Introduction
It is well established that a significant proportion of people with Parkinson's disease (PD) display cognitive deficits (Dubois & Pillon, Reference Dubois and Pillon1997). The reported prevalence of cognitive impairment in PD is variable; 20–50% have mild cognitive impairment (MCI) at disease onset (Caviness et al., Reference Caviness, Driver-Dunckley, Connor, Sabbagh, Hentz, Noble and Alder2007; Janvin, Larsen, Aarsland, & Hugdahl, Reference Janvin, Larsen, Aarsland and Hugdahl2006) with a further 10% developing PD dementia during the first 3 years of the disease (Williams-Gray, Foltynie, Brayne, Robbins, & Barker, Reference Williams-Gray, Foltynie, Brayne, Robbins and Barker2007), and ∼80% with dementia of those who survive for 20 years (Hely, Reid, Adena, Halliday, & Morris, Reference Hely, Reid, Adena, Halliday and Morris2008). The cognitive profile is characterized by impairments on visuospatial abilities (Pereira et al., Reference Pereira, Junque, Marti, Ramirez-Ruiz, Bargallo and Tolosa2009), working memory (Cooper, Sagar, Jordan, Harvey, & Suilivan, Reference Cooper, Sagar, Jordan, Harvey and Suillivan1991), attention (Ridenour & Dean, Reference Ridenour and Dean1999), and executive functioning (Zgaljardic, Borod, Foldi, & Mattis, Reference Zgaljardic, Borod, Foldi and Mattis2003) and as such the majority can be classified as non-amnestic MCI, although it has been reported that one third can display an amnestic MCI subtype (Aarsland, Brønnick, Larsen, Tysnes, & Alves, Reference Aarsland, Brønnick, Larsen, Tysnes and Alves2009). It has been recently suggested that different cognitive syndromes may reflect discrete pathological changes in PD with visuospatial and memory deficits reflecting dopamine-independent pathology that is associated with prodromal PD dementia, and executive dysfunction reflecting dopamine-dependent impairment of fronto-striatal circuitry (Kehagia, Barker, & Robbins, Reference Kehagia, Barker and Robbins2013).
One of the most frequently reported cognitive deficits in PD is in letter (phonemic) fluency (Azuma, Cruz, Bayles, Tomoeda, & Montgomery, Reference Azuma, Cruz, Bayles, Tomoeda and Montgomery2003; Bayles, Trosset, Tomoeda, Montgomery, & Wilson, Reference Bayles, Trosset, Tomoeda, Montgomery and Wilson1993; Flowers, Robertson, & Sheridan, Reference Flowers, Robertson and Sheridan1995; Green et al., Reference Green, McDonald, Vitek, Evatt, Freeman, Haber and DeLong2002; although see Auriacombe et al., Reference Auriacombe, Grossman, Carvell, Gollomp, Stern and Hurtig1993; Ivory, Knight, Longmore, & Caradoc-Davies, Reference Ivory, Knight, Longmore and Caradoc-Davies1999; Matison, Mayeux, Rosen, & Fahn, Reference Matison, Mayeux, Rosen and Fahn1982; Raskin, Sliwinski, & Borod, Reference Raskin, Sliwinski and Borod1992). Inconsistencies in the fluency literature in PD may, in part, reflect methodological differences and heterogeneity of cognitive impairment in PD. Studies investigating fluency performance for multiple letters and categories demonstrated that PD patients found some conditions significantly more difficult than others (Azuma et al., Reference Azuma, Bayles, Cruz, Tomoeda, Wood, McGeagh and Montgomery1997; Bayles et al., Reference Bayles, Trosset, Tomoeda, Montgomery and Wilson1993). Furthermore, some studies (e.g., Flowers et al., Reference Flowers, Robertson and Sheridan1995; Matison et al., Reference Matison, Mayeux, Rosen and Fahn1982) did not screen for dementia, while in any given cohort of non-demented patients, some will have MCI and others will not (Muslimovic, Post, Speelman, & Schmand, Reference Muslimovic, Post, Speelman and Schmand2005). Thus grouping all patients together for cognitive assessment may result in effects being exaggerated or attenuated respectively.
Although letter fluency deficits are commonly described in non-demented people with PD, the underlying cognitive cause remains unclear. There is debate as to whether the cognitive profile in PD is of predominant executive dysfunction or slowed processing speed (McDowd et al., Reference McDowd, Hoffman, Rozek, Lyons, Pahwa, Burns and Kemper2011). Impairments on tests of executive functions other than fluency have been readily reported, including; Wisconsin Card Sorting Test (Green et al., Reference Green, McDonald, Vitek, Evatt, Freeman, Haber and DeLong2002), Tower of London type paradigms (Morris et al., Reference Morris, Downes, Sahakian, Evenden, Heald and Robbins1988; Owen et al., Reference Owen, James, Leigh, Summers, Marsden, Quinn and Robbins1992), Trail Making tasks (Taylor, Saint-Cyr, & Lang, Reference Taylor, Saint-Cyr and Lang1986), Stroop test (Dubois, Boller, Pillon, & Agid, Reference Dubois, Boller, Pillon and Agid1991), see Zgaljardic et al. (Reference Zgaljardic, Borod, Foldi and Mattis2003) for a review of executive functioning in PD. Letter fluency tasks require internally generated responses with minimal environmental cues or triggers, and as such, are dependent upon executive processes (Abrahams et al., Reference Abrahams, Leigh, Harvey, Vythelingum, Grise and Goldstein2000; Abrahams et al., 2003; Azuma, Reference Azuma2004; Bittner & Crowe, Reference Bittner and Crowe2007).
Some have maintained that executive dysfunction is not the root of cognitive impairment in PD, but that patients exhibit bradyphrenia, a global slowing of processing speed (e.g., Shipley, Deary, Tan, Christie, & Starr, Reference Shipley, Deary, Tan, Christie and Starr2002). Slowed processing speed in PD has been demonstrated using both automatic perceptual processing (Johnson et al., Reference Johnson, Almeida, Stough, Thompson, Singarayer and Jog2004) and more controlled effortful processing tasks (Revonsuo, Portin, Koivikko, Rinne, & Rinne, Reference Revonsuo, Portin, Koivikko, Rinne and Rinne1993), and on tasks in which responses are not dependent on rapid motor functioning (discriminating verbal temporal order, Shipley et al., Reference Shipley, Deary, Tan, Christie and Starr2002; and visuospatial sequences, Sawamoto, Honda, Hanakawa, Fukuyama, & Shibasak, Reference Sawamoto, Honda, Hanakawam, Fukuyama and Shibasaki2002). The slowed processing speed account has been applied to fluency deficits in PD; one study reported that the curve of word production over 5 minutes for PD patients paralleled that of controls and the range of words was similar, but with simply less words produced per minute (Flowers et al, Reference Flowers, Robertson and Sheridan1995). More recently, McDowd et al. (Reference McDowd, Hoffman, Rozek, Lyons, Pahwa, Burns and Kemper2011) investigated fluency performance in healthy adults, Alzheimer's patients and PD patients and reported that processing speed was the single best predictor of fluency performance for all groups. Such findings are consistent with the work of Salthouse, Atkinson, and Berish (Reference Salthouse, Atkinson and Berish2003) in which fluency performance was suggested to be a function of perceptual speed and vocabulary abilities.
Performance in fluency tasks can be divided into discrete “clustering” (the generation of related words) and “switching” (the generation of new retrieval strategies to produce unrelated words akin to set-shifting) components. Clustering is thought to be a relatively automatic process within the lexical system whereas switching is an effortful process constrained by executive control (Troyer, Moscovitch, & Winocur, Reference Troyer, Moscovitch and Winocur1997). Subsequent studies have suggested that clustering is more sensitive to temporal lobe lesions whereas switching is more sensitive to frontal lesions (Baldo, Schwartz, Wilkins, & Dronkers, 2006; Troyer, Moscovitch, Winocur, Alexander, & Stuss, 1998).
Structural equation modeling of performance on standard tests of executive functions has supported the proposal of three dissociable executive functions; shifting, updating, and inhibition (Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000). Set-shifting is most akin to the concept of switching and has been implicated as a crucial process within fluency (Rende, Ramsburger, & Miyake, 2002). However, findings in PD cohorts have been inconsistent; some have reported that only demented PD patients switched less in letter fluency tests than controls (Tröster et al., Reference Tröster, Fields, Testa, Paul, Blanco, Hames and Beatty1998; Troyer, Moscovitch, Winocur, Leach, & Freedman, Reference Troyer, Moscovitch, Winocur, Leach and Freedman1998), while another study found a switching deficit in a non-demented PD sample with preserved clustering (Donovan, Siegert, McDowall, & Abernethy, Reference Donovan, Siegert, McDowall and Abernethy1999). However, simply reporting the number of switches does not take into account the number of words, or time spent retrieving words, within a cluster—if a participant spends a long time retrieving words within a cluster then consequently there will be less time for switching. Subsequently, Mayr (Reference Mayr2002) suggested that clustering and switching analysis should be quantitative in nature, using specific timing protocols to attain a precise measure of the amount of time spent retrieving words within clusters, and the amount of time spent switching between different clusters.
The present study aimed to apply quantitative clustering and switching analysis to the investigation of fluency performance in non-demented PD participants whilst controlling for motor dysfunction. This was achieved by incorporating a motor control condition in which the time to simply copy or read words aloud was recorded, allowing an estimation of the average time to think of each word (or fluency index, Fi) to be calculated. More specifically the study aimed to determine whether the letter fluency deficits in PD could be better explained by executive dysfunction or slowed processing speed. We predicted that a deficit in information processing speed would slow thinking times on both fluency components, whereas executive dysfunction should disproportionately affect the time taken to switch between clusters.
Methods
Participants
Parkinson's disease (PD)
Twenty-two people with idiopathic PD were recruited from the Department of Clinical Neurosciences at the Western General Hospital, Edinburgh. No PD participant had a history of head injury, alcohol abuse, or other neurological, medical, or psychiatric condition. Four PD participants were subsequently excluded: One participant with evidence of depression on screening for an affective disorder; two were being treated with deep brain stimulation, and one PD participant was unable to write and hence could not complete the Written Verbal Fluency test. Of the remaining cohort of 18 participants (12 male, 6 female), 15 were right-handed, and 3 were left-handed. All PD participants were tremor predominant and severity of disease was assessed using the Hoehn and Yahr scale (Hoehn & Yahr, Reference Hoehn and Yahr1967). None of the PD participants included had a history of other neurological problems, major medical or psychiatric illness, or learning disability. All PD participants were receiving treatment during the study (see supplementary information A). The study was approved by the ethics board for NHS Lothian and the University of Edinburgh and in accordance with the 1964 Declaration of Helsinki, informed consent was obtained for all participants.
Controls
Nineteen control participants (10 males, 9 females) were recruited via the University of Edinburgh, Psychology Department's volunteer participant panel. Controls were matched to PD participants in terms of age and years of education. Seventeen of the controls were right-handed, and two were left-handed. None of the controls had any history of neurological problems, major medical or psychiatric illness, or learning disability.
Participant Characteristics
PD and control groups did not significantly differ in terms of age (mean PD = 68.8; SD = 6.0; mean Control = 66.2; SD = 6.9; t = 1.25; p = .220) or years of education (mean PD = 14.1; SD = 2.9; mean Control = 15.6; SD = 4.1; t = −1.28; p = .210). PD participants had an average disease duration of 6.3 years (SD = 4.2), while the median disease severity as rated by the Hoehn and Yahr Scale was 1 (10/18 patients rated as 1, 4/18 rated as 3 and 4/18 rated as 4).
Neuropsychological Assessment
Addenbrooke's Cognitive Examination Revised (ACE-R; Mioshi, Dawson, Mitchell, Arnold, & Hodges, Reference Mioshi, Dawson, Mitchell, Arnold and Hodges2006) was used to screen for dementia. The Graded Naming Test (GNT; McKenna & Warrington, Reference McKenna and Warrington1983) provided a measure of confrontation naming to assess word finding abilities that could potentially confound verbal fluency performance. The Hayling Sentence Completion Test and Brixton Spatial Anticipation Test (Burgess & Shallice, Reference Burgess and Shallice1997) were used to assess executive functioning and measure response inhibition, rule detection and set shifting. Numerical Information Processing (NIP) task; The NIP task was based on the information-processing subtest of the Adult Memory Information-Processing Battery (AMIPB; Coughlan & Hollows, Reference Coughlan and Hollows1985) and used to assess processing speed. The task comprised of 30 rows of 5 double-digit numbers; in the first condition (NIP A) participants were required to cross out the highest digit from each row. In the second condition (NIP B), participants were required to identify the second-highest number. In the third condition (NIP Motor), participants were required to cross through numbers that were already identified as the correct responses. This condition served as a control for motor speed. The time taken to complete the NIP-Motor was deducted from the time taken to complete NIP-A and from NIP-B to give a measure of processing speed which accommodates for motor slowing called the NIP index (NIPi).
Experimental Measures
The Letter Fluency tests followed the procedure devised by Abrahams et al. (Reference Abrahams, Leigh, Harvey, Vythelingum, Grise and Goldstein2000) to investigate patients with amyotrophic lateral sclerosis (ALS) and were designed to control for individual variations in motor speed by producing a fluency index (Fi). Full details of the fluency procedure are displayed in supplementary information B. Both written and spoken letter fluency tests were used to maximize data collection in participants with motor impairment; proven successful in the study of ALS.
Spoken Letter Fluency Test (SLFT)
This test was adapted from Benton and Hamsher's (Reference Benton and deS Hamsher1976) Controlled Word Association Test (COWAT). The Generation Condition consisted of three trials in which participants said words beginning with the letters P, R, and W in 60-s time periods. Participants then undertook the Motor Control Condition in which they read out the previously generated words.
Written Letter Fluency Test (WLFT)
This test (Abrahams et al., 1996) was an adaptation of that described by Thurstone and Thurstone (Reference Thurstone and Thurstone1962). The Generation Condition consisted of two letter trials; in the first participants were asked to write down as many words as possible beginning with the letter “S” in 5 min. In the second trial, participants were asked to write down as many words as possible beginning with “C” in 4 min, however, the words produced had to contain only four letters. Participants then undertook the motor control condition in which they copied the previously generated words.
Clustering and switching thinking times
Clustering refers to the generation of words that are semantically or phonemically related (e.g., pat, past, pant, or pencil, paper, pen). Switching refers to the process of generating a new cluster, that is, words that are unrelated (e.g., from pant to press, or from paper to pull). Using the video film footage the following measures were produced: Clustering fluency index, representing the average time to think of a word within a cluster, calculated as follows;
 $${Cluster\,fluency\,index\, \cr\quad= \,\frac{{Cluster\,Generation\,Time\,{\rm{ - }}\,Cluster\,Read\,Time}}{{Number\,of\,Words\,in\,Cluster\, - \,{\it1}}}$$
$${Cluster\,fluency\,index\, \cr\quad= \,\frac{{Cluster\,Generation\,Time\,{\rm{ - }}\,Cluster\,Read\,Time}}{{Number\,of\,Words\,in\,Cluster\, - \,{\it1}}}$$
Switching fluency index, representing the average time to switch between clusters, calculated as follows;
 $${Switch\,fluency\,index\, \cr\quad= \,\frac{{Task\,Time\, - \,{}Total\,Cluster\,Generation\,Time}}{{Total\,Number\,of\,Clusters}}$$
$${Switch\,fluency\,index\, \cr\quad= \,\frac{{Task\,Time\, - \,{}Total\,Cluster\,Generation\,Time}}{{Total\,Number\,of\,Clusters}}$$
Differences in motor speed were accounted for by the same method described above in the fluency procedures methodology. Full details of the Clustering and Switching methodology are displayed in supplementary information C.
Statistical Design
The data were explored for normality and homogeneity of variance. Comparative group analyses of PD participant and control means were performed using t tests in normally distributed data, or Mann-Whitney U tests in populations that were not normally distributed. The one-tailed probability level was adopted for tests with a predicted directional result. Repeated measures analyses were performed using a two-way mixed analysis of variance (ANOVA) to investigate the effect of cognitive load between groups. In the subgroup analysis, one-way ANOVAs and Kruskal-Wallis tests (for non-parametric data) were administrated for between group comparisons, and any pairwise comparisons were Bonferroni corrected. Pearson's correlations and hierarchical regressions were used to investigate the contribution of background neuropsychological tests on fluency performance.
Results
Neuropsychological Assessment
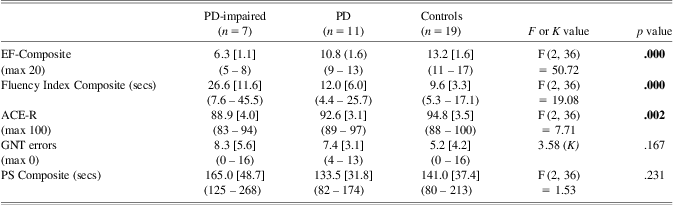
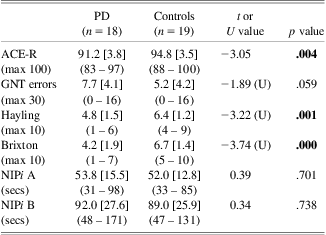
Comparative analyses (Table 1) revealed a significant difference between groups in ACE-R performance with the PD group performing worse than controls. However, none of the PD participants had an ACE-R score of less than 82 (cutoff for dementia). A significant group effect was found for scaled scores of the Hayling and Brixton tests, suggesting evidence of a difference between PD participants and controls in executive functioning.
Table 1 Neuropsychological assessment: means [SDs], (range), and exact p values for between group analyses
Note. Significant results are highlighted in boldface type.
ACE-R = Addenbrooke's Cognitive Examination-Revised (cutoff score for dementia = 82); GNT = Graded Naming Test; Hayling = Hayling Sentence Completion Test Scaled Score; Brixton = Brixton Test of Spatial Anticipation Scaled Score; NIPi = Numerical Information Processing index.
In the analysis of the NIP a repeated measures ANOVA was performed. The between-subjects factor was group (PD vs. controls) and the within-subjects factor was cognitive load; two levels; NIPi A (find the highest number), NIPi B (find the second highest number). The ANOVA revealed a significant main effect of cognitive load; F(1,35) = 174.15; p = .000, Effect Size (ES) = .83. However, there was no significant effect of group; F(1,35) = 0.14, p = .713, and no interaction; F(1,35) = 0.04, p = .840. This result confirmed that all participants found the NIP B task harder than the NIP A task, but there was no evidence that the PD group were impaired in either measure. In relation to motor speed there was no significant difference between PD and control groups in NIP-Motor condition.
Experimental Tests
Letter fluency
Scores from the spoken and written letter fluency tests were summed to produce composite scores for Total Word Output, Fluency Index (Fi), Errors (rule breaks), and Perseverations (repeated words). Analyses of the composite fluency scores are displayed in Table 2 and revealed significant differences between the PD group and control group in the number of words generated and the length of time taken to think of each word (Fi). There were no group differences in the amount of errors or perseverations made.
Table 2 Composite scores for Letter Fluency performance: means [SDs] and exact p values for between group analyses

Note. Significant results are highlighted in bold.
Fi = fluency index.
Clustering and switching thinking times
Composite scores were also calculated for the clustering and switching fluency indices as the sum of the two measures for the spoken and written tests. Composite clustering and switching indices were compared between groups using a repeated-measures mixed ANOVA. The between-subjects factor was Group (PD participants vs. controls) and the within-subjects factor was Fluency Component (clustering vs. switching). The group means for the composite clustering and switching fluency indices are presented in Figure 1.

Fig. 1 Composite clustering and switching fluency indices for Parkinson's disease (PD) group and controls.
The ANOVA revealed a significant main effect of Fluency Component; F(1,35) = 37.41, p < .001, ES = 0.52 and a significant main effect of Group; F(1,35) = 6.99, p = 0.012, ES = 0.17 but the interaction between Group and Fluency Component was not significant; F(1,35) = 1.67, p = .204, ES = 0.05. The results confirmed that switching was a more demanding process than clustering as all participants had longer thinking times when switching between clusters than when thinking of words within clusters. In addition, PD participants had longer thinking times than controls in both clustering and switching components of the task.
Correlations and Regression
The differential contribution of executive functioning and information processing speed to fluency performance, was investigated using correlational analyses with composite scores of the tests of Executive Functions (Hayling and Brixton scores summed) and Processing Speed (NIPi A and NIPi B scores summed). Pearson's correlations revealed that Fi was associated with ACE-R scores (r = −0.76; p < .001), Executive Functions scores (r = −0.66; p = .001) and Processing Speed scores (r = 0.47; p = .025). There was no association between the Executive Functions and Processing Speed scores (r = −0.26; p = .150).
The results of the correlational analyses were used to inform subsequent hierarchical regression on the Fi scores. ACE-R score was entered in block 1 of the analysis, followed by the Executive Functions and Processing Speed scores in block 2 and block 3 respectively. The level of multicollinearity was low; ACE-R VIF = 1.35, Executive Function VIF = 1.40, Processing Speed VIF = 1.08. Standardized Beta coefficients for ACE-R, β = −0.57, t = −3.39, p = .004; Executive Functions, β = −0.38, t = −2.24, p = .041; Processing Speed β = 0.28, t = 2.05, p = .060. The best fitting model for predicting the Fi scores was the linear combination of ACE-R and Executive Function scores; R = 0.827, R 2 = 0.684, F(1,15) = 5.01, p = .041. The addition of Processing Speed did not add significant predictive value to the model; R 2 change = 0.073, F(1,14) = 4.18, p = .060.
The relationship of motor speed to fluency performance was also subsequently investigated but there were no significant correlations between NIP-Motor and fluency indices.
Subgroup Analysis
To further investigate the effect of executive impairment on fluency performance, two subgroups of PD participants were identified based on the Executive Functions scores. PD participants with an Executive Functions score below 2.5 SDs of the control group mean formed the PD-impaired subgroup (n = 7). These were compared to the remaining PD participants (n = 11) and healthy controls (n = 19). Comparative analyses between the two PD groups and healthy controls are presented in Table 3 and Table 4 and confirmed a well-matched demographic profile with no significant difference in age, years of education, or disease duration.
Table 3 Subgroup demographics: means [SDs], (range), and exact p values for between group analyses
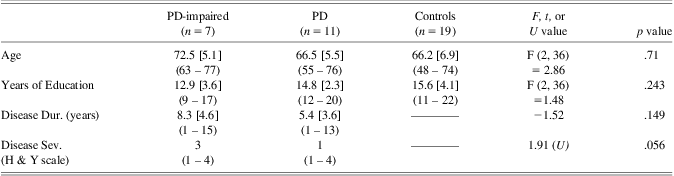
Note. Disease severity displays the median rating.
PD-impaired = impaired on tests of executive functions; PD = cognitively unimpaired patients; Dur. = duration; Sev. = Severity; H & Y = Hoehn and Yahr.
Table 4 Subgroup neuropsychological assessment: means [SDs], (range), and exact p values for between group analyses.

Note. Significant results are highlighted in bold.
ACE-R = Addenbrooke's Cognitive Examination-Revised (cutoff score for dementia = 82); GNT = Graded Naming Test; EF composite = Executive Functioning composite score; PS Composite = Processing Speed composite score.
As expected, one-way ANOVAs revealed that there was a significant difference in the Executive Functions scores with pairwise comparisons revealing that the PD-impaired group displayed lower performance than the control group (p = .000) and the PD group (p < .001). In addition, there were significant group differences in the Fi scores and the ACE-R; pairwise comparisons in the Fi analysis revealed that the PD-impaired group performed significantly worse than the control group (p = .018 Games-Howell corrected for unequal variances) and PD group (p = .036 Games-Howell corrected). In the ACE-R analysis, the PD-impaired group performed significantly worse than the control group (p = .001) but not the PD group (p = .093). By contrast, pairwise comparisons revealed no difference between the PD group and the control group in either test. There were no group differences in the Processing Speed scores.
Clustering and switching times were subsequently analyzed on the subgroups using a repeated measures mixed ANOVA. Group means and standard error bars are displayed in Figure 2. The ANOVA revealed a significant main effect of Fluency Component; F(1,34) = 62.56, p < .001, ES = 0.65, a significant main effect of Group; F(2,34) = 10.82, p < .001, ES = 0.39, and a significant interaction between Group and Fluency Condition; F(2,34) = 8.61, p = .001, ES = 0.34, indicating that the PD-impaired group had disproportionately slowed thinking times in the switching component of the fluency tasks. Post hoc pairwise comparisons indicated that the PD-impaired group differed significantly from the control group (p = .000) and PD group (p = .001) in the switching indices. By contrast, the PD-impaired group did not differ from the control group (p = .182 with Games-Howell correction for unequal variances) or the PD group (p = .648 Games-Howell corrected) in the clustering indices. The PD group and the control group did not differ in the clustering (p = .074 Games-Howell corrected) or switching indices (p = .750).

Fig. 2 Composite clustering and switching fluency indices for Parkinson's disease (PD) subgroups and controls. The asterisk denotes significant difference, p < .01.
Discussion
The current study set out to investigate the performance profile of PD participants on letter fluency tasks, with the aim of determining whether these commonly described deficits reflect an underlying executive dysfunction, or slowed processing speed. The findings revealed a letter fluency deficit in the PD group on both spoken and written fluency tasks; a result which is consistent with some studies (Azuma et al., Reference Azuma, Cruz, Bayles, Tomoeda and Montgomery2003; Bayles et al., Reference Bayles, Trosset, Tomoeda, Montgomery and Wilson1993; Flowers et al., Reference Flowers, Robertson and Sheridan1995; Green et al., Reference Green, McDonald, Vitek, Evatt, Freeman, Haber and DeLong2002), but in contrast to other studies that have reported intact letter fluency in PD patients without dementia (e.g., Auriacombe et al., Reference Auriacombe, Grossman, Carvell, Gollomp, Stern and Hurtig1993; Ivory et al., 1999; Matison et al., Reference Matison, Mayeux, Rosen and Fahn1982; Piatt et al., Reference Piatt, Fields, Paolo, Koller and Troster1999; see Henry & Crawford, 2004, for a review). However, none of the above studies accounted for motor slowing, and an implication of the current study is to suggest that this is an essential factor for the accurate assessment of patient populations with motor system pathology when administering tests that require a timed response.
Correlation and regression analyses suggested that performance on tests of executive functioning contributed to fluency performance in the PD group more than processing speed measures. This is in contrast to McDowd et al. (Reference McDowd, Hoffman, Rozek, Lyons, Pahwa, Burns and Kemper2011) who concluded that, although PD patients showed deficits in all measures of fluency tasks, these impairments were related to an underlying processing speed deficit, a conclusion based upon a regression analysis which saw processing speed tests predict the largest proportion of variance in the fluency performance (although inhibition was the second largest predictor). However, McDowd et al. (Reference McDowd, Hoffman, Rozek, Lyons, Pahwa, Burns and Kemper2011) did not control for individual variations in motor speed and slowing associated with PD in the processing speed or fluency tasks, thus it is difficult to elucidate whether the association between fluency performance and processing speed was independent of motor dysfunction.
Moreover, heterogeneity in PD was a determining factor. When analyzing the PD group as a whole, the participants were significantly slowed on both the executive component of the task (switching index) and the more automatic component of the task (clustering index), a result consistent with a slowed processing speed account of cognitive dysfunction in PD. However, when the PD group was subdivided into those with evidence of impairment in the tests of executive functioning and those without, subsequent analyses revealed a clear differential pattern; the impaired PD group performed significantly more slowly than the controls and the unimpaired PD group in the switching component of letter fluency, but did not exhibit a deficit in the clustering component. Furthermore, there were no significant differences between subgroups on the processing speed scores suggesting that processing speed was intact in both PD groups. The findings indicate a disproportionate role for executive dysfunction in letter fluency deficits in comparison with processing speed and lend support to the studies that have implicated the central executive, and specifically switching, in fluency performance (Rende et al., Reference Rende, Ramsberger and Miyake2002). The heterogeneity of executive functioning within the current PD cohort is consistent with the findings of Owen (Reference Owen2004) and Zgaljardic et al. (Reference Zgaljardic, Borod, Foldi and Mattis2003) and suggests that inconsistencies in the literature may reflect the ratio of impaired to non-impaired patients recruited by the studies.
The results of the current investigation are consistent with the findings of others studies using clustering and switching methodology in letter and semantic fluency tasks (e.g., Donovan et al., Reference Donovan, Siegert, McDowall and Abernethy1999), but are in contrast to others (Tröster et al., Reference Tröster, Fields, Testa, Paul, Blanco, Hames and Beatty1998; Troyer et al., Reference Troyer, Moscovitch, Winocur, Leach and Freedman1998) who reported that non-demented PD patients performed comparably to control participants in both types of fluency task. However, none of the studies above accounted for motor slowing or used quantitative fluency measurements, without which interpretation of the clustering and switching components remains ambiguous (Mayr, Reference Mayr2002). Specific switching impairments have also been reported in PD patients in semantic fluency only (Tröster, Woods, Fields Hanisch, & Beatty, Reference Tröster, Woods, Fields, Hanisch and Beatty2002), and letter and semantic fluency (De Gaspari et al., Reference De Gaspari, Siri, Di Giola, Antonini, Isella, Pizzolato and Pezzoli2006) after pallidal and deep brain stimulation surgery which is thought to disrupt fronto-striatal circuitry. Executive dysfunction in the PD group is consistent with reports of frontostriatal dysfunction in PD (Kehagia et al., Reference Kehagia, Barker and Robbins2013; Zgaljardic et al., Reference Zgaljardic, Borod, Foldi and Mattis2003). Frontostriatal circuits and in particular the dorsolateral prefrontal circuit, are affected by depleted dopamine levels within substantia nigra and the striatum (Owen, Reference Owen2004). Frontostriatal pathology is likely to disrupt the flow of information between the basal ganglia and the target frontal regions, which may have a knock-on effect on cognitive functioning as has been shown in PET studies (e.g., Dirnberger, Frith, & Jahanshahi, Reference Dirnberger, Frith and Jahanshahi2005).
Several limitations are noted for this study: Although our data quantitatively differentiated between clustering and switching, we were unable to determine whether long switching indices are a reflection of an inability to disengage from the current cluster (although lack of perseverations would suggest that this inflexibility was less likely to be the case), or an impairment in the activation and retrieval of a new cluster. It also remains unclear whether these findings would generalize to semantic fluency tasks, which purportedly impinge upon more temporal lobe functions and are more sensitive to Parkinson's disease dementia (Williams-Gray et al., Reference Williams-Gray, Foltynie, Brayne, Robbins and Barker2007). In addition, we did not include a background test of executive functioning that was specifically designed to assess set-switching ability and so we were unable to directly investigate this process outside of the fluency analyses. Furthermore, patients were tested in their “on” state of medication and it is noted that there was no significant difference between patients and controls in their motor speed on the NIP task and no significant relationship between motor speed and fluency indices. Levodopa equivalent data were also not available for our PD cohort so we were unable to investigate the potential effects of dopamine dose on cognitive performance. Further comparison of fluency performance between “on” and “off” states are required to shed light on the range of impairment which may be associated with PD. Finally, it is noted that the patients investigated were tremor-predominant and future investigation should include other presentations of PD including akinetic-rigid patients to determine whether this cognitive profile is indeed representative of the clinical spectrum of PD.
Conclusion
The current study demonstrated that executive dysfunction is likely to underlie letter fluency impairments in PD. Case analyses revealed that a subgroup of PD participants who performed poorly in tests of executive functioning showed particular difficulties with the switching component of the task. The study highlights the importance of controlling for motor dysfunction in neurodegenerative disorders such as PD, and has shown that it is crucial to consider the heterogeneity of these populations when making inferences on the specific cognitive profile of this disease.
Acknowledgments
L. Pettit and M. McCarthy were on the MSc in Human Cognitive Neuropsychology at the University of Edinburgh when undertaking this research. We thank all patients and volunteers who participated in this research. The authors have no conflicts of interest. This research was supported by funds from the MSc in Human Cognitive Neuropsychology, University of Edinburgh and there were no other sources of financial support.