Introduction
Anorexia nervosa (AN) is a serious mental illness with weighted annual mortality of 5.10 deaths per 1000 person-years, the highest mortality rate of any psychiatric disorder (Arcelus, Mitchell, Wales, & Nielsen, Reference Arcelus, Mitchell, Wales and Nielsen2011). The etiology of AN is complex and, despite ongoing research, the neurobiological mechanisms underlying AN symptomology remain poorly understood. Deficits in the flexible use of adaptive and situationally appropriate emotion regulation strategies to modulate negative affective states are characteristic of AN, and longitudinal studies have found emotion dysregulation to predict changes in AN symptom severity (Oldershaw, Lavender, & Schmidt, Reference Oldershaw, Lavender and Schmidt2018; Racine & Wildes, Reference Racine and Wildes2015). Relatedly, impairments in general aspects of emotion processing, such as emotional awareness and clarity, as well as an unwillingness to experience emotional distress in pursuit of meaningful activities have been found to contribute to the restricting of food intake in AN (Kucharska et al., Reference Kucharska, Kot, Biernacka, Zimowski, Rogoza, Rybakowski and Bednarska-Makaruk2019; Lavender et al., Reference Lavender, Wonderlich, Engel, Gordon, Kaye and Mitchell2015). There is also evidence to support that individuals with AN present aberrant physiological reactivity [e.g. heart rate and skin conductance response (SCR)] to affective stimuli, though it remains unclear whether a similar pattern holds true during emotion regulation (Clarke, Ramoz, Fladung, & Gorwood, Reference Clarke, Ramoz, Fladung and Gorwood2016; Monteleone, Treasure, Kan, & Cardi, Reference Monteleone, Treasure, Kan and Cardi2018).
The brain regions involved in, affect generation and emotion regulation, including the prefrontal cortex and the limbic system, undergo protracted structural and functional development during adolescence and early adulthood (Ahmed, Bittencourt-Hewitt, & Sebastian, Reference Ahmed, Bittencourt-Hewitt and Sebastian2015). Notably, peak onset of AN coincides with these periods, suggesting that the development of AN symptoms may be linked to disrupted maturation in these regions, leading to a maladaptive shift in emotion regulation (Golden, Reference Golden2003). This interpretation of the present evidence lends itself to a model of dysfunctional neural circuitry in AN in which the neural components underlying emotion regulation either fail to activate key regions required to successfully employ adaptive strategies, or in which abnormalities in the connectivity and coordination between prefrontal and subcortical regions obstruct volitional attempts to downregulate negative affective states (Steward, Menchon, Jiménez-Murcia, Soriano-Mas, & Fernandez-Aranda, Reference Steward, Menchon, Jiménez-Murcia, Soriano-Mas and Fernandez-Aranda2018). Indeed, recent research has found a neural response to implicit affective stimuli in the ventrolateral prefrontal cortex, a region associated with response inhibition and goal-appropriate response selection, to be blunted in women with AN compared to controls (Leppanen et al., Reference Leppanen, Cardi, Paloyelis, Simmons, Tchanturia and Treasure2017).
The few functional magnetic resonance imaging (fMRI) studies carried out to date examining effortful regulation of negative emotions in individuals with AN uphold that bottom-up alterations are partly responsible for the emotion regulation deficits in individuals with AN. For instance, Seidel et al. (2018a,Reference Seidel, King, Ritschel, Boehm, Geisler, Bernardoni and Ehrlichb) found that adolescent patients with AN displayed elevated amygdala reactivity to aversive pictures in comparison to healthy control (HC) group. Interestingly, no significant differences in neural activity during the downregulation of negative affect were found between groups, though the authors did find regulation of ventral striatum activity to be associated with increased body-related rumination and increased negative affect in AN, but not in controls (Seidel et al., Reference Seidel, King, Ritschel, Boehm, Geisler, Bernardoni and Ehrlich2018b). This is in contrast to a recent meta-analysis which found that patients with mood and anxiety disorders recruited regulatory frontoparietal networks involved in cognitive reappraisal, an adaptive emotion regulation strategy, to a lesser extent than controls (Picó-Pérez, Radua, Steward, Menchón, & Soriano-Mas, Reference Picó-Pérez, Radua, Steward, Menchón and Soriano-Mas2017). It has been posited that effective explicit emotion regulation involves the primary action of the frontoparietal and dorsal midline cortices, which are used in the implementation of an internal model to compute the value of emotion-regulatory actions, along with increased engagement of the dorsolateral prefrontal cortex (dlPFC) to make use of these models via working memory (Etkin, Büchel, & Gross, Reference Etkin, Büchel and Gross2015). Moreover, the strength of amygdala coupling with the ventral and dorsal medial prefrontal cortex has been found to be positively associated with the attenuation of negative affect during reappraisal, underscoring the importance of also considering functional connectivity patterns within fronto-subcortical circuitry as possible markers of clinical severity and treatment response (Banks, Eddy, Angstadt, Nathan, & Luan Phan, Reference Banks, Eddy, Angstadt, Nathan and Luan Phan2007).
Previous studies in other psychiatric disorders examining emotional functioning have suggested that increased prefrontal activation during response inhibition (Gyurak et al., Reference Gyurak, Patenaude, Korgaonkar, Grieve, Williams and Etkin2016) and the downregulation of conflict-responsive regions, such as the dorsal cingulate and anterior insulae, during the processing of emotional cues predict better treatment outcome (Fonzo et al., Reference Fonzo, Etkin, Zhang, Wu, Cooper, Chin-Fatt and Trivedi2019). Likewise, multimodal approaches have provided refined mechanistic insights into the specific neural pathways underlying the emotion regulation deficits that contribute to obesity (Steward et al., Reference Steward, Picó-Pérez, Mestre-Bach, Martínez-Zalacaín, Suñol, Jiménez-Murcia and Fernandez-Aranda2019) and borderline personality disorder (Zaehringer et al., Reference Zaehringer, Ende, Santangelo, Kleindienst, Ruf, Bertsch and Paret2019). Considering the evidence to suggest that emotion processing alterations, including emotion regulation, can predict treatment outcome in AN (Oldershaw et al., Reference Oldershaw, Lavender and Schmidt2018), further research is warranted to elucidate the relationship between emotion regulation circuitry and clinical variables in AN.
In this study, we aimed to build upon previous neuroimaging research on emotion regulation in AN (Seidelet al., Reference Seidel, King, Ritschel, Boehm, Geisler, Bernardoni and Ehrlich2018a) by examining the longitudinal clinical relevance of neural reactivity and functional connectivity during cognitive reappraisal in a sample of young adult women receiving day-hospital treatment for AN. Specifically, we aimed to test whether alterations in functional coupling between the prefrontal cortex and subcortical regions implicated in emotional reactivity are linked to endorsed emotion regulation impairments in AN patients. In addition, we sought to assess whether aberrant brain activity during emotion regulation would be associated with treatment outcome, defined by pre-post-treatment changes in body fat mass percentage. As in other psychiatric disorders (Picó-Pérez et al., Reference Picó-Pérez, Radua, Steward, Menchón and Soriano-Mas2017; Zilverstand, Parvaz, & Goldstein, Reference Zilverstand, Parvaz and Goldstein2017), we hypothesized that adult patients with AN would present prefrontal hypoactivation and prefrontal-subcortical hypoconnectivity compared to controls during cognitive reappraisal and that these brain-derived measures would be associated with clinical measures and treatment outcome in patients with AN.
Method
Participants
Twenty-two young female adults diagnosed with AN and 21 female HC participants between 18 and 28 years of age were recruited between 2016 and 2019 to join the study. AN patients were recruited from a highly structured, 12-week day hospitalization program, which has been described elsewhere (Fernández-Aranda & Turón-Gil, Reference Fernández-Aranda and Turón-Gil1998). This manualized intervention targets the nutritional and dietary needs of patients with AN and includes daily cognitive-behavioral therapy (CBT) sessions. HC participants were recruited from the same hospital catchment area and received financial compensation for participating. AN patients were diagnosed according to DSM-5 criteria (APA, 2013) via a semi-structured interview (18 patients with restrictive subtype and four with binge-eating/purging subtype).
All participants completed the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller and Dunbar1998) to screen for the presence of a psychiatric disorder. Participants were excluded if they had MRI contraindications, a history of head trauma, neurological disease, major medical illness, psychosis, or substance use disorders. HC were excluded if they met criteria for a current psychiatric disorder or if they had previously met diagnostic criteria for an eating disorder. All patients with AN were scanned 1 hour after consuming a hospital meal containing approximately 750 kcal and HC were instructed to consume a meal before scanning. Additional information regarding the AN group is available in the Supplementary Material (Table S1).
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the latest version of the Helsinki Declaration. All procedures involving human subjects/patients were approved by the Bellvitge University Hospital Research Ethics Committee.
Self-assessment
Participants completed validated Spanish versions of the Difficulties in Emotion Regulation Scale (DERS; Wolz et al., Reference Wolz, Agüera, Granero, Jiménez-Murcia, Gratz, Menchón and Fernández-Aranda2015) and the Eating Disorder Inventory-2 (EDI-2; Garner, Reference Garner1998). The DERS is a 36-item, self-report measure, which assesses emotion regulation difficulties using six separate subscales. It is based upon a prominent clinically derived model of emotion regulation, with higher scores indicating greater emotion regulation impairment (Gratz & Roemer, Reference Gratz and Roemer2004). The EDI-2 is widely used to assess clinical eating disorder psychopathology and it has been found to have high test-retest reliability (Thiel & Paul, Reference Thiel and Paul2006).
Body composition
Body composition in the AN group was measured weekly during treatment via bioelectrical impedance analysis (Tanita BC- 420MA, Tanita Corp. Tokyo, Japan). This leg-to-leg body composition analyzer is a noninvasive and validated method which utilizes an alternating voltage of 90 μA (50/60 Hz) via electrodes on metal footplates (Browning et al., Reference Browning, Mugridge, Dixon, Aitken, Prentice and Jebb2011). This instrument has been validated against dual-energy X-ray absorptiometry (DEXA) measures and other reference methods. Within the context of AN refeeding treatment, it has been argued that bioelectrical impedance provides more clinically useful indicators than body mass index (BMI) (Saladino, Reference Saladino2014). The change in total body fat mass percentage from day-hospital admission to the end of the 12-week program was used as the primary indicator of treatment response.
fMRI emotion regulation task
This block-design cognitive reappraisal task emulated the features of previously described emotion regulation tasks (Phan et al., Reference Phan, Fitzgerald, Nathan, Moore, Uhde and Tancer2005). The task is made up of three conditions — ‘LookNeutral,’ ‘LookNegative’ and ‘Regulate’— presented in an ABC design with four blocks per condition (i.e. a total of 12 blocks). At the beginning of each block, a word appeared in the middle of the screen for four seconds to provide instructions to participants for the upcoming block. If the instruction was ‘LookNeutral’, the images that followed were neutral in content and participants were required to simply attend to these images without trying to alter their affective response. If the instruction was ‘LookNegative’, the presented images that followed were negative and participants were instructed to sustain the negative emotions elicited by the images. Lastly, if the instruction was ‘Regulate,’ the images were negative in content and participants had to reduce the intensity of their negative affect using the previously trained reappraisal strategies (see the Supplementary Material for further information on pre-scanning training). All blocks consisted of two consecutive images (each image was presented on the screen for 10 s, with no interstimulus interval), immediately followed by a prompt to rate distress on a 1–5 scale (1 no distress; 5 very distressed). The task blocks were interspersed with 10-s rest periods in which participants viewed a fixation cross.
Stimuli
The task was displayed using Presentation (Version 18.3, build 03.11.16, www. neurobs.com). The LCD screen presenting (BOLDscreen 32, Cambridge Research Systems) stimuli were visible via a reverse mirror mounted to the participants' head coil and behavioral responses were captured using a button-box (Lumina 3 G Controller, Cedrus Corporation). Picture stimuli contained imagery from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, Reference Lang, Bradley and Cuthbert2008). In total, 16 negative and 8 neutral pictures were selected based on IAPS rating scores (see Supplementary Material for valence and arousal normative values).
Skin conductance response
In order to verify participant engagement in the cognitive reappraisal task (Sheppes, Catran, & Meiran, Reference Sheppes, Catran and Meiran2009), SCR was acquired during MRI scanning. Additional information on SCR acquisition and analysis can be found in Supplementary Figure 1.
fMRI analysis
Information regarding fMRI acquisition and preprocessing is available in the Supplementary Information.
Regulate v. LookNegative was defined as our contrast of interest for first-level (single-subject) analysis. This contrast allows for the delineation of brain activations associated with cognitive reappraisal (Phan et al., Reference Phan, Fitzgerald, Nathan, Moore, Uhde and Tancer2005). Conditions were modeled for 20 s and the displayed images did not include instruction, rating and rest periods. The BOLD response at each voxel was convolved with the SPM12 canonical hemodynamic response function (HRF) using a 128-s high-pass filter.
Contrast images from first-level comparisons were carried forward to second-level analyses, where independent t tests using the group as the main factor compared task-induced activations. In accordance with guidelines aimed at obtaining valid and reliable brain imaging results in eating disorder populations (Frank, Favaro, Marsh, Ehrlich, & Lawson, Reference Frank, Favaro, Marsh, Ehrlich and Lawson2018), global grey matter volume was extracted for each participant (see Supplementary Information) and included as a nuisance covariate in second-level comparisons. As a whole, activation patterns did not significantly differ when including global gray matter values as a covariate (Supplementary Figure 2), nor did activation patterns differ when covarying for psychotropic medication use (Supplementary Figure 3).
In order to explore differences in task-induced connectivity between the brain regions activated during the emotion regulation task, psychophysiological interactions (PPI) analyses were conducted using SPM12. The impact of the contrast of interest (the ‘psychological’ factor) on the strength of time-course correlations between our empirically obtained region of interest (ROI, the ‘physiological’ factor) was explored and functional connectivity maps were estimated for the selected seed by including the signal of interest (seed) in interaction with the task blocks while controlling for the raw signal of the seed and the task blocks. Resulting images were then included in a two-sample t test model (second-level) to assess between-group effects.
To correct for multiple comparisons for our whole-brain analyses, voxel-wise nonparametric permutation testing using 5000 permutations was performed via the threshold-free cluster enhancement (TFCE) method (Smith & Nichols, Reference Smith and Nichols2009) as implemented in the SPM-TFCE toolbox v174. TFCE has been shown to have greater sensitivity compared with voxel-based and cluster-based thresholding methods (Han, Glenn, & Dawson, Reference Han, Glenn and Dawson2019). Our TFCE significance threshold was set at p < 0.05, family-wise error (FWE) whole-brain corrected. Significant between-group effects have been detected with this cognitive reappraisal paradigm using similarly sized groups (Steward et al., Reference Steward, Picó-Pérez, Mata, Martínez-Zalacaín, Cano, Contreras-Rodríguez and Verdejo-García2016, Reference Steward, Picó-Pérez, Mestre-Bach, Martínez-Zalacaín, Suñol, Jiménez-Murcia and Fernandez-Aranda2019).
Statistical analysis of clinical and behavioral data
Statistical analysis for clinical and behavioral data was carried out with SPSS 21 (IBM Corp; Armonk, NY). Interactions between in-scanner ratings and SCR response for each condition (LookNeutral, LookNegative and Regulate) and subject groups were evaluated using a 2 × 3 repeated-measures ANOVA analysis in order to behaviorally confirm participants' ability to engage in the task. Derived peak activation differences from imaging analyses were extracted to assess their association with clinical and behavioral measures. Between-group comparisons were carried out using independent sample t tests, and linear associations were estimated using Pearson's correlations. Paired sample t tests were used to compare BMI and body fat mass percentage in the AN sample upon admission to treatment and at discharge. Shapiro–Wilk tests were performed to confirm normality of the variables of interest and variables were checked for the presence of outliers. For Pearson's correlations, associations were only considered significant if they obtained p values below 0.05 and if effect sizes were moderate to large (|r| > 0.24; Rosnow and Rosenthal, Reference Rosnow and Rosenthal1996).
Results
Demographic and clinical data
As expected, patients with AN had significantly lower BMI and body fat mass percentage levels than HC. AN patients also endorsed significantly greater emotion regulation difficulties on the DERS and eating-disorder-related severity on the EDI-2 than HC (Table 1). Six patients with AN were taking psychotropic medication at the time of scanning (Supplementary Table S1).
Table 1. Participant descriptive variables and behavioral measures

BMI: body mass index; EDI-2: Eating Disorders Inventory-2; DERS: Difficulties in Emotion Regulation Scale.
Paired sample t tests identified significant increases in BMI (t = 5.01, p < 0.001) and body fat mass percentage (t = 4.72, p < 0.001) from day-hospital admission to discharge. One patient from the AN group was excluded from the study sample due to excessive head movement during scanning. Three patients with AN who completed scanning dropped out of treatment and were not included in the longitudinal analysis.
Emotion regulation task results
In-scanner ratings and SCR
Analysis of distress ratings for each condition (LookNeutral, LookNegative and Regulate) revealed a main effect of condition (F = 198.36, p < 0.001). Post-hoc comparisons showed that LookNegative differed from LookNeutral, indicating negative emotion induction during this condition for both groups (LookNegative>LookNeutral: t = 20.9, p < 0.001). Similarly, post-hoc comparisons between Regulate and LookNegative identified differences between the two conditions (LookNegative>Regulate t = 4.78, p < 0.001), indicating participants endorsed lower distress levels during cognitive reappraisal blocks (Supplementary Figure 4). As in previous studies utilizing cognitive reappraisal paradigms in psychiatric populations (Picó-Pérez et al., Reference Picó-Pérez, Radua, Steward, Menchón and Soriano-Mas2017; Zilverstand et al., Reference Zilverstand, Parvaz and Goldstein2017), no significant effects of group (F = 2.55, p = 0.118) were found.
SCR data analysis yielded findings concurring with in-scanner ratings, indicating a linear SCR increase across conditions in both groups (t = 3.58, p = 0.002, Supplementary Figure 1). This is in agreement with previous reports (Sheppes et al., Reference Sheppes, Catran and Meiran2009), and can be interpreted as the result of increased attentional resources being allocated to emotional processing (LookNegative v. LookNeutral) and to the deployment of effortful emotion regulation (Regulate v. LookNegative). No effect of group was found (F = 3.25, p = .084).
Task activations and connectivity analyses
As in previous emotion regulation fMRI studies (Picó-Pérez et al., Reference Picó-Pérez, Radua, Steward, Menchón and Soriano-Mas2017; Zilverstand et al., Reference Zilverstand, Parvaz and Goldstein2017), participants presented widespread increased activation in posterior visual processing regions, along with subcortical affective processing regions during LookNeg>LookNeutral (Supplementary Figure 5A; Supplementary Table S2). During Regulate>LookNegative, participants exhibited increased activation in prefrontal regions, along with increased activation in the angular gyrus, precuneus and middle temporal gyrus (Supplementary Figure 5B; Supplementary Table S3).
Whole-brain level analyses using TFCE correction (p < 0.05; FWE) found that participants in the AN group presented reduced activation in the right dorsolateral prefrontal cortex (dlPFC; x = 42; y = 30; z = 36) during Regulate>LookNegative in comparison to HC (Fig. 1A).
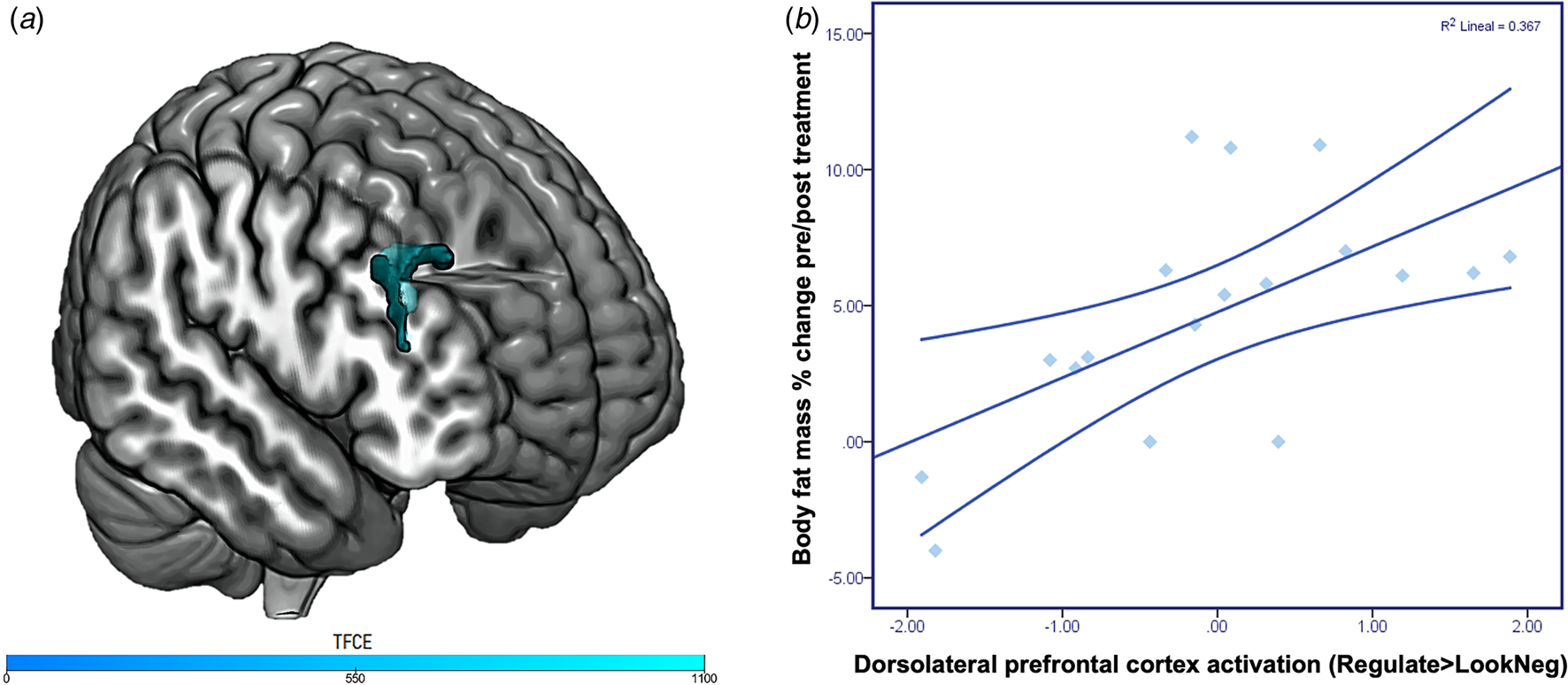
Fig. 1. Emotion regulation task activation and associations with treatment response. Panel A depicts differences in group activations during Regulate>LookNegative. Patients with anorexia nervosa presented significantly reduced activation in the right dorsolateral prefrontal cortex compared to healthy controls (x = 42; y = 30; z = 36; ke: 137 voxels; TFCE FWE corrected p < 0.05). TCFE values are presented in the color bar. Panel B depicts the positive correlation between body fat mass percentage change pre-post treatment and dorsolateral prefrontal cortex response in patients with anorexia nervosa during emotion regulation (R 2 = .367, p = .008).
In comparison to HC, PPI analyses found that AN patients presented lower connectivity between the above-mentioned right dlPFC peak with extended posterior cortical and limbic regions. Specifically, peaks for reduced connectivity from the dlPFC in the AN group were found in the left amygdala (x = −26; y = 2; z = −26) and the occipital peristriate area (x = −28; y = −68; z = −18) (Fig. 2B).
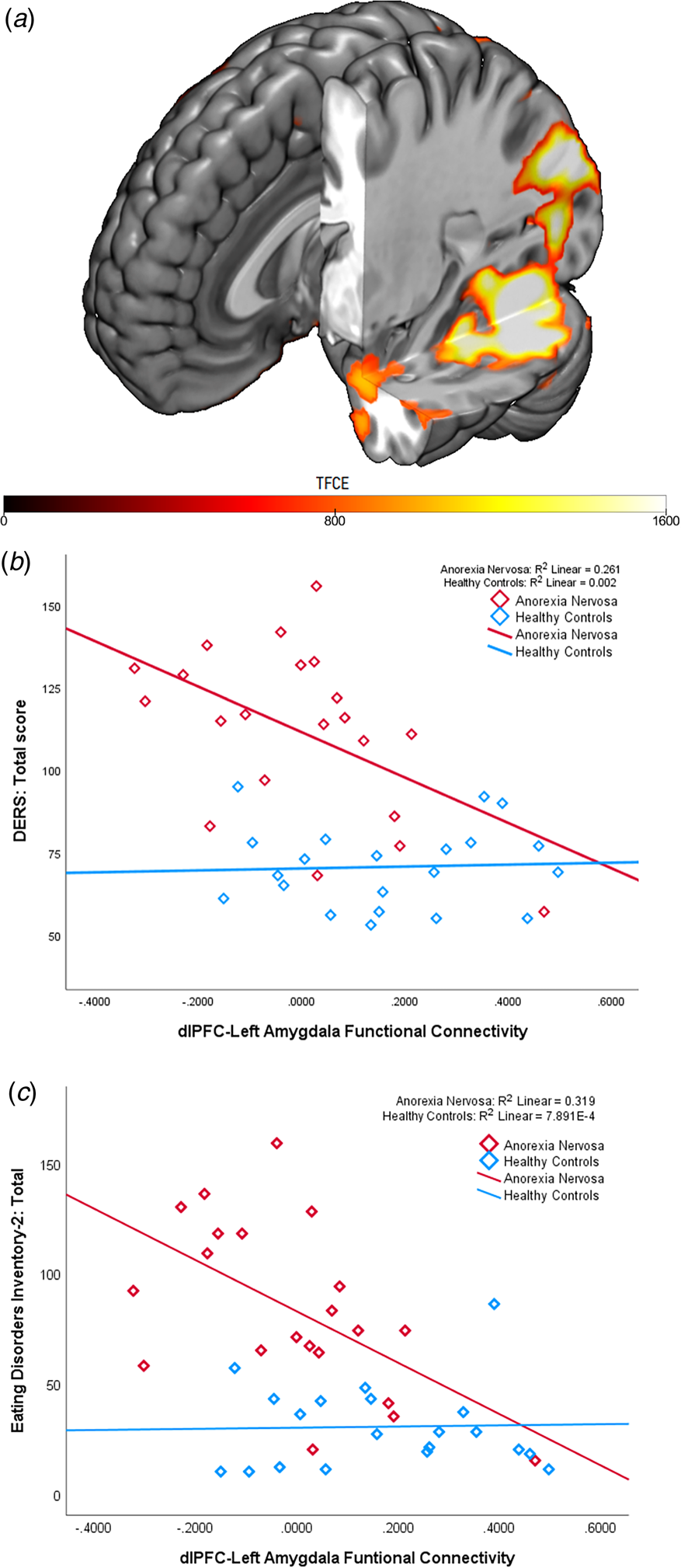
Fig. 2. Dorsolateral prefrontal cortex-amygdala functional connectivity during cognitive reappraisal and associations with self-report emotion regulation difficulties. Panel A depicts differences in group functional connectivity during Regulate>LookNegative. Patients with anorexia nervosa presented significantly reduced coupling between the right dorsolateral prefrontal cortex (dlPFC) and the left amygdala (x = −26 y = 2 z = −26; TFCE = 1007.13, FWE corrected, p < 0.05 and the extrastriate visual cortex (x = −28 y = −68 z = −18; TFCE = 2371.64, FWE corrected, p < 0.05). than controls during emotion regulation. Panel B depicts the correlations between dlPFC-amygdala connectivity levels and total scores on the Difficulty in Emotion Regulation Scale (DERS). A significant negative association between emotion regulation difficulties and dlPFC-amygdala connectivity was found in the anorexia nervosa group (R 2 = .261, p = .018), but not in the healthy control group (R 2 = .002, p = .839). Similarly, Panel C depicts the significant negative association between eating disorder severity, as assessed by the Eating Disorder Inventory-2, and dlPFC-amygdala connectivity in the anorexia nervosa group (R 2 = .319, p = .008). This association was not significant in the healthy control group (R 2<0.001, p = .906).
Relationship between brain response and clinical measures
Regarding brain activation estimates extracted from the emotion regulation task, a significant positive correlation was observed between dlPFC activation during emotion regulation in the AN group and pre-post-treatment changes in body fat mass percentage (r = .605, p = .008; Figure 1B) and BMI (r = .471, p = .042). No significant associations between dlPFC activation and total DERS (r = −.165, p = .474) or EDI-2 (r = −.347, p = .124) scores were found.
A significant negative correlation was identified between DERS total scores and dlPFC-amygdala connectivity during Regulate>LookNegative, to the extent that AN patients with greater dlPFC-amygdala coupling were less likely to endorse emotion regulation difficulties (r = −.511, p = .018; Figure 2B). This same pattern occurred with eating disorder severity levels; a significant negative correlation was found between dlPFC-amygdala connectivity and EDI-2 total scores (r = −.565, p = .008; Figure 2C). dlPFC-amygdala connectivity was not significantly associated with pre-post-treatment changes in body fat mass percentage (r = −.008, p = .975) or BMI (r = .158, p = .517)
Discussion
This study revealed that adult female patients with AN presented diminished activation in the dlPFC during volitional negative emotion regulation compared to controls. In addition, patients with AN presented less functional coupling between the dlPFC and extended posterior cortical and subcortical regions during emotion regulation. Importantly, fronto-amygdalar connectivity was negatively associated with overall eating disorder severity and endorsed difficulties in emotion regulation in patients with AN. Last, we identified an association between increased dlPFC activity during cognitive reappraisal and weight restoration following treatment.
These findings align with previous emotion regulation neuroimaging studies in other psychiatric disorders and lend credence to neural models contending that impairments in emotion regulation are underpinned by diminished recruitment of prefrontal control systems responsible for modulating activity in perceptual and affect networks as a function of one's regulatory goals (Ochsner, Silvers, & Buhle, Reference Ochsner, Silvers and Buhle2012; Zilverstand et al., Reference Zilverstand, Parvaz and Goldstein2017). We found that increased activity in the dlPFC during emotion regulation was associated with weight recovery over the course of treatment, suggesting that activity in the brain regions subserving the deployment of cognitive regulation strategies may serve as a predictor of treatment outcome. At present, the most robust predictor of treatment outcome in AN is early response (i.e. greater symptom change) to treatment (Nazar et al., Reference Nazar, Gregor, Albano, Marchica, Coco, Cardi and Treasure2017; Vall & Wade, Reference Vall and Wade2015), and our study offers a novel brain-derived measure which could be employed to guide AN patients with increased vulnerability to adjunct treatments. Our longitudinal findings also further support the involvement of emotion regulation deficits in contributing to AN psychopathology (Lavender et al., Reference Lavender, Wonderlich, Engel, Gordon, Kaye and Mitchell2015; Oldershaw, Lavender, Sallis, Stahl, & Schmidt, Reference Oldershaw, Lavender, Sallis, Stahl and Schmidt2015) and warrants future research examining whether the identified alterations in fronto-amygdalar connectivity persist after long-term weight recovery and remission.
Our emotion regulation paradigm revealed, in women with AN, reduced dlPFC activity during cognitive reappraisal in a manner comparable to previous findings in individuals with anxiety disorders and mood disorders (Zilverstand et al., Reference Zilverstand, Parvaz and Goldstein2017). This is worth noting given that up to 65% of patients with AN meet criteria for a lifetime anxiety disorder (Kaye, Bulik, Thornton, Barbarich, & Masters, Reference Kaye, Bulik, Thornton, Barbarich and Masters2004; Swinbourne et al., Reference Swinbourne, Hunt, Abbott, Russell, St Clare and Touyz2012), and supports the possibility that dlPFC hypoactivity when processing affective stimuli may represent a common vulnerability factor for both disorders. Within the context of emotion regulation, the dlPFC is understood to play a key role in supporting the manipulation of appraisals in working memory (Buhle et al., Reference Buhle, Silvers, Wage, Lopez, Onyemekwu, Kober and Ochsner2014). Deficits in the construction and re-evaluation of salient content, as indicated by reduced activity levels in the dlPFC and endorsed difficulties in emotion regulation, may be illustrative of malfunctioning within a core mechanism required for successful emotion regulation. Relatedly, there is converging evidence of dlPFC hypoactivation in patients with AN during paradigms assessing response inhibition (Lock, Garrett, Beenhakker, & Reiss, Reference Lock, Garrett, Beenhakker and Reiss2011) and cognitive flexibility (Sato et al., Reference Sato, Saito, Utsumi, Aizawa, Shoji, Izumiyama and Fukudo2013), suggesting that reduced dlPFC engagement may underlie the general executive dysfunction that is characteristic of AN. Indeed, there is preliminary longitudinal evidence of treatment-related increases in dlPFC activity in patients with AN (Brockmeyer et al., Reference Brockmeyer, Walther, Ingenerf, Wild, Hartmann, Weisbrod and Friederich2016), and a recent open-label case series targeting the dlPFC via repetitive transcranial magnetic stimulation (rTMS) found increased emotion regulation capacity in a sample of patients with eating disorders after an rTMS intervention (Woodside et al., Reference Woodside, Colton, Lam, Dunlop, Rzeszutek and Downar2017). This identified relationship with dlPFC hypoactivity during cognitive reappraisal and treatment outcomes may be an indication of an inability of an at-risk subset of patients with AN to recruit the necessary cognitive resources to put CBT-based skills into practice. As such, patients with AN who are unable to successfully apply their working memory and attention to goal-driven tasks may be less likely to respond to CBT-based treatment programs.
Still, other research has found individuals with AN to present increased activation in the dlPFC during the passive viewing of aversive pictures, but not during emotion regulation, leading the authors of this study to cautiously suggest increased dlPFC activity as a possible indicator of overactive control mechanisms in response to aversive stimuli despite the task instructions requiring none (Seidel et al., Reference Seidel, King, Ritschel, Boehm, Geisler, Bernardoni and Ehrlich2018a). Nevertheless, it should be considered that the sample in this study was made up of young adults, whereas the sample in the abovementioned study included adolescents (mean age = 16.63 years). It is understood that non-linear structural brain development trajectories during adolescence have consequences for brain function and behavior, with longitudinal research showing the use of reappraisal to increase throughout adolescence as a function of executive, verbal and cognitive skill development (Ahmed et al., Reference Ahmed, Bittencourt-Hewitt and Sebastian2015). For example, one study found greater cortical thinning of the dlPFC during adolescence to be significantly associated with greater cognitive reappraisal use in females, suggesting that cortical maturation may have played an important role in these differing results (Vijayakumar et al., Reference Vijayakumar, Whittle, Yücel, Dennison, Simmons and Allen2014).
We identified reduced functional coupling between the right dlPFC and the left amygdala and posterior cortical regions during cognitive reappraisal in patients with AN in comparison to controls. This is noteworthy since cytoarchitectural studies have found the density of direct projections from the dlPFC to amygdala to be weak (Ray & Zald, Reference Ray and Zald2012). As opposed to directly modulating amygdala response to salient stimuli, dlPFC signaling is understood to alter the competition between potential representations in the ventromedial prefrontal cortex through feedforward projections (Barbas, Reference Barbas2015). As such, reduced functional coupling between the dlPFC and the amygdala in individuals with AN may be reflective of biased computations being relayed to regions which exert a direct influence on the amygdala (i.e. medial prefrontal regions), thereby hindering adaptive responses by possibly biasing the allocation of attentional resources (Bang, Rø, & Endestad, Reference Bang, Rø and Endestad2017). Additionally, new research has specifically linked altered right dlPFC and left amygdala connectivity with alexithymia, the inability to identify and describe emotions (Kim et al., Reference Kim, Park, Lee, Jeon, Kim, Lee and Kim2019), which may partly underlie the negative association we observed between connectivity between these regions and endorsed emotion regulation impairments in our sample of AN patients. Similar conclusions can be reached regarding the negative correlation identified between fronto-amygdalar connectivity and overall AN severity. dlPFC-amygdala connectivity has been found to normalize following treatment in other psychiatric disorders (Shou et al., Reference Shou, Yang, Satterthwaite, Cook, Bruce, Shinohara and Sheline2017), and the identified association between connectivity levels and EDI and DERS scores may be indicative of a clinical state signature of dysfunctional affective processing and overall psychopathology. Moreover, one recent randomized clinical trial has revealed a causal role for dlPFC regulation of amygdala function in attentional capture by threatening stimuli in individuals with high trait anxiety (Ironside et al., Reference Ironside, Browning, Ansari, Harvey, Sekyi-Djan, Bishop and O'Shea2019), indicating that reduced dlPFC activity during affective processing may perpetuate the fear, hypervigilant scrutiny of one's body and unnatural fear of weight gain that is characteristic of AN (Murray et al., Reference Murray, Strober, Craske, Griffiths, Levinson and Strigo2018). Considering that previous research has found emotional avoidance to be a significant predictor of the treatment outcome (Oldershaw et al., Reference Oldershaw, Lavender and Schmidt2018), future research should aim to delineate the neural underpinnings of specific aspects of emotion regulation dysfunction in AN.
Although significant efforts were made to adhere to recently published guidelines for obtaining reliable imaging results in eating disorder samples (Frank et al., Reference Frank, Favaro, Marsh, Ehrlich and Lawson2018), the study at hand is not without its limitations. For example, it has been recommended that female participants be at least 8 weeks off oral contraceptive pills (OCPs) at the time of scanning, or if taking OCPs, then they be compared to a control population also taking OCPs. This factor was not considered in the original study design and future studies should examine and control for the effects of OCPs on affective processes. Also, weight recovery was only assessed upon discharge from day-hospital treatment and future studies should examine whether our identified associations persist long term. It should also be noted that our sample size was modest and that all patients with AN were females and recruited from the same university hospital setting, thereby receiving the same manualized treatment protocol. The lack of differences between AN patients and controls regarding in-scanner distress ratings and SCR could be driven by factors surrounding the patients' clinical state (i.e. sufficiently severe to warrant day-hospital treatment, but not inpatient treatment), though in-scanner behavioral ratings are not intended to provide an overall measure of emotion regulation capability, but rather to validate that participants are successfully engaging in the task (Zilverstand et al., Reference Zilverstand, Parvaz and Goldstein2017). The featured clinical sample was also limited to adults patients with AN under the age of 30 and our findings may not generalize to older populations. Therefore, our sample does not fully represent all individuals with AN in the general population and it would be beneficial to attempt to replicate the presented results in multiple treatment settings. Also, the block-design paradigm used in this study could have elicited potential confounds such as rapid habituation and anticipation in some participants. Last, considering the evidence of protracted neural alterations during emotion processing in women recovered from AN (Bang, Ro, & Endestad, Reference Bang, Ro and Endestad2016), it would be of interest to determine whether the observed differential neural activations patterns in patients with AN normalized following weight restoration and whether these correlated with changes in clinical measures.
Conclusions
This study marks a significant advancement in our understanding of the neural mechanisms underlying emotion regulation deficits in AN by providing evidence that impairments in emotion regulation in AN are likely underpinned by diminished fronto-amygdalar connectivity. Here, we, for the first time, demonstrate that prefrontal activation during cognitive reappraisal predicts favorable outcome to treatment, thereby delivering targets for brain-based treatments that augment the ability of patients with AN to resolve affective conflicts and to facilitate adherence to treatment.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720002457
Acknowledgements
We thank CERCA/the Generalitat of Catalunya for institutional support. This research was supported by grants from the Ministerio de Economía y Competitividad (PSI2015-68701-R), Ministerio de Sanidad, Servicios Sociales e Igualdad (PR338/17), Instituto de Salud Carlos III (ISCIII) (FIS PI13/01958, PI14/00290 and PI17/01167) and co-funded by FEDER funds/European Regional Development Fund (ERDF), a way to build Europe. CIBEROBN and CIBERSAM are both initiatives of ISCIII. This research was supported by a PNSD (PR338/17-MSSSI) grant. TS is supported by the University of Melbourne McKenzie Fellowship. CS-M is supported by a ‘Miguel Servet’ contract from the ISCIII (CPII16/00048). IM-Z is supported by a p-FIS grant from the ISCIII (FI17/00294). FFA is supported by ISCIII (INT19/00046). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. GMB is supported by a Fundación Ciudadanía y Valores postdoctoral grant.
Conflict of interest
All authors declare that they have no conflicts of interest.






