Detailed understanding of the dynamics of pathogen transmission requires simultaneous information on the presence and nature of pathogens in hosts and on contact patterns that drive transmission events between hosts. Until now, however, most studies have focused on only 1 of these 2 aspects.
In healthcare settings, the transmission of hospital-acquired influenza (HA-Flu) has been investigated via laboratory testing of patients and molecular phylogenetic analyses, possibly combined with coarse descriptions of contact patterns through surveys.Reference Eibach, Casalegno and Bouscambert1–Reference Wong, Lee and Li5 Better knowledge of patient and healthcare worker contact patterns is, however, a crucial element in understanding the spread of HA-Flu and, subsequently, the design and implementation of adequate prevention and control measures. In this respect, proximity-sensing technology based on wearable sensors has recently emerged as an appealing method for gathering information on contacts between individuals that uses high temporal and spatial resolution.Reference Barrat, Cattuto, Tozzi, Vanhems and Voirin6, Reference Read, Edmunds, Riley, Lessler and Cummings7 Such technologies have been deployed in contexts ranging from scientific conferences to primary schools, high schools, and hospitals,Reference Cattuto, Van den Broeck, Barrat, Colizza, Pinton and Vespignani8–Reference Vanhems, Barrat and Cattuto12 yielding detailed contact data useful in the modeling and understanding of communicable diseases, such as airborne infections.
In the present proof-of-concept study, and as advocated recently in Isella et alReference Isella, Romano and Barrat9 and Vanhems et al,Reference Vanhems, Barrat and Cattuto12 we explored the automated collection of high-resolution contact data by wearable sensors combined with virological data (ie, infection status of participants and partial phylogenetic analysis) on patients and healthcare workers in a geriatric unit. We show that such data, gathered during the peak of the influenza season in February–March 2012, can contribute to the investigation of influenza transmission, and we discuss the value of this approach in advancing our understanding of HA-Flu transmission mechanisms.
METHODS
Setting and Data Collection
Data were collected in an acute care geriatric unit (20 beds in 16 single rooms and 2 double rooms) of a university hospital in Lyon, France. The study was conducted from Monday, February 27, 2012, to Friday, March 9, 2012, during the seasonal peak of influenza in France (http://www.grog.org/). Individuals were categorized into 3 classes according to their role in the ward: patients, medical doctors (physicians and residents), and paramedical staff (nurses and nurses’ aides). Medical doctors and nursing professionals comprised the healthcare worker group. During this period, 48 healthcare workers worked in the unit, and 44 patients were hospitalized. In the remainder of the text, we refer to Monday, February 27, 2012, as day 1 (D1) of the study and Friday, March 9, 2012, as D12.
Contact Data
We recorded close-range encounters between individuals in the ward, during which a communicable disease (eg, influenza) could be transmitted, for example, by coughing, sneezing or hand contact. These data were obtained and processed using a proximity-sensing platform developed by the SocioPatterns collaboration (http://www.sociopatterns.org/). This system, previously described in detail,Reference Barrat, Cattuto, Tozzi, Vanhems and Voirin6–Reference Isella, Romano and Barrat9, Reference Vanhems, Barrat and Cattuto12 is based on wearable radio-frequency identification proximity sensors (“tags”) embedded in unobtrusive badges that exchange ultra-low-power radio data packets. Participating individuals were asked to wear the badges on their chests with lanyards. The sensors were set so that they assessed “contact” when the persons wearing them were facing each other within 1–1.5 m for at least 20 s. Information on face-to-face proximity events detected by the devices was relayed to radio receivers installed in the ward.
We measured close-range contacts between participants from D1 at 1:00 p.m. to D12 at 1:00 p.m. Tags were given to participants in the afternoon of D1 and were retrieved in the afternoon of D12. All 48 healthcare workers (33 nurses and 15 medical doctors) and 38 patients participated in data collection. The participation rate was 86%. Overall, data on contacts among 32 nurses, 15 medical doctors, and 37 patients were collected and analyzed; 2 badges (1 of a nurse and 1 of a patient) were defective.
Virological Data
Nasopharyngeal swabs were taken to confirm influenza infection. Samples were collected at admission and discharge of each patient. In patients already hospitalized at the start of data collection, the first sample was taken when the study began. Swabs were taken from healthcare workers on D1 of the study (or on the first day of duty for healthcare workers not on duty on D1) and on D12, at the end of the study. An additional swab was taken if an individual presented 1 or more symptoms usually associated with influenza-like infection, such as fever, feverishness, chills, cough, nasal congestion, weakness, asthenia, loss of appetite, sore throat, pharyngitis, headache, or myalgia.Reference Monto, Gravenstein, Elliott, Colopy and Schweinle13
Nasopharyngeal swabs were sent to the Southern French National Reference Laboratory for Influenza (Lyon, France) and were analyzed for influenza A and B viruses by real-time polymerase chain reaction (PCR; Respiratory Multi-Well system R-gene®, Argene, Verniolle, France). Culture-based influenza subtyping and phylogenetic analysis were not originally planned in this study but were attempted for some PCR-confirmed influenza A (H3N2) samples. Culture-positive viral isolates were subjected to amplification of overlapping fragments of neuraminidase and hemagglutinin genes by reverse transcription-PCR.
Data Analysis
Each admitted patient or healthcare worker was considered susceptible (ie, at risk of acquiring influenza) until the first laboratory-confirmed positive test or until discharge or the end of the study for individuals with negative laboratory-confirmed test results.
Incubation of influenza was considered to last up to 5 days, and the duration of contagiousness was considered to last up to 6 days (ie, 1 day before influenza-like infection onset, the day of influenza-like infection onset, and the 4 following days).Reference Carrat, Vergu and Ferguson14 According to the influenza laboratory test results, the duration of contagiousness was adopted as follows: if a test was positive more than 5 days after the onset of influenza-like infection symptoms, we assumed that the infected person was contagious up to the date of the positive test result. In asymptomatic individuals, the contagious period was assumed to begin from the date of the first positive result up to the last positive result. After the contagious period, individuals were assumed to be immune and no longer at risk.
Moreover, we analyzed detailed information on healthcare workers and patient contact patterns occurring in the ward to detect contacts between contagious and susceptible individuals, ie, contacts compatible with potential transmission. For individuals who became positive for an influenza-like infection during the stay, all contacts with contagious individuals in the 5 previous days were considered potential transmissions.
Ethics and Privacy
All participants signed an informed consent form and were given a proximity-sensing badge that they were asked to wear at all times. Data collected included date of birth, gender, influenza vaccination status, dates of admission and discharge (for patients), work timetable (for healthcare workers), and medical and clinical histories (for patients). Data on patients were provided by the hospital and were collected by means of healthcare worker interviews. The study was approved by the French national bodies responsible for ethics and privacy: the Commission Nationale de l'Informatique et des Libertés (http://www.cnil.fr) and the Comité de Protection des personnes (http://www.cppsudest2.com/) of the hospital.
RESULTS
Contact Data
A total of 18,765 contacts were recorded during the study period, with a cumulative duration of 904,560s (ie, ~15,076 min or 251 h). The total number and cumulative duration of contacts between individuals belonging to specific classes are reported as contact matrices in Figure 1. Contacts were most frequent between nurses (10,107; 54%), between 1 nurse and 1 patient (3,634; 19%), and between medical doctors (2,546; 14%). Detailed contact data, namely, individual contacts, pair contacts, contact matrices, and temporal evolution of contacts, are not shown and exhibited patterns similar to those reported in a previous study.Reference Vanhems, Barrat and Cattuto12

FIGURE 1 Contact matrices defined in classes of individuals. Matrices give the total number and duration of contacts, in seconds, between pairs of individuals belonging to specific classes. Abbreviations: NUR, paramedical staff (nurses and nurses’ aides); PAT, patients; MED, medical doctors, ie, physicians and residents).
Socio-Demographic and Virological Data
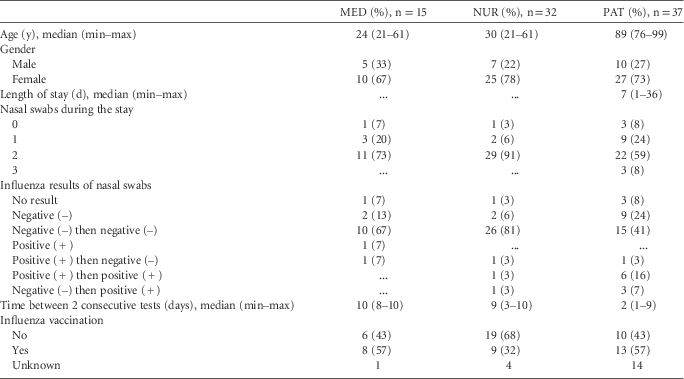
Socio-demographic and clinical data on medical doctors, nurses, and patients are presented in Table 1. A total of 148 nasal swabs were taken: 25 among 15 medical doctors, 60 among 33 nurses, and 63 among 38 patients. A total of 15 individuals (10 patients and 5 healthcare workers) had influenza A (H3N2) at some point during the study. Among them, 11 study participants (7 patients, 2 medical doctors, and 2 nurses) were positive at the beginning of data collection or at admission, and 4 study participants (patients #600, #612, #657, and nurse #644) acquired laboratory-confirmed influenza [ie, tested influenza negative (–) and then influenza positive (+)] during the study.
TABLE 1 Virological, Socio-Demographic, and Clinical Data

Note. MED, medical doctor; NUR, nurse; PAT, patient.
Among the 15 PCR-confirmed influenza cases, samples from 9 cases (60%) were cultured. Of these, 3 (20%) were culture positive and were sequenced; they showed genetically identical influenza A (H3N2) viruses for the 3 corresponding individuals (nurses #640 and #644 and patient #612). This finding strongly suggests the possibility that the infectious individuals were part of a single influenza cluster, but no further information on genetic similarity of the viruses in other infected individuals was obtained.
Combining Virological and Contact Data
Figure 2 highlights the contact network between laboratory-confirmed influenza cases and those negative for influenza. Interestingly, people that were contagious and at some point in time were laboratory-confirmed with influenza (in orange) are also individuals who had a higher number and longer duration of contacts. Especially, patients #657, #612, #600, and nurse #644, the 4 individuals (in yellow) who acquired laboratory-confirmed influenza during the study, were among those with a higher number and longer duration of contacts.

FIGURE 2 Contact network between laboratory-confirmed influenza cases and those negative for influenza. Only the contacts for which either individual is considered contagious are shown. Lines represent interactions between 2 individuals. Orange lines represent interactions between persons that were laboratory confirmed and contagious for influenza; gray lines represent persons that were not infected. Height of the inner ring represents the total duration of the contacts this person had with other persons on a log scale. The longest contact duration was 12 h 44 min. The height of the outer ring represents the total number of contacts this person had with other persons on a log scale. The higher number of contacts was 822. Orange nodes represent people that were laboratory-confirmed as infected and were contagious before the study started; yellow represents people who became infected and were laboratory-confirmed for influenza during the study period.
Figure 3, a synoptic chart that combines time, contact, and virological data for 15 individuals with influenza A (H3N2), highlights potential transmission events among 4 individuals who acquired influenza during the study (patients #657, #612, #600, and nurse #644). These contacts are described here in detail.
∙ Contact data analysis suggested a likely source of infection for patient #657, namely, medical doctor #640: 7 contact events with a cumulated duration of 8 min occurred on D3 between these individuals, while medical doctor #640 was infectious. Afterward, on D5, patient #657 developed symptomatic laboratory-confirmed influenza.
∙ The proximity-sensing system recorded 2 contacts with infectious individuals for patient #612, who presented laboratory-confirmed influenza on D5: a 20-s contact on D2 with nurse #626 and another 20-s contact on D4 with nurse #663. The fact that the swab of patient #612 tested negative for influenza on admission (D2) indicates that the observed contacts with infectious nurses could have been responsible for disease transmission despite their short duration.
∙ Patient #600 presented asymptomatic laboratory-confirmed influenza on D8. He was already hospitalized but negative for influenza at the beginning of the study (6 days earlier). The contact data suggested nurse #663 as a potential source of infection, as she took care of this patient on D7. On that day, 5 contact events were recorded between these 2 individuals with a cumulated duration of 3 min.
∙ Nurse #644 presented laboratory-confirmed influenza on D10. This nurse had repeated contacts with 2 infectious patients (#602 and #612) and 1 nurse (#663) on D5. On D8, she had repeated contacts with infectious patients #612, #675, #677, and further contacts with infectious patients #675 and #677 on D9. Moreover, nurse #644 and patient #612 had genetically identical influenza viruses, strongly indicating that these 2 individuals were part of a single influenza cluster.

FIGURE 3 Analysis of 15 influenza A(H3N2) cases with electronic contact data and virological data. Note. RFID, radio-frequency identification; PAT, patient; MED, medical doctor, NUR, nurse. Monday, February 27, 2012 was day 1 (D1) of the study and Friday, March 9, 2012, was D12. †Study participants 640, 612, and 644 had a positive (pos) culture, were phylogenetically linked and highlighted in orange color. For other individuals, influenza was not sequenced because culture was not done (nd) or negative (neg). *Study participants 657, 612, 600, and 644 were cases with hospital-acquired influenza. **Contacts were not analyzed on D1.
DISCUSSION
The objective of the present study was to investigate how combining high-resolution contact data and virological data can help describe potential routes of influenza transmission in a hospital ward.
A previous study in the same wardReference Régis, Gorain and Pires-Cronenberger15 identified potential transmission chains of laboratory-confirmed influenza during 3 influenza seasons by combining virological data and survey-based contact data. Here, we took a further step by using a proximity-sensing system that provides high-resolution contact data. This system does not rely on the memory of observers and limits the impact of missing information or recall biases.Reference Smieszek, Burri, Scherzinger and Scholz16 The investigation was, moreover, partially completed with phylogenetic analysis of influenza viruses.
Overall, analysis of contacts that occurred between healthcare workers and patients in the geriatric ward yielded results similar to those of previous investigations.Reference Isella, Romano and Barrat9, Reference Vanhems, Barrat and Cattuto12, Reference Régis, Gorain and Pires-Cronenberger15, Reference Bernard, Fischer, Mikolajczyk, Kretzschmar and Wildner17–Reference Hornbeck, Naylor, Segre, Thomas, Herman and Polgreen20 The present study illustrates that most contacts occurred between nurses or between a nurse and a patient, highlighting the role of nurses in potential HA-Flu spread.Reference Bernard, Fischer, Mikolajczyk, Kretzschmar and Wildner17 Indeed, infectious healthcare workers were identified as potential sources of influenza infection for patients, and infectious patients were identified as likely sources for healthcare workers. However, while it could be expected from contact matrices that transmission would occur between healthcare workers, only 1 potential transmission between nurses was apparent in our study. In addition to contact data, sequence analysis revealed that 2 nurses (#640 and #644) and 1 patient (#612) had genetically identical influenza A(H3N2) viruses and, therefore, were epidemiologically linked cases.Reference Eibach, Casalegno and Bouscambert1
Given the large number of laboratory-confirmed influenza cases during the study period (ie, 15 contagious sources over 12 days), it might be surprising that only 4 transmissions occurred within the ward, since estimation of the transmissibility of seasonal influenza suggests that a contagious individual would generate an average of 1–2 cases.Reference Truscott, Fraser and Hinsley21 Moreover, during that influenza season, a mismatch between vaccine strains and circulating H3N2 strains was observed, suggesting that vaccination did not prevent transmission. However, during the study, recommended control measures,Reference Siegel, Rhinehart, Jackson and Chiarello22 such as hand hygiene, wearing masks, and isolation of contagious patients, were applied, which likely contributed to the limited transmission. Also, individual behavioral responses to the large number of positive influenza cases may have played a role; it has been shown that risk perception leads to protective behavior.Reference Funk, Gilad, Watkins and Jansen23, Reference Funk, Salathe and Jansen24
Even with detailed contact and virological data, understanding whether or not transmission actually occurred remains a challenge because various other factors modulate the probability of transmission for both source and susceptible host. These factors include individual characteristics (eg, severity of disease, immunosuppression, immunosuppressive therapies, or influenza vaccination), microbial agent features (eg, virulence or inoculum size) and environment (eg, ward specialty or compliance with hygiene protocols), which could provide useful contextual information in addition to contacts and virological data.
Of course, the major limitations of this proof-of-concept study are the small numbers of patients, transmissions, and phylogenetic confirmations of transmission, which limit the generalizability of these findings to other care units. The small number of samples only allowed us to assess that some infected individuals belonged to the same influenza cluster, giving more weight to the hypothesis that transmission events effectively occurred within the ward. More frequent systematic virological sampling (eg, at least 3 or 4 times per week for each individual) would have helped to better estimate incubation and infectious periods and more precisely determine influenza-like infection onset. Most importantly, not all influenza samples could be cultured and subsequently sequenced; further studies should put more effort into collecting genetic data.
The important contribution of electronic contact data lies in the heterogeneity of contacts between individuals belonging to different groups. Future works could include comparison of results between hospitals, which may highlight how different epidemiological situations may influence contacts patterns. For example, it may be interesting to compare contact patterns during different interventions (eg, vaccination or masks). In addition, insights can be gained from mathematical simulations using the collected data, which may help to assess the impact of various prevention or infection control interventions.
While describing the precise routes of influenza transmission remains challenging,Reference Régis, Gorain and Pires-Cronenberger15, Reference Brankston, Gitterman, Hirji, Lemieux and Gardam25–Reference Vanhems, Voirin and Roche27 the collection of contact data has already been shown to represent an important tool in outbreak investigations.Reference Cauchemez, Bhattarai and Marchbanks28, Reference Gardy, Johnston and Ho Sui29 Here, we developed a promising method, combining high-resolution contact data and virological data, to better understand influenza transmission dynamics. It will be interesting to apply this method for longer periods and in larger populations with more complete use of phylogenetic analyses to confirm transmissions.
Acknowledgments
We thank all patients and healthcare workers who consented to participate in this study. We are grateful to Stanley Plotkin for his useful comments during manuscript preparation and to Mr. Ovid Da Silva (IRTC, Inc.) for editing our manuscript. We also thank Jan Willem Tulp (http://tulpinteractive.com/) for providing Figure 2.
Financial support: This work was supported in part by the FINOVI Foundation (Lyon) and the INSERM IMI Pandemic Program (to NV and PV), the French ANR project HarMS-flu (Grant ANR-12-MONU-0018 to AB), and the EU FET project (Multiplex Grant 317532 to AB and CC).
Potential conflicts of interest: BL has received travel grants from Roche; research grants from Roche and Sanofi Pasteur, and consulting fees from Roche, GlaxoSmith-Klein, Biocryst, Merck, and Sanofi Pasteur. NV has received a research grant and statistical analysis and publication writing fees from Sanofi Pasteur. The other authors have no conflicts of interest.