Reference Furuta, Takasu, Fukuda, Sato-Matsumura, Inuyama and Hondo7 Liston and KleidReference Liston and Kleid8 undertook a post-mortem study of histological lesions caused by supposed viral inflammation of the facial nerve. The main lesions observed were: serious inflammation and intraneural oedema of the internal acoustic meatus; an absence of causes of vascular impairment (e.g. thrombosis or compression) (in contrast to Kettel's findings);Reference Kettel2 and signs of axonal degeneration, with marked alteration in the axonal density of the nerve (the number of axonal fibres was considerably diminished between the proximal part of the nerve, at the entrance to the internal acoustic meatus, and the distal part, at the entrance to the facial canal at the meatal foramen). A decrease in axonal density at the meatal foramen was also observed by Jackson et al.,Reference Jackson, Johnson, Hyams and Poe9 McKeever et al. Reference McKeever, Proctor and Proud10 and Fisch and Esslen.Reference Fisch and Esslen11, Reference Fisch12 All believe that such a change in the axonal density at this point exists because it's the narrowest diameter along the intrapetrous nerve path (0.6 mm in diameter) and this aera could be the initial site of axonal degeneration. An unusual extensive haemorrhage is also present in this zone.Reference Fisch and Esslen11 Nerve inflammation, intraneural oedema, decrease of axonal density at the meatal foramen have been reported by numerous authors, in both animalsReference Sugita, Murakami, Yanagihara, Fujiwara, Hirata and Kurata6 and humans.Reference O'Donogue, Michaels and Portmann13
We are in agreement with the previous findings. In all our patients, we observed inflammation and intraneural oedema on the geniculate ganglion (GG) within the tympanic and labyrinthine segments.
Role of electromyography and radiology in surgical decision-making
Electromyographic examination provides essential information regarding the severity and prognosis of Bell's palsy, according to the type of neural involvement observed: axonal or myelinic.Reference Sittel and Stennert14 An incomplete nerve conduction block with blink reflex impairment indicates the presence of neurapraxia, with a good prognosis. Axonal lesions (axonotmesis or electrophysiological neurotmesis) have a less favourable prognosis, and are indicated by the presence of inexcitability, which develops more rapidly as the intensity of neural damage worsens. In cases of severe neurotmesis or axonotmesis, the first signs of reinnervation are only visible from the third post-palsy month, if they appear at all. This anticipated reinnervation will usually be of poor quality. The difficulty is to locate, out of the extensive denervations which appear between one and two weeks (corresponding to severe axonotmesis lesions), those which are likely to result in satisfactory, spontaneous reinnervation. According to Fisch,Reference Fisch12 detection of more than 95 per cent non-excitable fibres on the 14th day is associated with a less than 50 per cent chance of obtaining satisfactory facial recuperation. We consider that a loss of total excitability before the 14th day corresponds either to severe axonotmesis or to electrophysiological neurotmesis and should favour surgical treatment, since a good quality of reinnervation is unlikely.
Contrast-enhanced MRI contributes to the confirmation of idiopathic Bell's palsy and can help identify the sites affected, aiding surgical planning. The zones of contrast enhancement are usually the geniculate ganglion and the tympanic segment.Reference Martin-Duverneuil, Sola-Martinez, Miaux, Cognard, Weil and Mompoint15, Reference Charachon, Bebear, Sterkers, Magnan and Soudant16 Some authors believe that enhancement of the labyrinthine and meatal segments and the petrous nerves generally represents an inflammatory, pathological process involving the nerve.Reference Martin-Duverneuil, Sola-Martinez, Miaux, Cognard, Weil and Mompoint15, Reference Brandle, Satoretti-Schefer, Bohmer, Wichmann and Fisch17 Kress et al. Reference Kress, Griesbeck, Stippich, Bähren and Sartor18 have stated that major intrameatal enhancement in the first week after onset of Bell's palsy is a poor prognostic factor. However, there is currently no consensus as to the prognostic value of MRI in Bell's palsy cases.Reference Brandle, Satoretti-Schefer, Bohmer, Wichmann and Fisch17
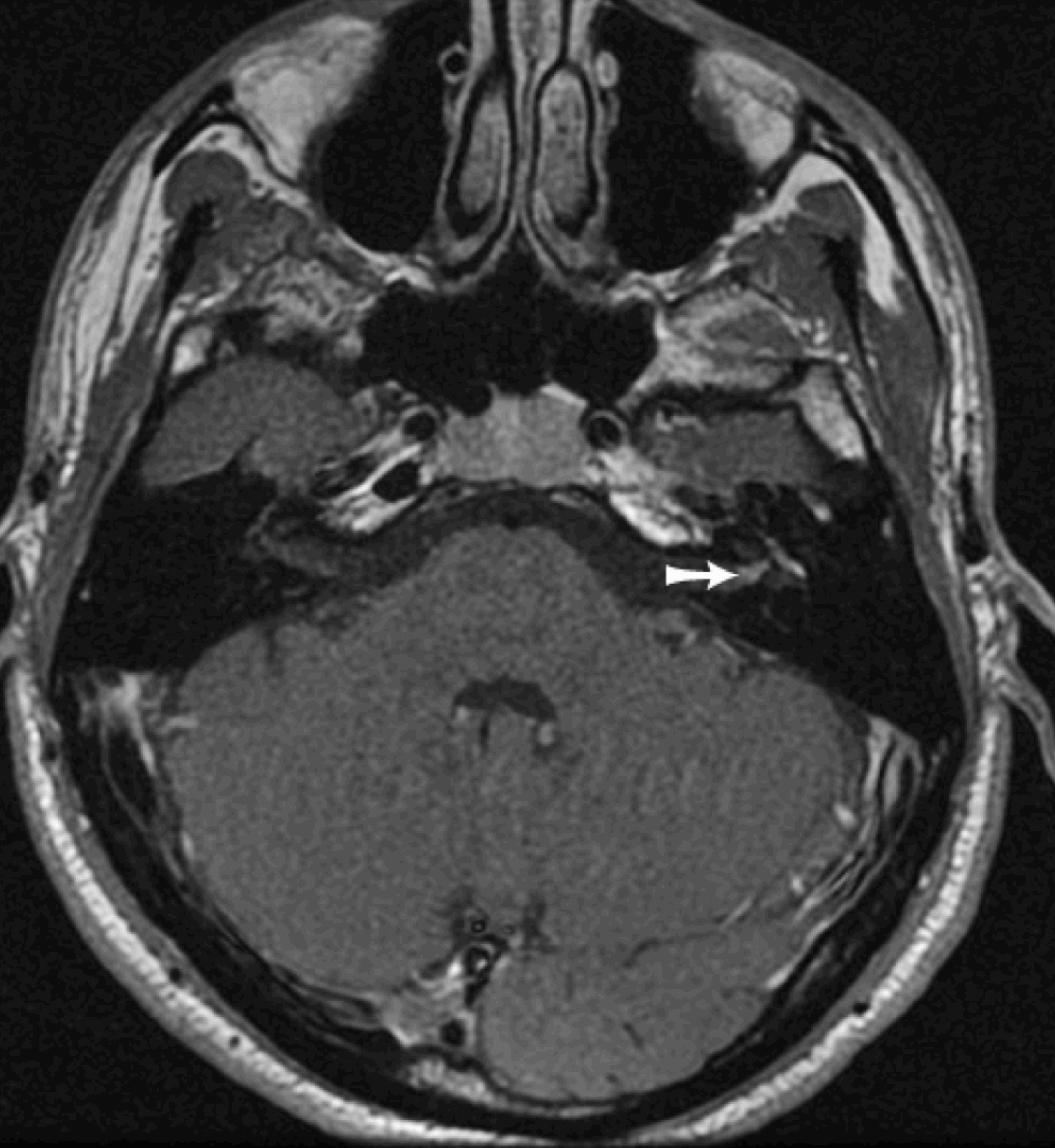
Computed tomography is not routinely recommended for Bell's palsy patients, apart from those suffering a recurrence (in whom a narrow facial canal may be identified). Its main advantage lies in presurgical study of the facial canal ratiosReference Sennaroglu19 and of the distance between the cochlea and the labyrinthine segment and the geniculate ganglion, thus enabling an assessment of the risk of labyrinthine involvement during a subpetrous approach (Figure 2).

Fig. 2 Axial computed tomography scan of the pre-operative left petrous bone, showing the narrow distance (arrow) between the base of the cochlea and the labyrinthine segment.
Surgical treatment
Currently, no consensus exists regarding the advantages of, and indications for, surgery for Bell's palsy.Reference Adour20–Reference Yanagihara, Hato, Murakami and Honda24 Surgical indications should be based on precise criteria, after failure of well conducted medical treatment. According to the findings of the current study and others, only a very small number of patients require surgical treatment – the majority make a good recovery with or without medical treatment.Reference Charachon, Bebear, Sterkers, Magnan and Soudant16, Reference Grogan and Gronseth22, Reference Sullivan, Swan, Donnan, Morrison, Smith and McKinstry25–Reference Hato, Yamada, Kohno, Matsumoto, Honda and Gyo27 In a recent study of 551 patients, Sullivan et al. Reference Sullivan, Swan, Donnan, Morrison, Smith and McKinstry25 reported good recovery at nine months in 94.4 per cent of patients treated with prednisolone and in 85.2 per cent of those left untreated. The 13 patients included in our study represent only 3.4 per cent of patients who with idiopathic Bell's palsy graded IV or more who were hospitalised in our unit over the same period. In 18 years, out of 1492 patients treated by Yanagihara and colleagues,Reference Yanagihara, Hato, Murakami and Honda24, Reference Yanagihara, Gyo, Yumoto and Tamaki28 surgical treatment was proposed for only 6.8 per cent. Twenty-three per cent of the patients treated by Gantz et al. Reference Gantz, Rubinstein, Gidley and Woodworth29 over a 15-year period underwent surgery; this study probably had a larger cross-section of recruited patients, and broader surgical indications, than our own.
Sittel and StennertReference Sittel and Stennert14 and May and colleaguesReference May, Klein and Taylor23, Reference May, Blumenthal and Taylor30 recognised the prognostic contribution of electrophysiological tests undertaken before the 14th day of Bell's palsy onset, but remained fervent defenders of less invasive medical treatment, whatever the final results. Today, most authors opt for surgery based on a high percentage of denervations detected in the first two or three weeks; however, controversy exists concerning the optimal delay before decompression. Yanagihara and colleaguesReference Yanagihara, Hato, Murakami and Honda24, Reference Yanagihara, Gyo, Yumoto and Tamaki28 proposed surgical treatment before day 30 in patients with more than 95 per cent degeneration on electroneurography in the first three weeks. The benefit of early surgery is clear in these studies, although the authors noted that, paradoxically, a positive result for decompression could be obtained in some patients operated upon in the three to six months after onset of palsy. Gantz et al. Reference Gantz, Rubinstein, Gidley and Woodworth29 reported improvement to House–Brackmann grade I or II in all patients with more than 90 per cent degeneration on electroneurography and absence of voluntary contraction on EMG who underwent decompression before day 14; patients operated upon after this time had greatly inferior outcomes (grade III or more). FischReference Fisch12 used the same electrophysiological criteria as Gantz et al., and recommended surgery in the two to three weeks after onset of symptoms.
In our previous experience,Reference Lejeune, Bernat, Vitte, Lamas, Willer and Soudant31 the presence of 10 per cent stimulatable nerve fibres is sufficient to enable spontaneous, good quality axonal regrowth. The proposal that decompression be performed in all patients with 90 per cent denervation or more is, we believe, excessive, and others concur with this opinion.Reference Fisch12 We consider that, in view of the morbidity rates of surgery and anaesthesia, it is only permissible to propose decompression in patients with total denervation before day 14. However, we do agree that, in theory, early decompression of a patient with subtotal denervation (i.e. 95 per cent) could save a few fibres and therefore encourage axonal regrowth. This indication can, in our view, be extended to patients seen belatedly, with severe Bell's palsy and total denervation, with no sign of axonal regrowth after two months or more. In our study, in view of the small number of patients included, it is difficult to demonstrate the benefits of early decompression, since the result was identical for decompressions undertaken at 11 days versus four months after the onset of palsy. Late surgery is precisely proposed in order to limit spastic sequelae.
Surgically, the choice of facial nerve approach depends on the presumed lesion site(s). Consequently, surgical approaches have varied over the decades, as have the proposed aetiologies for idiopathic Bell's palsy.Reference Friedman21
More than 40 years ago, KettelReference Kettel2 used only the mastoid approach to reach the third segment and the stylomastoid foramen, believed to be the initial site of vascular compression responsible for nerve ischaemia. BrownReference Brown32 and PulecReference Pulec33 used varying decompression sites, according to the results of localisation tests.
Yanagihara and colleaguesReference Yanagihara, Hato, Murakami and Honda24, Reference Yanagihara, Gyo, Yumoto and Tamaki28 have for many years advocated decompression via the mastoid route with disarticulation of the incus. The facial nerve is thus exposed from the stylomastoid foramen to the distal part of the labyrinthine segment. YanagiharaReference Yanagihara, Hato, Murakami and Honda24 refers to the findings of Pulec,Reference Pulec33 who observed labyrinthine segment impairment in only 15 per cent of cases, compared with second and third segment impairment in 80 per cent and this finding should not justify the subpetrous route, more invasive than the mastoid route. Conversely, Liston and KleidReference Liston and Kleid8 insisted on the necessity to decompress the whole of the nerve route, including oedematous lesions in all segments of the nerve. In their view, it was imperative to decompress the labyrinthine segment up to the meatal foramen, the narrowest part of the facial canalReference Liston and Kleid8, Reference McKeever, Proctor and Proud10 and most probably the initial site of axonal degeneration. Many authors also agree with this theory of pathogenesis, including Gantz et al.,Reference Gantz, Rubinstein, Gidley and Woodworth29 Fisch and Esslen,Reference Fisch and Esslen11 Jackson et al.,Reference Jackson, Johnson, Hyams and Poe9 Knox,Reference Knox34 and Ge and Spector,Reference Ge and Spector35 as do we. We approached the nerve via the subpetrous route, as do most authors.Reference Fisch and Esslen11, Reference Fisch12, Reference Gantz, Rubinstein, Gidley and Woodworth29, Reference Lee and Lee36 Decompression should be undertaken without damaging the nerve; this is helped by taking certain precautions,Reference Devriese37 such as the use of diamond-headed drills around the nerve and continuous irrigation.
Frank oedema with herniation of the nerve fibres is frequently observed on opening the nerve sheath,Reference Kettel2, Reference Liston and Kleid8, Reference Fisch and Esslen11, Reference Yanagihara, Gyo, Yumoto and Tamaki28, Reference Gantz, Rubinstein, Gidley and Woodworth29, Reference Pulec33, Reference Knox34 as was the case in all our patients. In our opinion, surgical intervention eliminates nerve compression, particularly in the meatal foramen segment, and above all favours axonal regrowth by reducing ischaemia. Revascularisation decompression of the stylomastoid foramen appears to have now been abandoned by most authors. However, there is great interest in the concept of opening the epineurium of the geniculate ganglion or in order to limit axonal degeneration to the few intact fibres which may be saved in the process,Reference Liston and Kleid8 or even to promote axonal regrowth.Reference Yanagihara, Hato, Murakami and Honda24, Reference Yanagihara, Gyo, Yumoto and Tamaki28 FischReference Fisch12 and EsslenReference Fisch and Esslen11 and Gantz et al. Reference Gantz, Gmur and Fisch38 have used direct intra-operative stimulation of the facial nerve to demonstrate a nerve conduction block situated before the geniculate ganglion, at the meatal foramen, the narrowest point of the nerve. Intra-operative stimulation of the nerve (theoretically impossible to undertake in cases of total denervation) to facilitate localisation and removal of the conduction block appears to us a very interesting procedure. It also constitutes an argument in favour of surgical treatment before total denervation occurs.
The main operative risk of the subpetrous approach is accidental opening of the bony labyrinth.Reference Sennaroglu19, Reference Lee and Lee36 Precise information should be given to the patient regarding the risks involved and the expected benefits of such surgery, based on current findings. Brown estimated the risk of labyrinthine opening at 5 per cent;Reference Brown32 however, this complication was not observed by either Gantz et al. Reference Gantz, Rubinstein, Gidley and Woodworth29 or Fisch.Reference Fisch12
Comparison of results, and proposed new therapeutic approach to severe Bell's palsy
The series of Bell's palsy patients published thus far have been heterogeneous in terms of populations studied, clinical classifications, delay in post-operative evaluation, indications for surgery and decompression sites. Such variation makes it difficult to assess the benefits of other approaches and to compare them with our own. In addition, few recently published studies appear to be statistically valid.Reference Grogan and Gronseth22
May et al. Reference May, Klein and Taylor23 did not demonstrate any benefit of surgical decompression, in a group of 38 patients (a number too small for valid statistical analysis). These authors performed decompression along the whole of the nerve route via a mastoid approach, apart from the meatal foramen. This latter fact could, in part, explain the study results, in view of the importance granted to this site by other anatomical and clinical studies.Reference Liston and Kleid8–Reference Fisch12, Reference Gantz, Gmur and Fisch38 Nevertheless, Yanagihara et al.,Reference Yanagihara, Hato, Murakami and Honda24, Reference Yanagihara, Gyo, Yumoto and Tamaki28 using the same procedure, reported that 85 per cent of patients operated upon in the first month of onset achieved House–Brackmann grades of I and II. The chances of obtaining good quality recovery would be reduced by 23 per cent in the absence of surgical treatment and results are markedly more mediocre when decompression is undertaken after one month.Reference Yanagihara, Hato, Murakami and Honda24,Reference Yanagihara, Gyo, Yumoto and Tamaki28 Fisch,Reference Fisch12 in a series of 25 patients, showed the benefits of surgery via the subpetrous approach, compared with medical treatment alone, in patients with more than 95 per cent degeneration seen on electroneurography performed within the first two weeks of palsy onset: of those receiving medical treatment, 64 per cent achieved good quality facial recovery, compared with 78.8 per cent for those receiving surgical treatment. However, Fisch did not observe any difference in results between the two treatment modalities in patients in whom denervation was observed in the third week after onset. The more recent study by Gantz et al. Reference Gantz, Rubinstein, Gidley and Woodworth29 appears to us the most interesting one in terms of comparison of results, since the surgical procedure used matched our own. Gantz et al. reported better functional results than ours: in their study, 91 per cent of 19 patients undergoing surgery obtained House–Brackmann grades I or II, versus only 42 per cent of non-surgically treated patients (both groups had identical electrophysiological criteria). To our knowledge, these are the only statistically significant results in the literature, which testify to the true benefits of surgical treatment.Reference Grogan and Gronseth22
All our patients obtained a post-treatment House–Brackmann grading of III, a rather disappointing result; other authors have reported achieving grades of I or II. This discrepancy could be explained in several ways. Firstly, in these other studies, surgery was taken to be indicated at a lower denervation percentage, compared with our study (e.g. 90 per cent denervation, versus 100 per cent denervation in our study). It seems likely to us that some patients who still had approximately 10 per cent functional fibres could have shown an identical outcome without surgical treatment, as was our experience.Reference Lejeune, Bernat, Vitte, Lamas, Willer and Soudant31 Furthermore, Gantz et al.Reference Gantz, Rubinstein, Gidley and Woodworth29 performed decompression in the first two weeks after the onset of palsy, compared with five weeks on average in our study. This shorter delay appears ideal, of course, but difficult to obtain in actual clinical practice. Finally, definitive evaluation of results sometimes took place at an earlier time point (e.g. at seven months in Gantz and colleagues' study),Reference Gantz, Rubinstein, Gidley and Woodworth29 whereas all our patients were evaluated at one year or more. In our experience, evaluate the patients at seven months is premature, since spastic signs and synkinesis (linked to delayed regrowth of the most affected fibres) can appear at any time up to 12 months after onset of palsy, thus decreasing the final functional result.
• Surgical decompression for serious idiopathic facial palsy remains a highly controversial issue
• Surgical indications can only be considered when based on precise clinical and electromyographic criteria, and after providing the patient with detailed information as to the potential risks and benefits of surgery
• The literature describes very good and sometimes surprising functional results, which are better than those obtained in this retrospective study
• Further, statistically valid studies are needed to resolve the controversy over the use of surgery in the treatment of this condition
To improve our results, we propose a new therapeutic procedure for the management of severe Bell's palsy, summarised in Figure 3.

Fig. 3 Proposed new therapeutic procedure for the treatment of severe idiopathic Bell's palsy. FP = facial palsy; EMG = electromyography; D15 = first fifteen days after onset of palsy
Conclusion
Surgical decompression for serious idiopathic Bell's palsy remains a highly controversial issue. Indications can only be considered when based on precise clinical and electromyographic criteria, after providing the patient with detailed information as to the potential risks and benefits of surgery. The literature describes very good, and sometimes surprising functional results, which are better than those obtained in our retrospective study. It is possible that our results could be improved by extending our operative indications to patients with 95 per cent denervation, by shortening the timing of surgery to before day 15 and by optimising post-operative facial re-education. Further, statistically valid studies will be necessary to resolve the controversy over the use of surgery in the treatment of Bell's palsy.