Introduction
Antipsychotics are the foundational treatment for schizophrenia and schizoaffective disorder, and an important option for the treatment of bipolar disorder. However, even if a medication can reduce symptoms and is tolerable for that individual,Reference Volavka and Citrome1 adherence issues often emerge.Reference Valenstein, Ganoczy and McCarthy2 Long-acting injectable (LAI; depot) formulations of antipsychotics can assist with adherence, leading to better outcomes. Many patients prefer LAI formulations, especially after gaining some experience with this modality.Reference Caroli, Raymondet and Izard3 In the most recent iteration of the guidelines for the treatment of patients with schizophrenia published by the American Psychiatric Association, the authors advocate that patients receive treatment with a LAI antipsychotic medication if they prefer such treatment or if they have a history of poor or uncertain adherence.Reference Keepers, Fochtmann and Anzia4 Regarding the latter, nonadherence to oral antipsychotic medication treatment (defined by some investigators as a failure to take at least 80% of prescribed medication) is common and has been observed in approximately half of all patients with schizophrenia, schizoaffective disorder, and bipolar disorder.5-7 Lack of adherence extends out to treatments targeting comorbidities, especially when the underlying psychiatric disorder remains poorly managed.Reference Dolder, Lacro and Jeste8, Reference Shafrin, Silverstein and MacEwan9 Given the consequences of nonadherence to antipsychotic medication, including relapse, hospitalization, aggressive behavior, suicide, substance abuse, and disease progression,10-18 efforts to identify and address poor adherence is crucial. LAI antipsychotics can eliminate the guesswork about adherence status and allow the clinician to focus on other reasons why symptoms may be exacerbated, such as psychosocial stressors or substance use. In the end, preventing a relapse today can make a difference for a lifetime.
What are the available LAI options?
In the United States, there are several LAI antipsychotics to select from and they are listed in Table 1.19-21 Older first-generation antipsychotics such as fluphenazine decanoate (administered every 2 weeks) and haloperidol decanoate (administered every 4 weeks) remain available.Reference Citrome19 Both can be injected in either the deltoid or gluteal muscle. They are dissolved in sesame seed oil and may be more challenging to inject than the second-generation intramuscular LAI formulations which are all suspended in water. Nevertheless, fluphenazine decanoate and haloperidol decanoate are relatively inexpensive and commonly prescribed. For fluphenazine decanoate the initial dose is 12.5 to 25 mg and can be increased in increments of 12.5 mg but the total amount administered at one time should not exceed 100 mg.22 The initial dose for haloperidol decanoate should be 10 to 20 times the previous daily dose in oral haloperidol equivalents but the initial injection is limited to 100 mg, followed by the balance 3 to 7 days later. The usual maintenance range is 10 to 15 times the previous daily dose in oral haloperidol equivalents depending on clinical response. Clinical experience with haloperidol decanoate at doses greater than 450 mg/month is limited.23 Other first-generation LAI antipsychotics have been available outside the United States (eg, flupentixol, perphenazine, pipotiazine, and zuclopenthixol).Reference Taylor24 The biggest limitation to using first-generation LAI antipsychotics is the common occurrence of drug-induced parkinsonism and thus the use of anticholinergic medication. This contributes to an increased risk of developing tardive dyskinesia.Reference Citrome25 In addition, anticholinergic medications can also be associated with worsened cognitive impairment.Reference Vinogradov, Fisher and Warm26 Drug-induced parkinsonism and the need for anticholinergic medications can be minimized by using second-generation LAI antipsychotics.
Table 1. Long-Acting Injectable Antipsychotics Available in the United States as of December 2020

There are three different second-generation antipsychotics currently available in LAI formulations: risperidone/paliperidone, aripiprazole, and olanzapine. For each of these second-generation antipsychotic categories, there may be several different products to consider. The different formulations containing the same active molecule can be differentiated in terms of their “amenities of care” such as dosing intervals (eg, every 2, 4, 6, 8, or 12 weeks), availability of different dose strengths, choice of injection site, size of the needle, injection volume, storage and reconstitution requirements, need for oral supplementation, guidance regarding early or late dosing, and approved indications. A list of these pragmatic considerations is contained in Table 2.
Table 2. Long-Acting Injectable Antipsychotics: “Amenities of Care”

Risperidone/paliperidone containing formulations
Table 3 outlines the four different formulations available that contain risperidone or paliperidone. They differ broadly in approved indications, dosage forms/strengths, reconstitution requirements, injection sites, needle gauge/length, injection volume, injection interval, requirement for oral supplementation, need for refrigeration when stored, and instructions for early or late dosing.
Table 3. Summary of Characteristics of Risperidone- or Paliperidone-Containing Long-Acting Injectable Antipsychotics Commercially Available in the United States
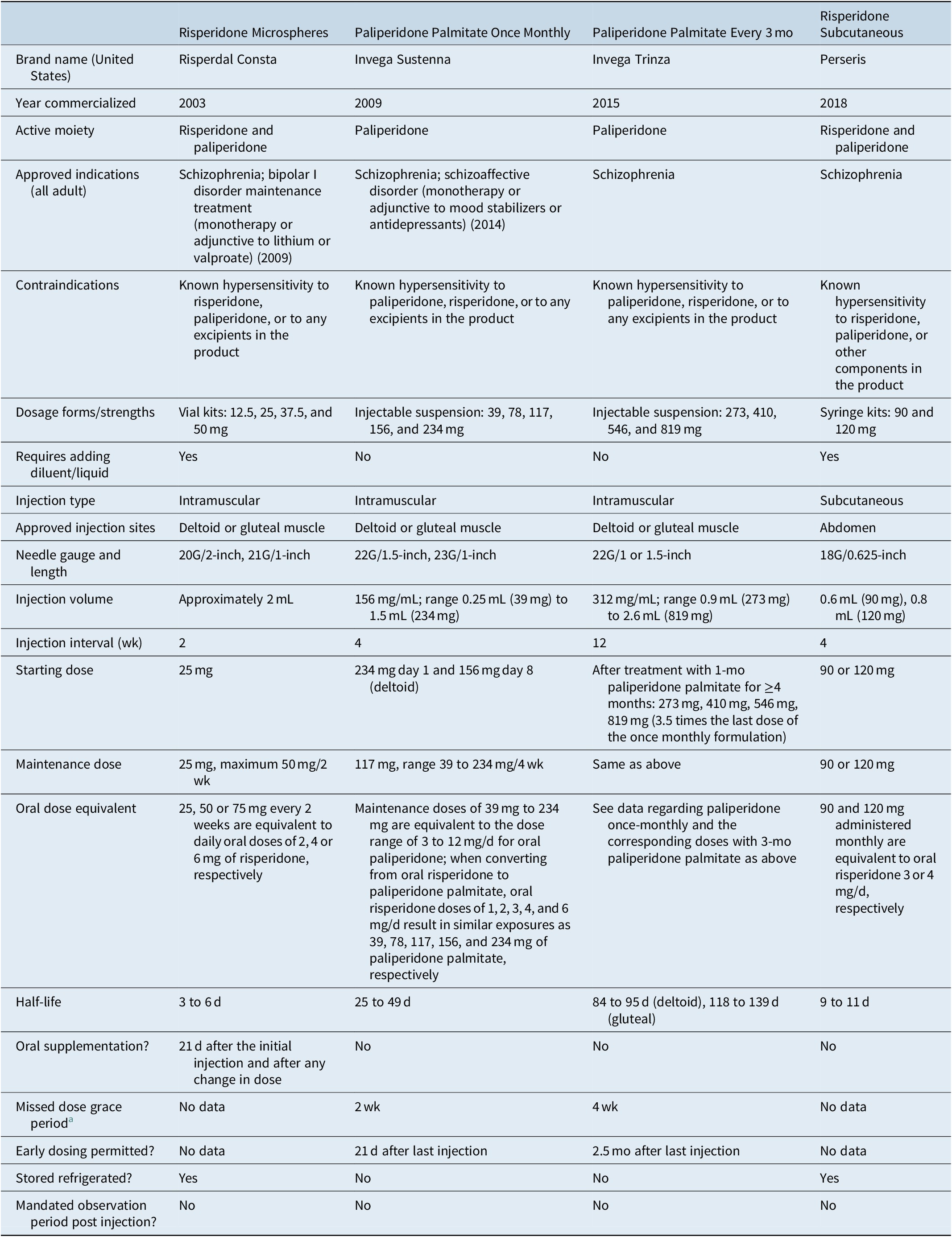
a Defined as the amount of time that can elapse after an injection is due before any supplemental oral medication is required (risperidone microspheres), or in the case of LAI antipsychotics where oral supplementation is not used, the amount of time that can elapse after an injection is due before any extra dose(s) of LAI antipsychotic is required (paliperidone palmitate).
In 2003, risperidone microspheres became the first second-generation antipsychotic to be available in a LAI formulation.Reference Ehret and Fuller27, Reference Harrison and Goa28 In addition to the indication for schizophrenia, risperidone microspheres are also approved as monotherapy or as adjunctive therapy to lithium or valproate for the maintenance treatment of bipolar I disorder.Reference Citrome20, 29 Of note, storage of the product requires refrigeration.29 Because the main release of the drug does not begin until 3 weeks after administration, supplemental oral risperidone is required for 21 days after the first injection and after any dose increase.29 After mixing the risperidone microspheres powder with the supplied aqueous diluent, it can be administered in the deltoid or gluteal muscle. The recommended starting dose is 25 mg/2 weeks, and the maximum recommended dose is 50 mg/2 weeks. With respect to total exposure, injections of 25, 50, or 75 mg every 2 weeks were found to be equivalent to daily oral doses of 2, 4, or 6 mg of risperidone, respectively.Reference Eerdekens, Van Hove and Remmerie30 Another formulation of an intramuscular risperidone LAI is under development (risperidone ISM); although reconstitution of risperidone ISM is needed and the maximum dose is limited to the equivalent of oral risperidone 4 mg/day, it can be administered monthly and does not require oral supplementation or loading doses.Reference Correll, Litman and Filts31, Reference Clark and Taylor32
The use of risperidone microspheres administered every 2 weeks has been replaced in many instances with paliperidone palmitate administered monthly or every 3 months. Paliperidone (9-OH risperidone) is the main active metabolite of risperidone and a once-monthly injectable formulation became available in the United States in 2009.33-35 In contrast to risperidone microspheres which must be reconstituted as well as stored in a refrigerator, paliperidone palmitate is an aqueous suspension that comes in prefilled syringes and does not require refrigeration. The product has relatively small needle bores to choose from. Instead of using oral supplementation, the initiating doses are all by injection: 234 mg on treatment day 1 and 156 mg 1 week later (±4 days), both administered in the deltoid muscle. Although the recommended monthly maintenance dose is 117 mg for the treatment of schizophrenia, the maintenance doses can be within the range of 39 to 234 mg, equivalent to the dose range of 3 to 12 mg/day for oral paliperidone.33 When converting from oral risperidone to paliperidone palmitate, oral risperidone doses of 1, 2, 3, 4, and 6 mg/day result in similar exposures as 39, 78, 117, 156, and 234 mg of paliperidone palmitate, respectively.Reference Russu, Kern Sliwa and Ravenstijn36 The regular monthly maintenance doses can be administered in either the deltoid or gluteal muscle. In addition to the indication for treatment of schizophrenia, paliperidone palmitate once monthly received approval for use in schizoaffective disorder as monotherapy or as an adjunct to mood stabilizers or antidepressants.Reference Citrome20, 33, Reference Greenberg and Citrome35 In 2015, a 3-month formulation was approved for the treatment of schizophrenia in individuals who have been treated with the once-monthly formulation of paliperidone palmitate for ≥4 months.Reference Citrome20, 37 In contrast to the once-monthly preparation, this longer-acting formulation of paliperidone palmitate does not have approval for schizoaffective disorder at the present time. The 3-month formulation is packaged in water-based prefilled syringes; however, the product is denser than the once-monthly formulation and has a larger particle size.Reference Ravenstijn, Remmerie and Savitz38 The doses that are available remain sufficiently small in volume so that they can be administered in the deltoid muscle, although gluteal injection remains an option. Dose for the 3-month formulation is calculated by multiplying the once-monthly dose by 3.5. The 3-month formulation requires the use of special-purpose thin-walled needles that come packaged with the product and these needles cannot be interchanged with those supplied with the once-monthly formulation or with other regular commercially available needles. A 6-month version of paliperidone palmitate is under development (NCT03345342, NCT04072575).
In 2018, a sustained release of risperidone was approved for subcutaneous injection in the abdomen.Reference Citrome21, 39 This is a novel method of administration for psychiatric medications (although fluphenazine decanoate is also approved for subcutaneous use, it is generally thought of as an intramuscular medication). Storage of the product requires refrigeration. The injection process requires preparing the product by mixing the risperidone powder with the liquid vehicle using two syringes coupled together. The needle itself is 18 gauge but short (5/8 inch). The available doses strengths are 90 and 120 mg administered monthly, which are equivalent to oral risperidone 3 or 4 mg/day, respectively. Another formulation of a subcutaneous risperidone LAI is under development (TV-4600); reconstitution of TV-4600 is not required, and injections can be administered every 1 or 2 months.Reference Clark and Taylor32
Aripiprazole containing formulations
Table 4 outlines the three different formulations available that contain aripiprazole, with one of them (aripiprazole lauroxil nanocrystal dispersion) reserved for the initiation of aripiprazole lauroxil.Reference Citrome20, 40-46 Principal differences between the formulations include approved indications, dosage forms/strengths, reconstitution requirements, injection sites, needle gauge/length, injection volume, injection interval, requirement for oral supplementation, concomitant use instructions with CYP3A4 inducers, and instructions for early or late dosing.
Table 4. Summary of Characteristics of Aripiprazole-Containing Long-Acting Injectable Antipsychotics Commercially Available in the United States
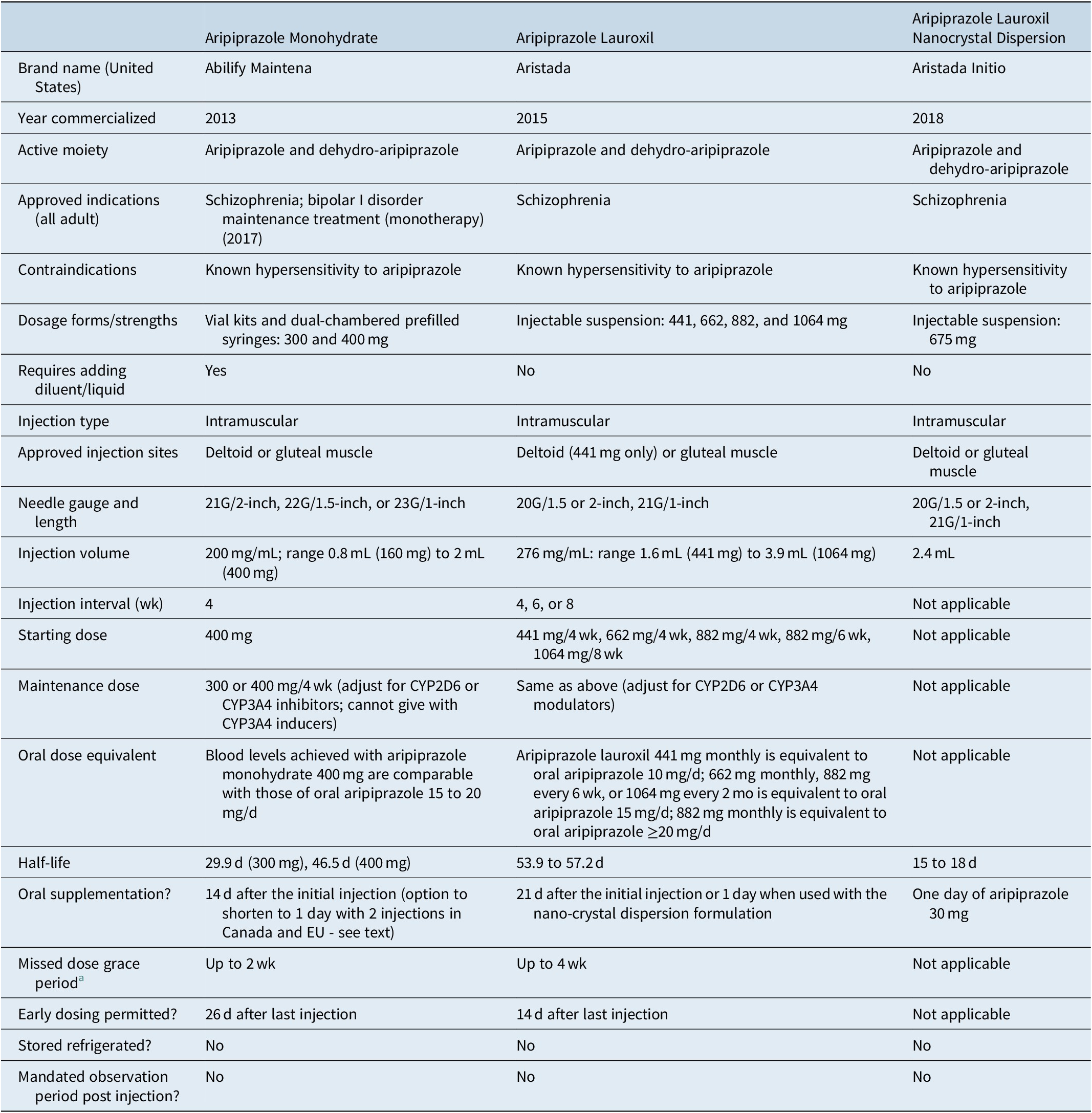
a Defined as the amount of time that can elapse after an injection is due before any supplemental oral medication (or aripiprazole lauroxil nano-crystal dispersion) is required. Details may vary depending on the dose of the last injection (as with aripiprazole lauroxil) or may vary by how many consecutive injections have already been administered (as with aripiprazole monohydrate).
Aripiprazole monohydrate became available in the United States in 2013.Reference Citrome19, Reference Citrome20, Reference Citrome40, 43 Following reconstitution with water using either a vial kit or a pre-filled dual-chambered syringe, the monthly injection can be administered in either the deltoid or gluteal muscle. Oral supplementation with aripiprazole or any other antipsychotic is required for 14 days after the initial injection. In Canada and the EU, but not in the US, product labeling permits initiation of aripiprazole monohydrate by administering two separate injections of 400 mg at different injection sites, along with one 20 mg dose of oral aripiprazole, all on the first day of treatment (https://www.newswire.ca/news-releases/health-canada-approves-otsuka-and-lundbeck-s-abilify-maintena-r-aripiprazole-for-prolonged-release-injectable-suspension-alternative-initiation-regimen-850848524.html). The recommended initial and maintenance doses are 400 mg, although a reduction to 300 mg can be considered to manage tolerability concerns. Blood levels achieved with aripiprazole monohydrate 400 mg are comparable with those of oral aripiprazole 15 to 20 mg/day.Reference Raoufinia, Peters-Strickland and Nylander47 In addition to being approved for the treatment of schizophrenia, aripiprazole monohydrate is also approved for maintenance monotherapy treatment of bipolar I disorder.Reference Citrome20, 43 A 2-month formulation of aripiprazole monohydrate is under development (NCT04030143).
Aripiprazole lauroxil was introduced in the United States in 2015.Reference Citrome20, Reference Citrome40, 44 Aripiprazole lauroxil is supplied in prefilled syringes as an aqueous suspension.44 Once injected into the deltoid muscle (approved for the 441 mg dose) or gluteal muscle (approved for any of the doses), the conversion of aripiprazole lauroxil to aripiprazole is governed by the slow dissolution of aripiprazole lauroxil and subsequent enzyme-mediated cleavage by esterases. When the product was launched, dose strengths of 441, 662, and 882 mg were initially available. These doses, when administered monthly, yield exposures to aripiprazole equivalent to oral aripiprazole 10, 15, and ≥20 mg/day, respectively. The dose of 882 mg administered every 6 weeks yields similar exposures as 662 mg administered monthly. In 2017, a dose strength of aripiprazole lauroxil 1064 mg administered every 2 months became available and yields equivalent exposures as 662 mg monthly or 882 mg every 6 weeks. Instructions for the use of aripiprazole lauroxil suggest that any dose can be initiated, including 1064 mg every 2 months (in contrast to 3-month paliperidone palmitate which must be preceded by ≥4 months of exposure to 1-month paliperidone palmitate). Initiation of aripiprazole lauroxil requires either 21 days of supplementation with oral aripiprazole or the use of the aripiprazole lauroxil nanocrystal dispersion (ALNCD) formulation, available since 2018.Reference Ehret, Davis and Luttrell41, Reference Hard, Wehr and von Moltke42, 45, Reference Hard, Wehr and Du46 ALNCD contains nanometer-sized particles instead of the micrometer-sized particles contained in standard aripiprazole lauroxil; the smaller particles have faster dissolution properties when injected into the muscle.Reference Hard, Wehr and von Moltke42 An injection of the ALNCD formulation into either the deltoid or gluteal muscle, plus administration of oral aripiprazole 30 mg that same day, can substitute for the 21 days of oral supplementation that would otherwise be required when starting aripiprazole lauroxil.Reference Hard, Wehr and Du46 The first injection of standard aripiprazole lauroxil may be administered on the same day as the ALNCD formulation or up to 10 days thereafter. The ALNCD formulation can also be used in lieu of oral supplementation when the regularly scheduled injection is unduly delayed.44 Because there is only one strength of the ALNCD formulation available (675 mg), adjustments to the dose are not possible in the presence of potential drug–drug interactions, such as with strong CYP2D6 or CYP3A4 inhibitors and strong CYP3A4 inducers, and thus the ALNCD formulation cannot be used under those circumstances and a 21-day oral supplementation period will then be necessary.44, 45
Olanzapine containing formulations
Table 5 outlines the characteristics of olanzapine pamoate.48-50 There are currently no alternative LAI formulations of olanzapine commercially available. Olanzapine pamoate was approved in the United States in 2009. It differs from the other LAI antipsychotics in that its use is governed by a Risk Evaluation and Mitigation Strategy program (United States) or Risk Minimization Plan (Europe), requiring a 3-hour post-injection monitoring period after each injection.Reference Citrome50 This is to better manage the potential risk of Post-injection Delirium Sedation Syndrome (PDSS), as described below.
Table 5. Summary of Characteristics of Olanzapine-Containing Long-Acting Injectable Antipsychotics Commercially Available in the United States
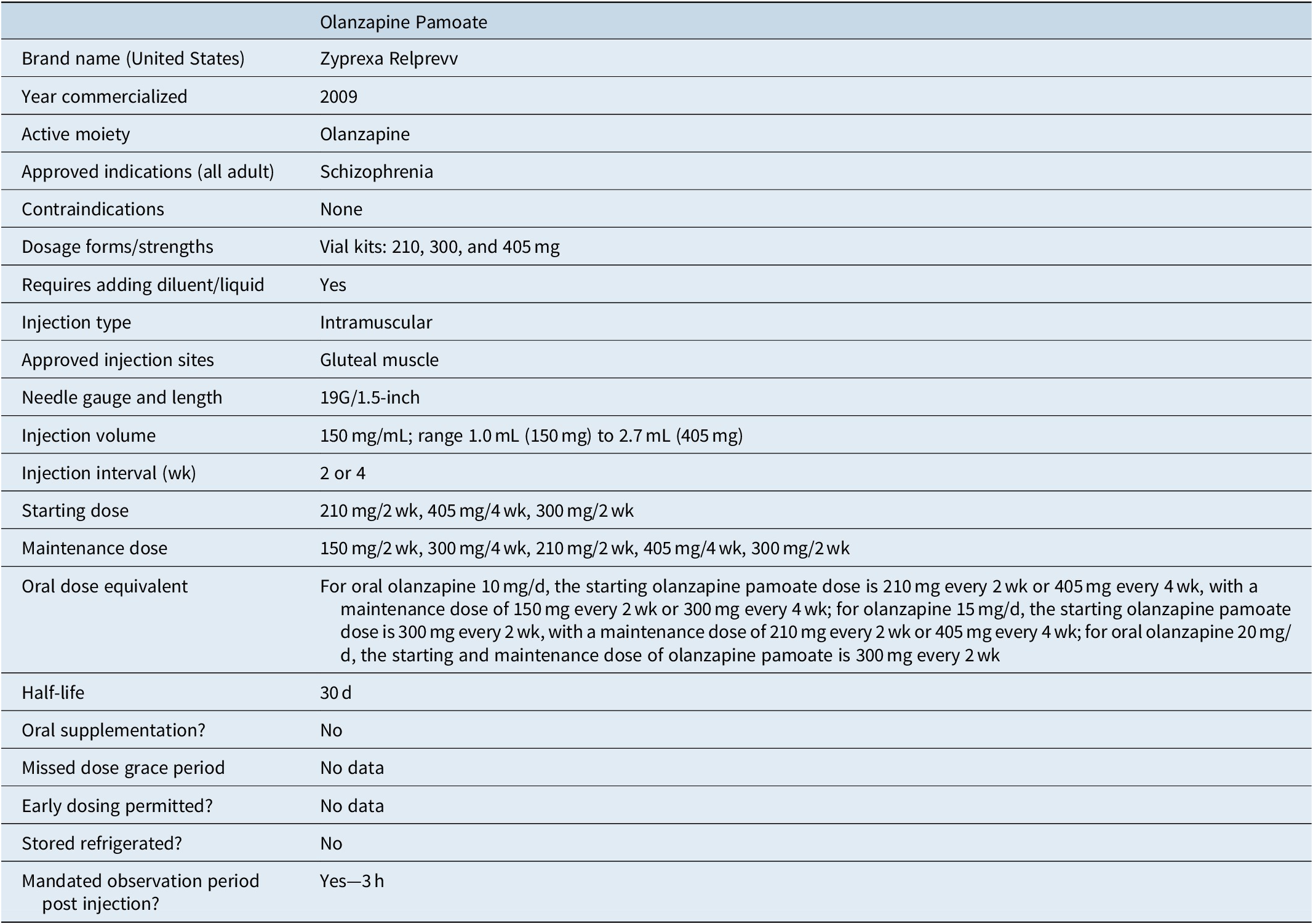
Olanzapine pamoate is a crystalline salt formulation composed of olanzapine and pamoic acid.48, Reference Citrome49 After reconstitution in water, it is injected into the gluteal muscle and the salt slowly dissolves, releasing olanzapine over a period of weeks. However, when olanzapine pamoate comes into contact with a substantial amount of blood or plasma, the salt dissolves more quickly, resulting in the release of a larger amount of olanzapine, potentially leading to PDSS characterized by sedation, confusion, slurred speech, altered gait or unconsciousness. PDSS can be expected to occur in approximately 0.07% of injections and is time-limited but may require symptomatic treatment.Reference Detke, McDonnell and Brunner51, Reference Luedecke, Schöttle and Karow52 Because there are no clear identifiable risk factors, olanzapine pamoate can only be provided at registered healthcare facilities and patients must be monitored by appropriately qualified staff for at least 3 hours after injection. In the United States, there is the additional requirement that patients must be accompanied to their next destination upon leaving the facility. Because the risk of PDSS is cumulative, patients receiving olanzapine pamoate every 2 weeks can decrease their risk of PDSS by 50% by switching to monthly injections. PDSS is not common; from a provider perspective, a clinic with 60 patients receiving an injection every 2 weeks might expect approximately one event per year.Reference Detke, McDonnell and Brunner51
Initiation of olanzapine pamoate does not require oral supplementation. The recommended starting and maintenance dose is dependent on the dose of oral olanzapine required for stabilization: for patients requiring olanzapine 10 mg/day, the starting olanzapine pamoate dose is 210 mg every 2 weeks or 405 mg every 4 weeks, and then if clinically indicated, patients can be evaluated 2 months later for a reduction to a maintenance dose of 150 mg every 2 weeks or 300 mg every 4 weeks; for patients requiring oral olanzapine 15 mg/day, the starting olanzapine pamoate dose is 300 mg every 2 weeks, and then if clinically indicated, patients can be evaluated 2 months later for a reduction to a maintenance dose of 210 mg every 2 weeks or 405 mg every 4 weeks; for patients requiring oral olanzapine 20 mg/day, the recommended starting and maintenance dose of olanzapine pamoate is 300 mg every 2 weeks.48
Acute treatment: what is the evidence?
Oral risperidone/paliperidone, aripiprazole, and olanzapine are all efficacious medications when used to manage individuals with acute exacerbations of schizophrenia. They have been well characterized, with their major differences being their tolerability profiles when comparing groups enrolled in clinical trials.Reference Leucht, Cipriani and Spineli53, Reference Huhn, Nikolakopoulou and Schneider-Thoma54 For example, olanzapine is associated with greater weight gain and metabolic abnormalities, aripiprazole with higher rates of akathisia, and risperidone/paliperidone with elevation in prolactin levels. However, there is considerable individual variation in how people respond to or tolerate antipsychotics.
It would be expected that the LAI formulations would be as efficacious in the acute setting as their oral counterparts. Efficacy in acutely exacerbated patients with schizophrenia has been formally evaluated for once-monthly paliperidone palmitate,55-58 olanzapine pamoate,Reference Lauriello, Lambert and Andersen59 aripiprazole monohydrate,Reference Kane, Peters-Strickland and Baker60 aripiprazole lauroxil,Reference Meltzer, Risinger and Nasrallah61 and risperidone subcutaneous injection.Reference Nasser, Henderson and Fava62 Although the initiation procedures vary among the different products, starting a LAI antipsychotic while hospitalized with an acute exacerbation of schizophrenia consistently demonstrated robust superiority over placebo in reducing psychotic symptoms. Of additional interest is the ability to reduce hostility and agitation.Reference Citrome, Du, Risinger and Stankovic63, Reference Citrome and Volavka64
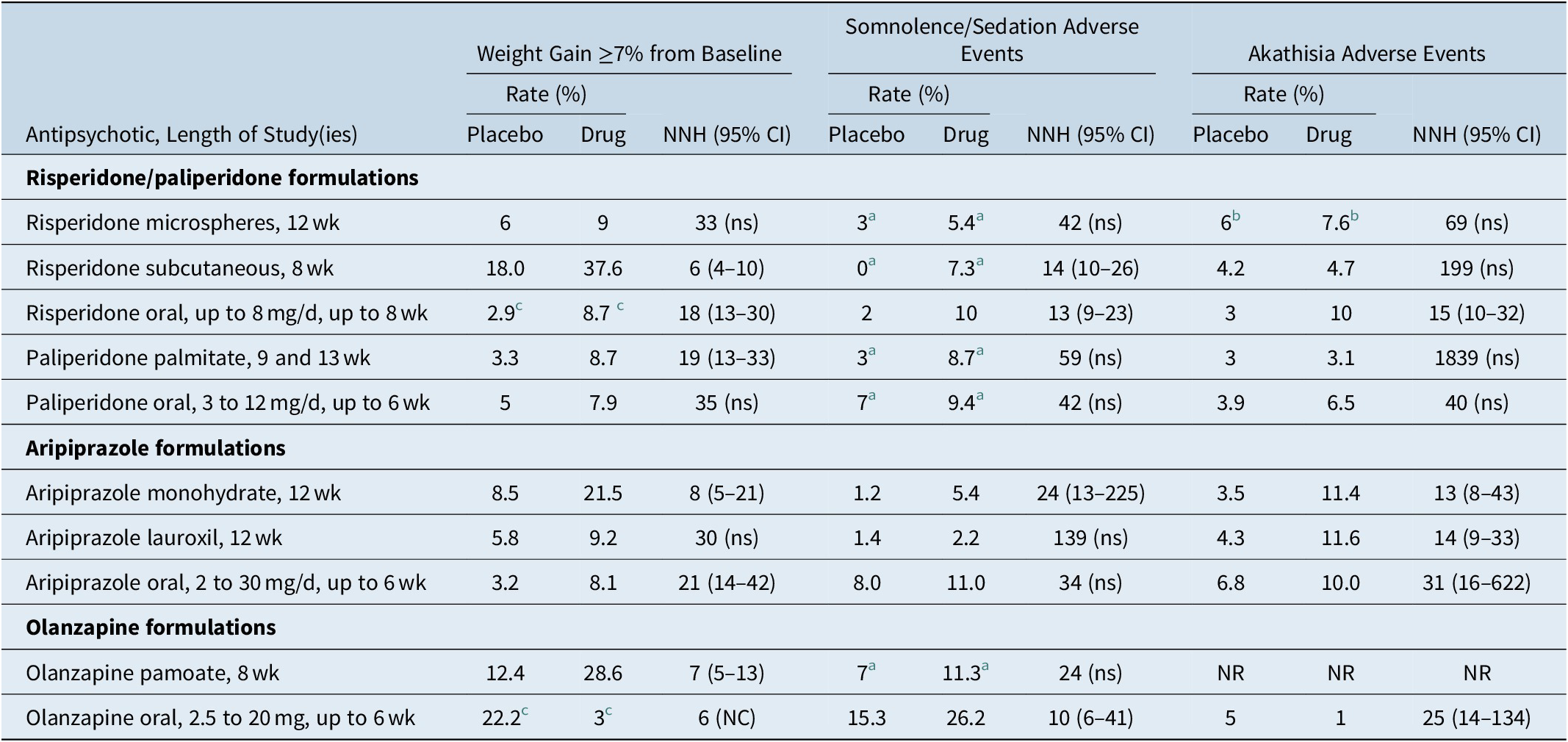
Aside from potential adverse effects related to the injection itself (such as pain, redness, induration), it would be expected that the LAI formulations would have similar tolerability profiles in the acute setting as their oral counterparts. Number need to harm (NNH) vs placebo can be used to indirectly compare risk for weight gain, sedation, and akathisia (Table 6).Reference Citrome21, 29, 33, 39, 43, 44, 48, 65-69 NNH values less than 10 denote events that would be more commonly encountered; this would be the case for weight gain ≥7% from baseline for risperidone subcutaneous injection, aripiprazole monohydrate, and olanzapine pamoate, as calculated from their short-term acute registration studies. The weight gain data is counter-intuitive for risperidone subcutaneous injection and aripiprazole monohydrate and appears to differ somewhat from what has been calculated from registration studies of the oral formulations of risperidone and aripiprazole, where the NNH vs. placebo estimates for weight gain ≥7% were 18 and 21, respectively66, 68; this could be a reflection of study design where patients remained hospitalized throughout the study and potential skewing of the characteristics of the study participants towards those more prone to weight gain.Reference Kane, Peters-Strickland and Baker60, Reference Nasser, Henderson and Fava62
Table 6. Rates and Number Needed to Harm vs Placebo for Weight Gain, Somnolence/Sedation, and Akathisia, for Approved Long-Acting Injectable Second-Generation Antipsychotics and Their Oral Counterparts in Adults as Observed in Acute Short-Term Studies for Schizophrenia (Doses Pooled)

Abbreviations: CI, confidence interval; NC, the 95% CI is not calculable as denominators were not provided in product labeling; NNH, number needed to harm; NR, not reported (did not meet threshold for reporting); ns, not significant at the P < .05 threshold and thus the 95% CI is not shown.
a Pooled term of somnolence/sedation as reported in the product label.
b Pooled term of akathisia/restlessness as reported in the product label.
c Pooled schizophrenia and bipolar as reported in the product label.
Prevention of relapse: what is the evidence?
Although LAI antipsychotics can be used acutely, LAI antipsychotics are more often considered as part of a long-term treatment strategy. Evidence supporting this goes back to the notion of the need to improve adherence to medication treatment in order to optimize outcomes. Real-world prospective and retrospective studies comparing LAI antipsychotics vs oral antipsychotics generally demonstrate decreases in relapse, hospitalization, and all-cause discontinuation for patients receiving LAI antipsychotics.Reference Kirson, Weiden and Yermakov70
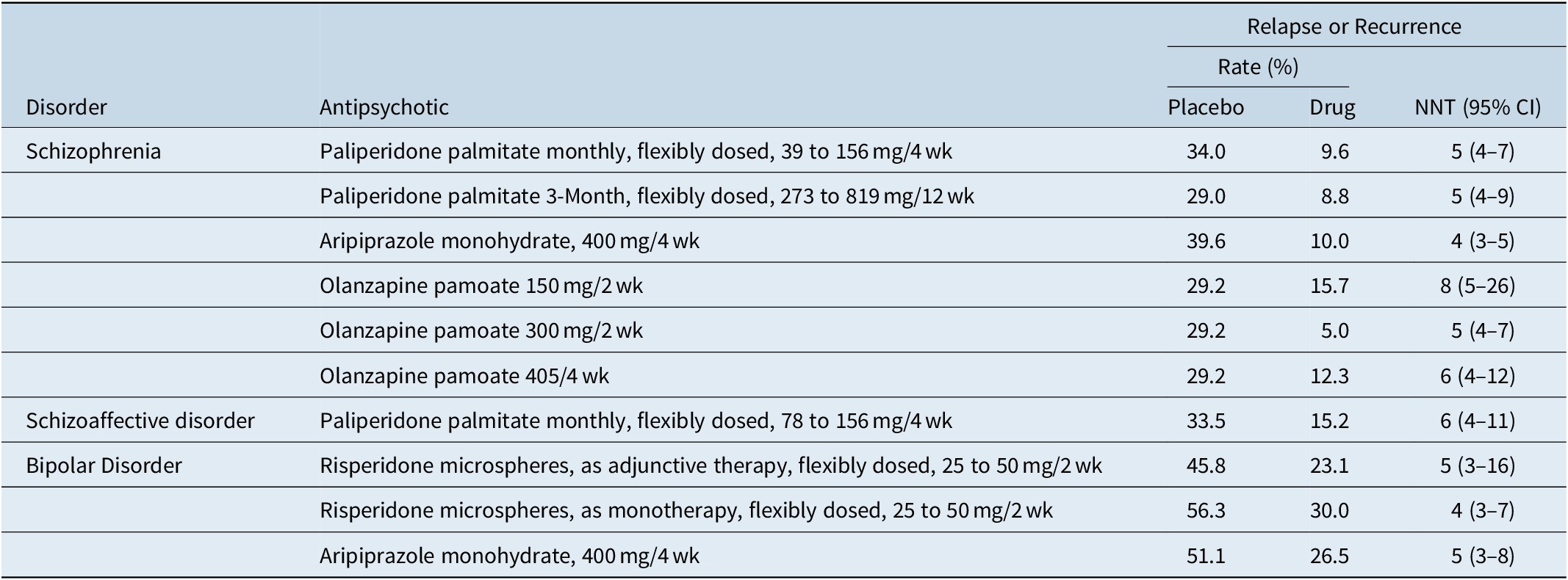
Although not without controversy,Reference Emsley, Fleischhacker and Galderisi71 placebo-controlled randomized withdrawal study designs are often used to establish efficacy for the maintenance indication. The typical study design would be one where patients with the disease of interest are stabilized on the test medication and then subsequently randomized to either continue the test medication or receive placebo. The primary outcome measure is usually time to relapse, impending relapse, or recurrence, depending on the disorder and the study. This has been formally assessed vs. placebo in registration studies in individuals with schizophrenia for paliperidone palmitate administered monthlyReference Hough, Gopal and Vijapurkar72 or every 3 months,Reference Berwaerts, Liu and Gopal73 olanzapine pamoate,Reference Kane, Detke and Naber74 and aripiprazole monohydrate.Reference Kane, Sanchez and Perry75 Registration studies were also done in individuals with bipolar disorder for risperidone microspheres (monotherapy or adjunctive use)Reference Macfadden, Alphs and Haskins76, Reference Quiroz, Yatham and Palumbo77 and aripiprazole monohydrate (monotherapy),Reference Calabrese, Sanchez and Jin78 and in individuals with schizoaffective disorder for once monthly paliperidone palmitate (monotherapy or adjunctive use).Reference Fu, Turkoz and Simonson79 Number needed to treat vs placebo for prevention of relapse or recurrence for any of the tested medications for any of the indications range from 4 to 8, with overlap of the 95% confidence intervals (Table 7).Reference Citrome20, Reference Citrome34, Reference Greenberg and Citrome35, Reference Citrome40, Reference Citrome49, Reference Citrome50, 80 These effect sizes are consistent with the broader literature in schizophrenia.Reference Ceraso, Lin and Schneider-Thoma81
Table 7. Prevention of Relapse or Recurrence as Quantified Using Number Needed to Treat vs Placebo (or vs. 45 mg/4 wk for Olanzapine Pamoate), Data from U.S. Registration Studies
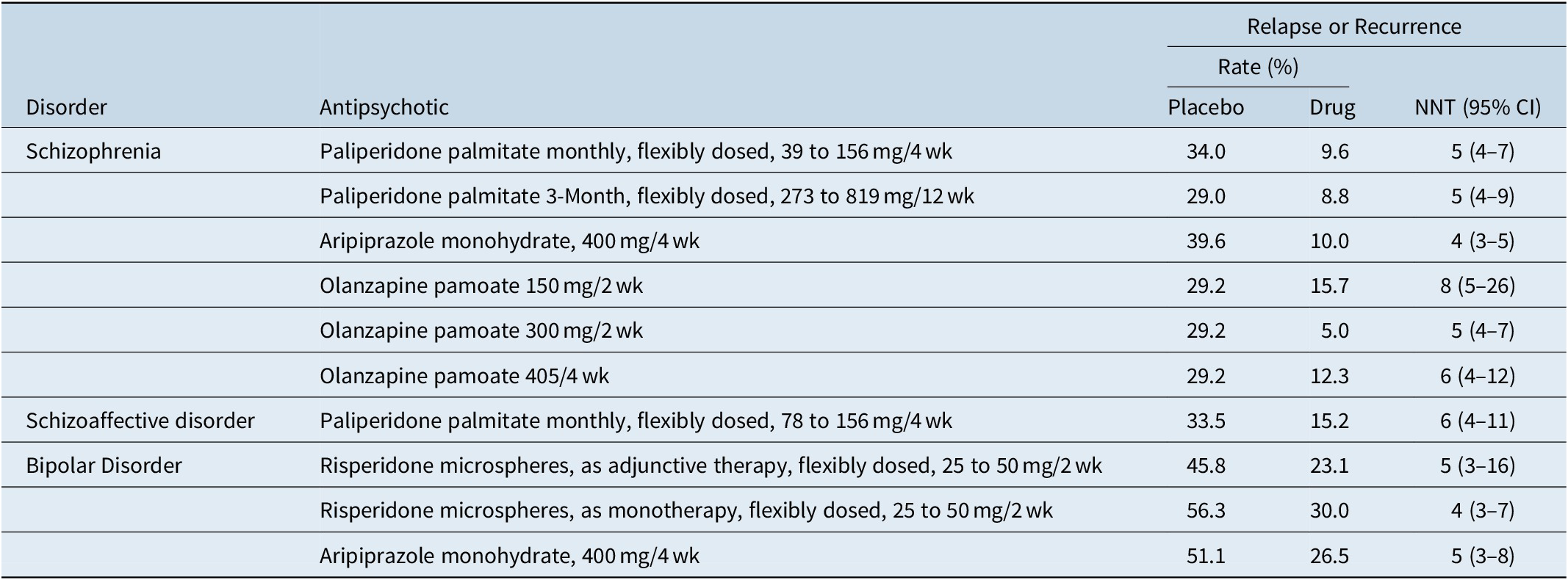
Abbreviations: CI, confidence interval; NNT, number needed to treat.
When should LAIs be offered?
Although LAI antipsychotics are often thought of as a last resort for chronically ill individuals who have been unable to adhere to oral medications, there is strong evidence supporting the use of LAI antipsychotics earlier in the disease process.Reference Stahl82, Reference Gardner and Nasrallah83 Early episode patients may have the most to gain from LAI antipsychotics, at a time when schizophrenia is most treatable and when avoidance of recurrences and rehospitalizations may lead to the biggest improvements in outcome. Using LAI antipsychotics may potentially decrease the percentage of time spent experiencing psychotic symptoms, reduce disability, and perhaps avoid some of the decrease in treatment response that can occur with subsequent exacerbations. Neuropathological brain changes can progress with subsequent clinical episodes.Reference Lieberman, Alvir and Koreen84 The first 2 to 3 years of illness may be the most critical.Reference Birchwood, Todd and Jackson85 LAI antipsychotics allows for the swift identification of overt nonadherence and can eliminate covert nonadherence.
The evidence supporting the early use of LAI antipsychotics is from both real-world studies and from controlled clinical trials. For example, in a nationwide cohort study in Finland of 2588 patients diagnosed with schizophrenia following a first hospitalization, the risk of rehospitalization for patients receiving LAI antipsychotics was about one-third of that for patients receiving oral antipsychotics.Reference Tiihonen, Haukka and Taylor86 This is not surprising given that relapse rates are as high as about 80% within 5 years of initial recovery from first-episode schizophrenia,Reference Robinson, Woerner and Alvir87 often related to stopping medication.Reference Robinson, Woerner and Alvir88 The case for using LAI antipsychotics in first-episode or early-phase schizophrenia has also been supported by randomized clinical trials of risperidone microspheres,Reference Subotnik, Casaus and Ventura89 paliperidone palmitate,Reference Schreiner, Aadamsoo and Altamura90 and aripiprazole monohydrate.Reference Kane, Schooler and Marcy91
Of interest is the inclusion in treatment guidelines of the importance of patient preference when selecting medications, including LAI formulations.Reference Keepers, Fochtmann and Anzia4 However, LAI antipsychotics are not being consistently offered and thus patients who may want them may not even know of the existence of this modality of treatment. In a survey of attitudes towards LAI antipsychotics among patients, relatives, and psychiatrists in Switzerland, about 67% of the patients did not receive information about depot antipsychotics from their psychiatrist and less than 10% of psychiatrists offer depot treatment after a first psychotic episode.Reference Jaeger and Rossler92 This is a disservice because data suggests that once provided, patients receiving LAI antipsychotics want to continue them. In a survey conducted in France in patients with schizophrenia who had received at least 3 months’ treatment with a LAI antipsychotic, injections were the favored dosage form, and 67% said they felt better having received an injectable treatment than they felt before, with about half the patients (51%) considered injectable therapy to be more effective than other medication.Reference Caroli, Raymondet and Izard3 Moreover, 70% felt better supported in their illness by virtue of regular contact with the doctor or nurse who administered their injection.
How to offer LAIs
Motivational interviewing can be a useful method of encouraging active participation in treatment planning and increase levels of adherence to whatever decision is reached.Reference Haque and D’Souza93, Reference Lewis-Fernández, Coombs and Balán94 This technique is not unique to psychiatry, as motivational interviewing is also encouraged for the management of hypertensionReference Ren, Yang and Browning95 where levels of treatment nonadherence are also as high as 50%.Reference Naderi, Bestwick and Wald96, Reference Briesacher, Andrade and Fouayzi97 In addition to “meeting patients where they are,” shedding negative attitudes toward LAI antipsychotics is important, as evidenced in a study examining psychiatrists’ ambivalence regarding the value of LAI antipsychotics and the perceived difficulty with patient acceptance of this modality of treatment.Reference Weiden, Roma and Velligan98
A useful opening is asking “How would you like to receive your medication once a month (or every 2 months) (or every 3 months)? I know I would!” Other remarks that can be made include:
• “You know, I have high blood pressure and take pills for that, and sometimes I forget. How often does that happen to you with your pills?”
• “It must be hard to hear your Mother constantly ask all day long if you have taken your medicine…”
• “It must be hard to remember if you had taken your medicine last night.”
The injection process can be perceived as stigmatizing by some patients who have received intramuscular medication for behavioral emergencies and over their objection; in this instance, the voluntary nature of routine maintenance treatment will need to be emphasized. It can be helpful to “normalize” injection treatments in general by mentioning that many people with diabetes need insulin injections and that depot medications have been used for other purposes in medical care, such as hormonal treatments and opioid use disorder. Another source of stigma may be that the patient has received care in settings where only the most severely ill received LAI antipsychotics; in this case it would be helpful for the patient to converse with a peer advocate who has been successful with LAI antipsychotic treatment.
Demystification of the injection process can be accomplished by showing the syringe and needle to be used, and in the case of intramuscular administration, discussing the similarities with the mechanics of a routine “flu shot.” Concerns over gluteal injection can often be allayed by mentioning that it can be administered in the upper outer quadrant of the buttocks, which is relatively easy to access without disrobing, and is also a far quicker process than injection in the deltoid muscle during the cold winter months when they are all bundled up in multiple layers of clothing.
Ultimately if acceptance of the LAI antipsychotic appears tenuous, there is no harm done by suggesting “Would you like to give it a try? If you do not want it again, you do not have to have it.”
Patient and family education on schizophrenia, schizoaffective disorder and bipolar disorder, and the risks of relapse/recurrence are important. The National Alliance on Mental Illness (NAMI) make a Family-to-Family educational program available in many communities in the United States (see https://www.nami.org/Support-Education/Mental-Health-Education/NAMI-Family-to-Family).
Lastly, who gives the injection can also be a driver for both the offer of a LAI antipsychotic by the treating clinician, and the acceptance of this treatment by the patient. Several options exist as to who administers the injection: the prescriber, another member of the office staff, another provider in the building that has agreed to do this, or the pharmacist in States that allow this.
It needs to be acknowledged that LAI antipsychotics are not for everyone. Since LAI antipsychotics are usually administered every 2 to 12 weeks, depending on the product, this prevents the flexibility that is ordinarily present with dosing of oral medications and when optimal dose for the individual is not known. There are some patients with delusions of being controlled and for whom LAI antipsychotics seem to be particularly threatening, no matter how strong the therapeutic alliance. In the event of adverse reactions, slow distribution from the muscle or subcutaneous tissue may lead to prolonged effects, making management of side effects challenging.Reference Remington and Adams99 Some individuals may be sensitive to injection site reactions—pain, erythema, swelling and discomfort, particularly with sesame oil-based products such as fluphenazine decanoate or haloperidol decanoate.
More about choices
The simplest scenario is if the patient is already receiving an antipsychotic that is available as a LAI formulation. Then it is a matter of educating the patient (and caregiver) about the availability of this different way of receiving medication. If there are competing LAI formulations for the same or related molecule, then a review of the “amenities of care” (Table 2) is in order. There may be a preference for a specific injection interval that is available with only some of the products. Patients and caregivers sometimes need to be reassured that a longer interval between injections does not necessarily mean that visits will be scheduled less often.
In some situations, it may be unrealistic to expect adherence to oral supplementation and alternatives should be considered. Some choices require an infrastructure (refrigeration for storage of risperidone microspheres or risperidone subcutaneous injection, examination table for administering risperidone subcutaneous injection in the abdomen, ability to observe the patient for 3 hours after each injection of olanzapine pamoate). For patients receiving oral fluphenazine or haloperidol and a switch to a LAI is being considered, despite the relatively low cost of haloperidol decanoate or fluphenazine decanoate, the clinician needs to weigh the potential disadvantages of using concomitant oral anticholinergics as discussed earlier. On occasion, the supply chain for older generic medications sometimes gets interruptedReference Demers, Bilodeau and Laberge100; the American Society of Health-System Pharmacists maintains a web resource that tracks drug shortages and is available at https://www.ashp.org/Drug-Shortages/Current-Shortages.
If the patient is receiving acute treatment, paliperidone palmitate, risperidone subcutaneous, and olanzapine pamoate are options that can be administered in the inpatient setting and no oral medication is required upon their initiation. However, the other options, notably aripiprazole monohydrate and aripiprazole lauroxil, have demonstrated robust efficacy in the acute setting, provided that supplemental medication be administered after the first injection: 14 days for aripiprazole monohydrate (or 1 day in Canada or the EU if the two-injection start regimen is used), and either 1 day or 21 days for aripiprazole lauroxil depending if the ALNCD formulation is used. Prior knowledge of tolerability and efficacy is important, because once injected, the medication cannot be withdrawn. Oral or intramuscular short acting antipsychotic medications are in most situations the most prudent way to initiate antipsychotic treatment in an individual who is treatment-naïve or if a medication history cannot be reliably obtained. In general, weight gain and metabolic adverse effects are a common concern with second-generation antipsychotics, especially with olanzapine; first-generation LAI antipsychotics could possibly be considered under these circumstances and where a switch among the second-generation LAIs was not helpful. If prolactin-related adverse effects are a clinical concern, one of the aripiprazole LAI formulations would be the first choice; to be avoided under these circumstances would be paliperidone palmitate, risperidone microspheres, or the first-generation LAI antipsychotics.
Cost considerations for branded products are sometimes obstacles to their use and access to patient-assistance programs can be helpful in many instances.
Conclusion
Poor adherence to antipsychotic medication is common and this can result in suboptimal outcomes. LAI antipsychotics can address the guesswork about adherence status and patients may prefer them if they are offered this as a choice, including individuals early in their disease course. Although not every oral antipsychotic is available in a LAI formulation, there are now more options than existed 20 years ago. Choosing among the different LAI antipsychotics is partly based on pragmatic concerns. For example, olanzapine pamoate would not be a practical option if the mandatory 3-hour post-injection observation period cannot be provided. For patients receiving oral risperidone, using risperidone microspheres can be inconvenient as that formulation is administered every 2 weeks, requires refrigeration and reconstitution, and must be accompanied by oral supplementation for the first 3 weeks after the initial injection. Instead of risperidone microspheres, paliperidone palmitate can be considered as it does not require oral supplementation, entails less frequent injections (either monthly or every 3 months), is supplied in prefilled syringes, has a needle gauge as small as 23G, a small injection volume, and does not need to be refrigerated. Regarding aripiprazole LAI, there are 2 competing formulations available in the United States; they differ in terms of oral supplementation, frequency of injections, requirement for reconstitution, and needle gauge. Additional approved indications in the US for LAI antipsychotics include bipolar I disorder maintenance treatment for risperidone microspheres and aripiprazole monohydrate, and schizoaffective disorder for paliperidone palmitate once monthly.
Disclosures
Leslie Citrome has had the following disclosure information in the past 12 months, consultant: AbbVie, Acadia, Alkermes, Allergan, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Eisai, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Luye, Lyndra, Medavante-ProPhase, Merck, Neurocrine, Noven, Osmotica, Otsuka, Relmada, Sage, Shire, Sunovion, Takeda, Teva, University of Arizona, and one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research.
In the past 12 months, speaker: AbbVie, Acadia, Alkermes, Allergan, Angelini, Eisai, Intra-Cellular Therapies, Janssen, Lundbeck, Merck, Neurocrine, Noven, Otsuka, Sage, Shire, Sunovion, Takeda, Teva, and CME activities organized by medical education companies such as Medscape, NACCME, NEI, Vindico, and Universities and Professional Organizations/Societies.
Stocks (small number of shares of common stock): Bristol-Myers Squibb, Eli Lilly, J & J, Merck, Pfizer purchased >10 years ago.
Royalties: Wiley (Editor-in-Chief, International Journal of Clinical Practice, through end 2019), UpToDate (reviewer), Springer Healthcare (book), Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics).
In the past 5 years Leslie Citrome has engaged in collaborative research with, or received consulting or speaking fees, from: AbbVie, Acadia, Alexza, Alkermes, Allergan, Angelini, Astellas, AstraZeneca, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Bristol-Myers Squibb, Cadent Therapeutics, Eisai, Eli Lilly, Forum, Genentech, Impel, Indivior, Intra-Cellular Therapies, Janssen, Jazz, Karuna, Lundbeck, Luye, Lyndra, Medavante-Prophase, Meiji, Merck, Medivation, Mylan, Neurocrine, NeuroRx, Novartis, Noven, Osmotica, Otsuka, Pfizer, Reckitt Benckiser, Relmada, Reviva, Sage, Shire, Sunovion, Takeda, Teva, University of Arizona, Valeant, Vanda, and one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research.