Introduction
In mammals, the ovary is a metabolically active organ that provides the necessary microenvironment for germ cell development via folliculogenesis. During this process, any functional disruption may lead to reproductive problems that ultimately affect the reproductive potential of an individual. Di-(2-ethylhexyl)phthalate (DEHP) is one of the most common endocrine-disrupting chemicals (EDCs) and is widely used to make vinyl plastics softer and more flexible (Bauer & Herrmann, Reference Bauer and Herrmann1997; Kambia et al., Reference Kambia, Dine, Gressier, Dupin-Spriet, Luyckx and Brunet2004; Sampson & de Korte, Reference Sampson and de Korte2011). A metabolite of DEHP, mono(2-ethylhexyl)phthalate (MEHP), has been found in human urine samples (Kato et al., Reference Kato, Silva, Reidy, Hurtz and Malek2004; Heudorf et al., Reference Heudorf, Mersch-Sundermann and Angerer2007; Hogberg et al., Reference Hogberg, Hanberg, Berglund and Skerfving2008), amniotic fluid (Huang et al., Reference Huang, Kuo, Chou, Lin and Lee2009), breast milk (Hogberg et al., Reference Hogberg, Hanberg, Berglund and Skerfving2008) and ovarian follicular fluid (Krotz et al., 2012) indicating that even a limited exposure to DEHP in daily life from any source may become a potential risk factor to modulate ovarian physiology. As the major route for exposure to DEHP in the general population is food and water intake, this indicates its ubiquitous presence and therefore it seems impossible for many mammalian species including human to avoid daily exposure to DEHP.
It has been reported that exposure to DEHP leads to depletion of primordial follicle pools, affects oocyte maturation, decreases sex steroid hormone production and changes the DNA methylation status of imprinted genes (Ambruosi et al., Reference Ambruosi, Filioli, Sardanelli, Pocar and Martino2011; Li et al., Reference Li, Liu, Lai, Liu, Zhang and Dyce2016). Studies in animals have suggested that DEHP exposure impairs mouse primordial follicle assembly (Zhang et al., Reference Zhang, Zhang, Li, Feng, Chen, Ma, Huynh, Shi, De Felici and Shen2013, Reference Zhang, Li, Qin, Zhou, Zhang, Wang, De Felici, Chen, Qin and Shen2014) and the heritable modification of imprint gene DNA methylation in mouse oocytes (Li et al., Reference Li, Zhang, Qin, Ge, Ma, Sun, Hou, Chen, Chen, Qin, Shen and Zhang2014; Liu et al., Reference Liu, Lai, Li and Sun2017). DEHP has shown hepatotoxicity, reproductive toxicity, and developmental toxicity in experimental animals (Sircar et al., Reference Sircar, Albazi, Atallah and Pizzi2008; Engel & Wolff, Reference Engel and Wolff2013; Abd-Ellah et al., Reference Abd-Ellah, Aly, Mokhlis and Abdel-Aziz2015; Hannon et al., Reference Hannon, Brannick, Wang, Gupta and Flaws2015). Recently, Sun et al. (Reference Sun, Zhou and Wang2018) reported that DEHP has the ability to interfere with the hypothalamic–pituitary–thyroid axis, with downregulation of the homeostasis of thyroid-related hormones in adolescent rat. Although, the exact mechanism by which DEHP alters ovarian physiology remains unknown, a few studies have indicated that reactive oxygen species (ROS) plays an important role during DEHP-mediated toxicity (Ames, Reference Ames1999; Erkekoglu et al., Reference Erkekoglu, Rachidi, Yuzugullu, Giray and Favier2010). ROS acts as a signalling molecule in various physiological processes and has a potential role in ovarian physiology (Tripathi et al., Reference Tripathi, Khatun, Pandey, Mishra, Chaube, Shrivastav and Chaube2009). Oocytes, such as other aerobic cells, produce ATP and ROS by mitochondrial oxidative phosphorylation. Therefore, any perturbation in mitochondria or in the activity of scavenger systems can lead to oxidative stress (OS) induction and therefore ROS generation and mitochondrial cytochrome c release, which is an important sign of apoptosis. It seems that generation of ROS and/or depletion of the antioxidant system due to DEHP toxicity could result in OS and therefore apoptosis in follicular cells and/or oocytes. However, very little information is available on the precise mechanisms, timing and toxicity of DEHP that are responsible for several aberrations in reproductive functions in mammalian species.
Granulosa cells act as a potent antioxidant system and provide nutrient and growth factors to the developing oocyte via crosstalk between granulosa cells and oocyte (Huang & Wells, Reference Huang and Wells2010; Shaeib et al., Reference Shaeib, Banerjee, Maitra, Diamond and Abu-Soud2013; Tiwari et al., Reference Tiwari, Tripathi and Chaube2017). Further granulosa cells also protect the oocytes from harsh environments (Shaeib et al., Reference Shaeib, Banerjee, Maitra, Diamond and Abu-Soud2013; Tiwari et al., Reference Tiwari, Tripathi and Chaube2017). Therefore, in this study, we hypothesized that encircling granulosa cells could protect against DEHP-induced oocyte toxicity.
Materials and methods
Chemicals
All chemicals used in this study were obtained from Sigma Chemical Co. (St. Louis, MO, USA) unless stated otherwise. DEHP (99% purity, analytical grade; cat no. 36735) was purchased from Sigma–Aldrich (St. Louis, MO, USA). The DEHP stock solution (2.56 mM) was prepared by diluting 1 mg of DEHP powder in 1.0 ml of dimethyl sulfoxide (DMSO). The stock solution was immediately aliquoted and kept at −20°C until use.
Experimental animal
Sexually immature female rats (50 ± 5 g body weight), Charles-Foster strain, were purchased from the Central Animal House, Institute of Medical Science, Banaras Hindu University, Varanasi, India and housed in air-conditioned, light-controlled rooms, with food and water ad libitum.
Preparation of culture medium and DEHP working concentrations
Culture medium (TCM-199) was prepared according to a published protocol (Tripathi & Chaube, Reference Tripathi and Chaube2015). TCM-199 was supplemented with sodium bicarbonate (0.035% w/v), penicillin (100 IU/ml) and streptomycin (100 mg/ml) and the pH was adjusted to 7.2 ± 0.10. To find out the optimum effective dose of DEHP, different working concentrations (0.0, 25.0, 50.0, 100, 200, 400 and 800 μM) of DEHP were prepared by diluting the stock solution (2.56 mM) with freshly prepared working culture medium. The final concentrations of DEHP did not alter the osmolarity (290 ± 5 mOsmol) and pH 7.2 ± 0.10 of the culture medium. As DMSO was used as a solvent for the DEHP stock solution, an equivalent dilution of the highest concentration (0.02% DMSO) was used in the control group.
Collection of COCs and denuded oocytes
The ovary along with the oviduct was collected from experimental animals that had been subjected to a superovulation induction protocol (Tripathi et al., Reference Tripathi, Shrivastav and Chaube2013). Ovulated cumulus-enclosed oocyte (COCs) were isolated in pre-warmed culture medium under a dissecting microscope (Wild (Heerbrugg), Switzerland) by puncturing the oviduct using a 26-gauge needle attached to a 1 ml syringe. Ovulated cumulus-enclosed oocytes were picked up using microtubing (inner diameter 2 mm) attached to a glass micropipette (inner diameter, 100 μm; Clay Adams, NJ, USA) and transferred to culture medium with or without 0.01% hyaluronidase at room temperature. After 2 min of treatment, denuded and cumulus containing oocyte were removed, washed three times with fresh culture medium and used for in vitro studies.
In vitro exposure of DEHP to COCs and denuded oocytes
Groups of COCs and denuded oocytes (4–6 per group) were placed in culture medium containing different concentrations of DEHP (0.0, 25.0, 50.0, 100, 200, 400 and 800 μM) for 3 h in a CO2 in air incubator (Model; Galaxy 170 R, New Brunswick, Eppendorf AG, Hamburg, Germany). For the dose- and time-dependent study, oocytes from different treatments groups were evaluated every 1 h for morphological changes using a Phase-Contrast Microscope (EVOS FL, Life Technologies) at ×400 magnification. Based on the dose- and time-dependent study, the dose of 400 µM DEHP was selected to analyze the levels of intracellular ROS, OS, mitochondrial membrane potential (JC1 staining), and analyse apoptotic maker gene expression in both denuded and COCs. All experiments were performed in triplicate.
Oxidative stress detection
The redox status of control/stressed cells was assessed using an OS (ENZ-51042; Enzo Life Sciences, NY, USA) assay kit according to the manufacturer’s instructions. In brief, after 3 h DEHP (400 μM) treatment, denuded oocytes and COCs were washed with phosphate-buffered saline (PBS) and incubated with an OS detection mix for 30 min at 37°C in a CO2 in air incubator, respectively. After incubation, cells were washed with PBS and observed under a fluorescence microscope (EVOS FL, Life Technologies). As per the manufacturer’s instructions, OS were detected using a fluorescein isothiocyanate (FITC) filter at 490/525 nm. The ROS inducer (Pyocyanin) was used as the positive control and a sample without the detection mix was used as the negative control for the assay. The experiment was repeated three times to confirm the results.
Intracellular ROS detection
For analysis of intracellular ROS levels in DEHP-treated denuded oocytes and COCs, 10 μM dichlorofluorescein diacetate (DCFH-DA, Sigma Chemical) were used. In detail, after 3 h of DEHP (400 μM) exposure, denuded oocyte and COCs were washed in PBS and incubated with 10 μM DCFH-DA prepared in PBS for 15 min at 37°C respectively. After incubation, oocytes were observed under a fluorescence microscope (EVOS FL, Life Technologies) for fluorescence intensity measurement. The negative control was prepared without adding DCFH-DA in incubation solution.
Assessment of mitochondrial membrane potential
DEHP-treated denuded oocytes and COCs were stained with inner mitochondrial membrane potential reporter dye JC-1 (5,5,6,6-tetra-chloro-1,1,3,3-tetra-ethyl-benz-imidazolo-carbocyanine iodide) (BD Bioscience) as per manufacturer’s instruction. In brief, JC-1 was prepared to a final concentration of 1 µM in diluting buffer and denuded oocytes and COCs were incubated for 20 min. The stained oocytes were examined under an epifluorescence microscopy in the FITC and rhodamine-B isothiocyanate (RITC) channels using an inverted fluorescence microscope (EVOS FL, Life Technologies). The specific characteristics of JC-1 dye are as follows. When the mitochondrial membrane potential is a low, JC-1 exists as a monomer, and green fluorescence can be detected in the FITC channel. By contrast, when the potential increases, JC-1 monomers assemble into arrays termed J-aggregates that exhibit red fluorescence. Therefore, at high mitochondrial membrane potential (ΔΨm), red fluorescence is detected in the RITC channel. Fluorescence analysis was carried out by measuring the total fluorescence of the entire oocyte. The mean value for each fluorescence was normalized to area of measurement using the corrected total cell fluorescence (CTCF) method (Parry & Hemstreet, Reference Parry and Hemstreet1988). Values were expressed as total red CTCF for individual oocytes from three different treatments.
Acridine orange/propidium iodide nuclear staining
Acridine orange (AO) and propidium iodide (PI) staining were performed to confirm the apoptotic profile as a result of morphological changes in the DEHP-treated denuded oocytes and COCs. In brief, DEHP-treated oocytes and COCs were rinsed with PBS and fixed with 3.7% formaldehyde in PBS (pH 7.4) at room temperature for 20 min. Then, cells were rinsed in PBS twice and stained with AO/PI mix (10 μg/ml in PBS) for 10 min. Photographs were taken at ×200 and ×400 magnification under an inverted fluorescence microscope (EVOS FL, Life Technologies) from three separate experiments.
Annexin-V/PI staining
Apoptosis was detected in all treated groups using an annexin V-FITC/PI apoptosis detection kit. In detail, the denuded oocytes and COCs were harvested after DEHP (400 µM) treatment and incubated with 200 μl of binding buffer (HEPES-buffered PBS supplemented with 2.5 mM CaCl2), 2 μl of annexin V-FITC and 5 μl of PI at room temperature for 15 min in the dark, following the manufacturer’s instructions. After a final wash in PBS, denuded oocytes and COCs were immediately analyzed under a fluorescence microscope. Cells were excited by light at a wavelength of 488 nm with barrier filters of 525 nm and 575 nm for FITC fluorescence and PI detection, respectively. Data were analyzed and plotted for annexin V-FITC using appropriate software.
Detection of Bax, Bcl-2 and cytochrome c expression
DEHP-treated denuded oocytes and COCs were immediately fixed with 3.7% formaldehyde for 10 min at room temperature. The fixed cells were exposed to sodium tricitrate (0.01 M; pH 6.0) for antigen retrieval. After washing with Triton X-100 (0.01% in PBS) supplemented PBS, cells were then incubated with blocking buffer (5% PBS–BSA solution) at 37°C for 30 min. Thereafter, cells were exposed to 50 µl of their respective primary antibodies (Bax, Bcl2, Cyt-c, 1:500 dilution in blocking buffer; Santa Cruz Biotechnology) at 37°C for 2 h. After five washes with pre-warmed PBS, slides were exposed to 500 µl fluorescein isothiocyanate (FITC)-labelled secondary antibody (1:1000 dilution in blocking buffer; Santa Cruz Biotechnology) for 1 h at 37°C in a humidified chamber. After 1 h of incubation, cells were washed three times with pre-warmed PBS and then observed under an inverted fluorescence microscope (EVOS FL, Life Technologies).
CTCF measurement for oocytes and COCs was carried out as per the published protocol (Tiwari et al., Reference Tiwari, Prasad, Tripathi, Pandey, Singh, Shrivastav and Chaube2016). All parameters were kept constant for each oocyte, fluorescence intensity was analyzed using ImageJ software (version 1.44 from the National Institutes of Health, Bethesda, USA). For this purpose, a minimum of three different areas for each oocyte cytoplasm, as well as its corresponding background, was selected. Total fluorescence per oocyte was calculated on a Microsoft Excel® sheet, by applying the measurements obtained from the analyzed cell using the formula: CTCF=integrated density − (area of selected cell × mean fluorescence of background readings).
Gene expression analysis by PCR
mRNA was isolated from control as well as DEHP-treated denuded oocytes and COCs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and then reversed transcribed using a cDNA synthesis kit (Thermo Scientific Inc). RTPCR for different genes: Bax, Bcl2, cytochrome c and GAPDH was performed using the Maxima hot start green master mix (Thermo Scientific, Rockford, IL, USA) in a thermal cycler (Applied Biosystem). Semi-quantitative RT-PCR was chosen to estimate the transcript level of analyzed genes according to Dubey et al. (Reference Dubey, Tripathi, Singh, Saikumar, Katiyar, Pratheesh, Gade and Sharma2012). To control variation in the efficiencies of the RT step among different experimental samples, mRNA concentrations of GAPDH, a housekeeping gene presumed to be expressed at constant amounts in all samples, were also calculated, along with the mRNA concentrations of targeted genes, by densitometry analysis using ImageJ 1.43U software (NIH, Bethesda, Maryland, USA). Relative expression was determined as arbitrary units, defined as the ratio of mRNA level to the corresponding GAPDH mRNA level after subtraction of background intensity:
Mean values of three measurements for each gene in each sample band were taken for analysis.
Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM) of three independent experiments. All percentage data were subjected to arcsine square-root transformation before statistical analysis. Data were analyzed by one-way analysis of variance (ANOVA) using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA) followed by Bonferroni post hoc analysis. A probability of P<0.05 was considered to be statistically significant.
Results
DEHP-induced morphological apoptotic changes in denuded oocyte (DOs) and COCs
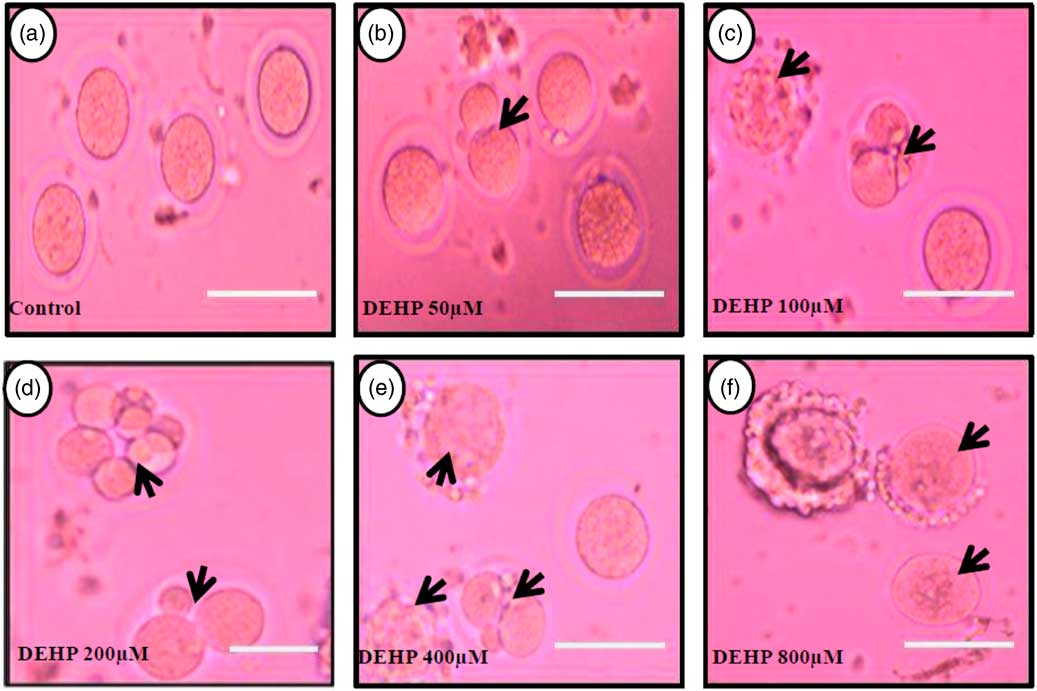
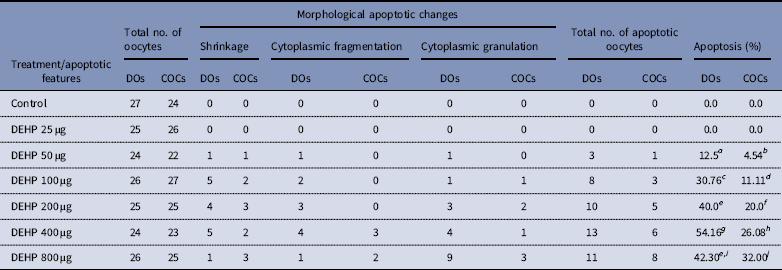
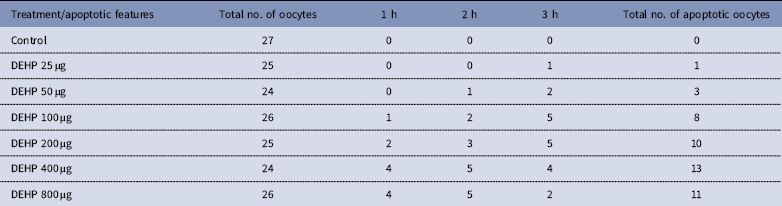
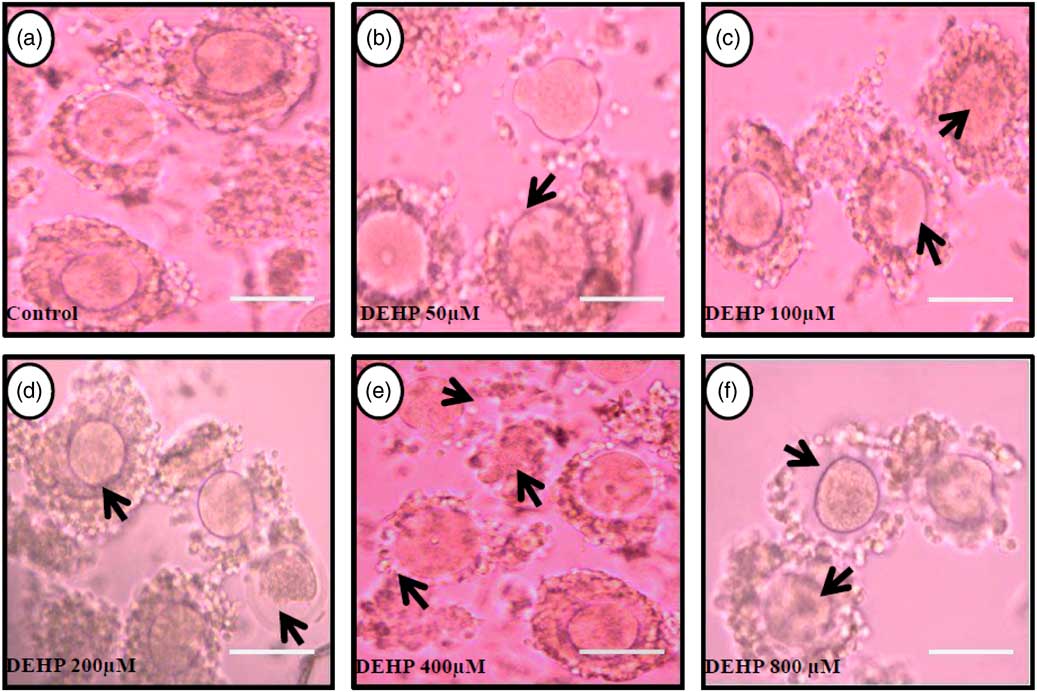
DEHP (100, 200 and 400 µM) significantly (P<0.05) induced morphological apoptotic changes (shrinkage, cytoplasmic granulation and fragmentation) in denuded oocytes (DOs) as well as in COCs in a dose-dependent manner (one-way ANOVA, F=114.28, P<0.05), while higher doses of DEHP (800 µM) induced degeneration in few denuded oocytes (Fig. 1 and Tables 1 and 2). We did not observe any apoptotic features in denuded oocytes or in COCs at the lower dose of DEHP (25 µM). However, we observed less morphological apoptotic changes in DEHP-treated COCs (Fig. 2) compared with denuded oocytes. Based on dose- and time-dependent studies (Tables 1 and 2), we selected the concentration of 400 µM DEHP for analysis of cell survival, intracellular ROS level, OS, mitochondrial membrane potential (JC1 staining), apoptosis and gene expression in denuded oocytes, as well as in COCs.
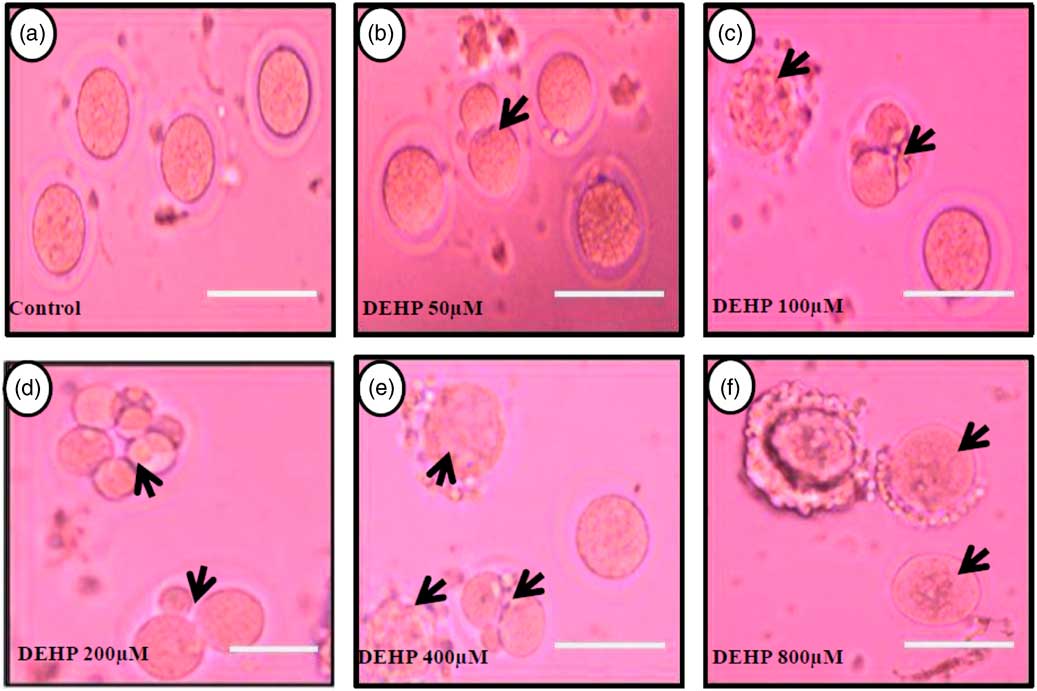
Figure 1 Representative photographs showing DEHP-induced morphological apoptotic changes in a dose-dependent manner in denuded oocytes cultured in vitro. Higher doses of DEHP (100, 200, 400 and 800 µM) induced shrinkage, cytoplasmic fragmentation, granulation and degeneration (c–f; black arrows) compared with control and lower dose of DHEP (50 µm) treated oocyte showing normal morphology (a, b). Three independent experiments were conducted to confirm the results. Bar represent 200 µm.
Table 1 Effect of DEHP exposure on morphological apoptotic changes in rat DOs and COCs cultured in vitro
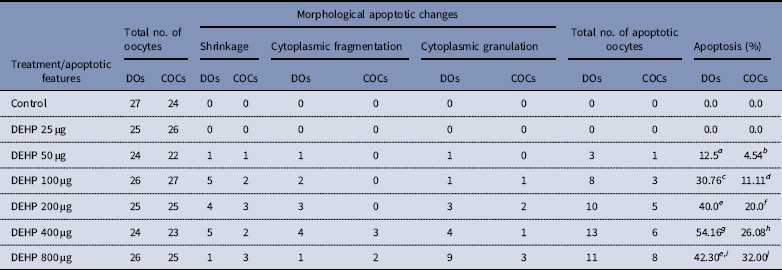
a–j Different superscript letters represent significant difference (DOs vs. COCs) to each other (P<0.05).
Table 2 Time and dose-dependent effect of DEHP exposure on morphological apoptotic changes in rat denuded oocytes (DOs) cultured in vitro
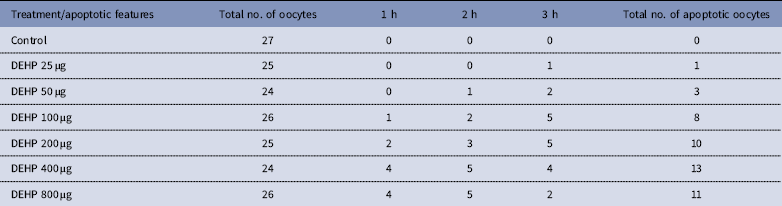
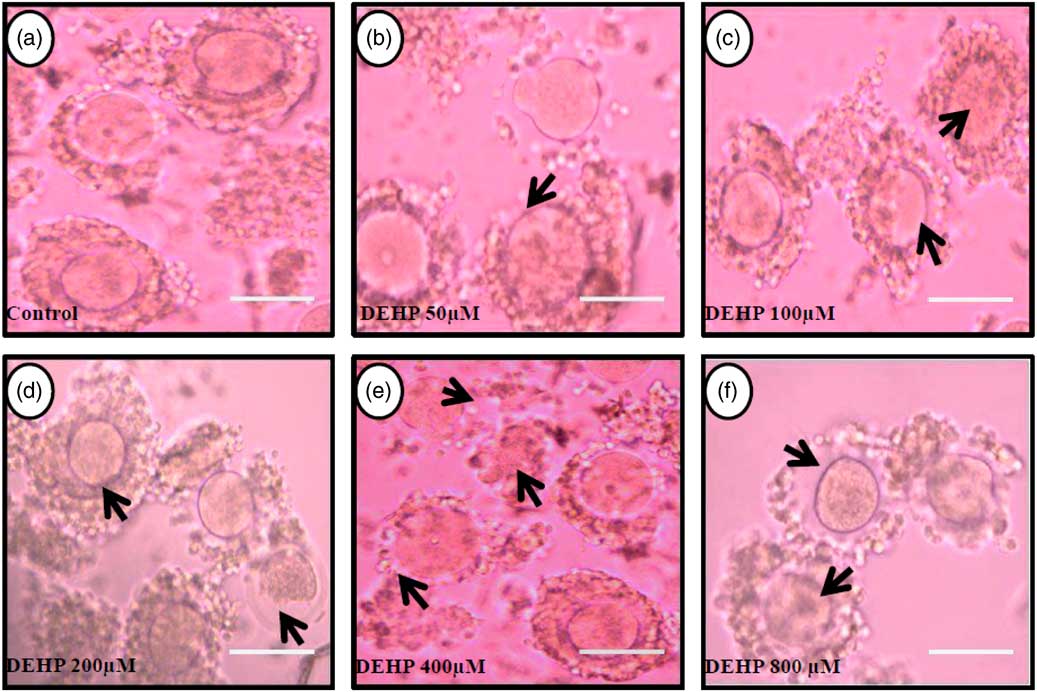
Figure 2 Representative photographs showing the protective effects of encircling granulosa cells on DEHP-induced morphological apoptotic changes in COCs cultured in vitro. The presence of encircling granulosa cells protected the oocyte against higher doses (100, 200, 400 and 800 µM) of DEHP-induced morphological apoptotic changes (c–f; black arrows) compared with control and lower dose of DHEP (50 µm) treated COCs showing normal morphology (a, b). Encircling granulosa cells protected DEHP-induced morphological apoptotic features in DEHP-treated oocytes. Three independent experiments were conducted to confirm the results. Bar represents 200 µm.
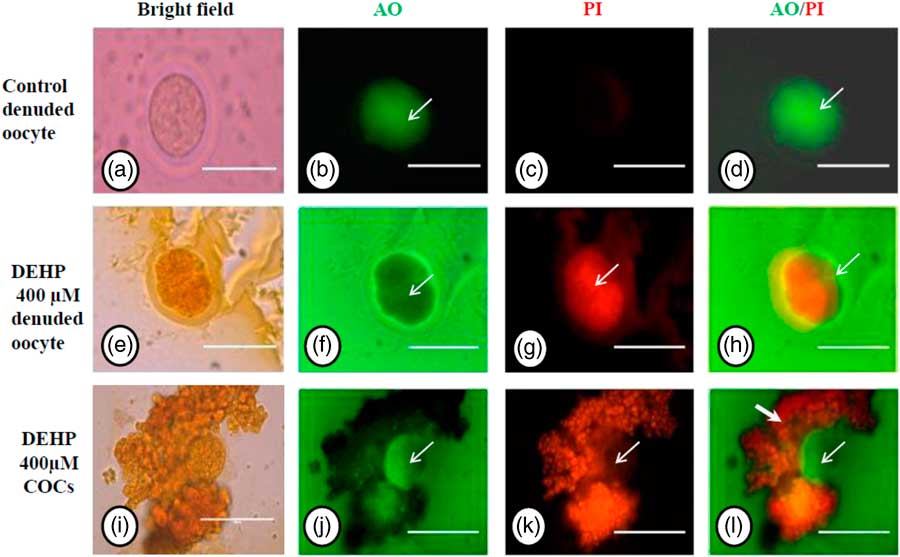
Acridine orange/propidium iodide nuclear staining
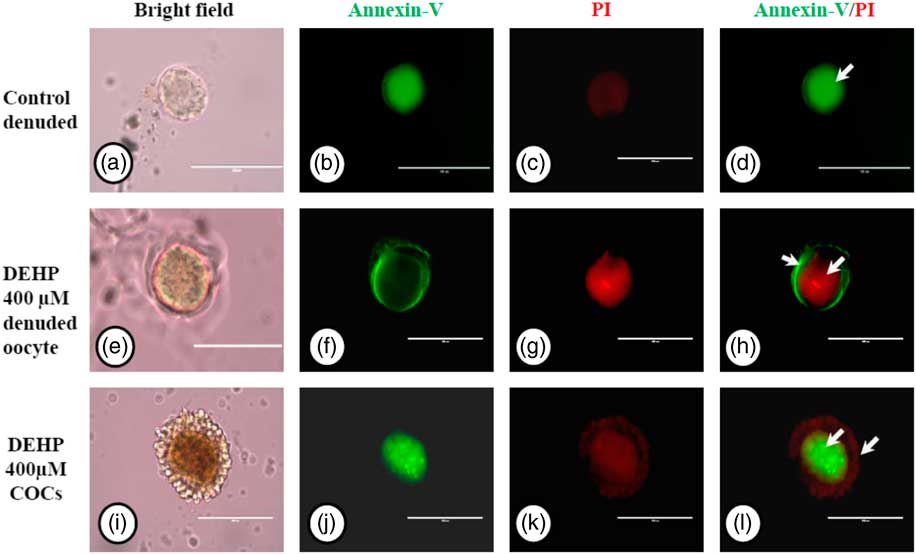
We performed AO/PI staining to confirm apoptosis as a result of morphological changes in DEHP-treated (400 µM) denuded oocytes and COCs. The results showed that DEHP treatment induced apoptosis in denuded oocytes (Fig. 3g) as evidenced by a more red fluorescence intensity for PI compared with control denuded oocytes (Fig. 3c). However, as shown in Fig. 3j, oocytes within the COCs showed AO green fluorescence compared with PI red fluorescence in the same encircling granulosa cells, indicating the protective role of encircling granulosa cells towards oocyte. Interestingly, as shown in Fig. 3l, granulosa cells in COCs showed more intense PI red fluorescence, while oocytes did not take up the PI stain.

Figure 3 Representative photographs showing acridine orange/PI staining for analysis of cell viability after DEHP treatment in denuded oocytes and in COCs. DEHP treatment (400 µM) induced apoptotic morphological apoptotic changes in denuded oocyte as evidenced by increased PI staining and reduced AO staining (e–h) compared with control denuded oocyte (a–d). The presence of encircling granulosa cells protected oocytes (green fluorescence in oocyte) from DEHP-induced apoptotic changes (j, white arrows). However, encircling granulosa themselves showed deterioration in their morphology because of DEHP toxicity as evidenced by higher PI staining (i, k, l). Three independent experiments were conducted to confirm the results. Bar represents 200 µm.
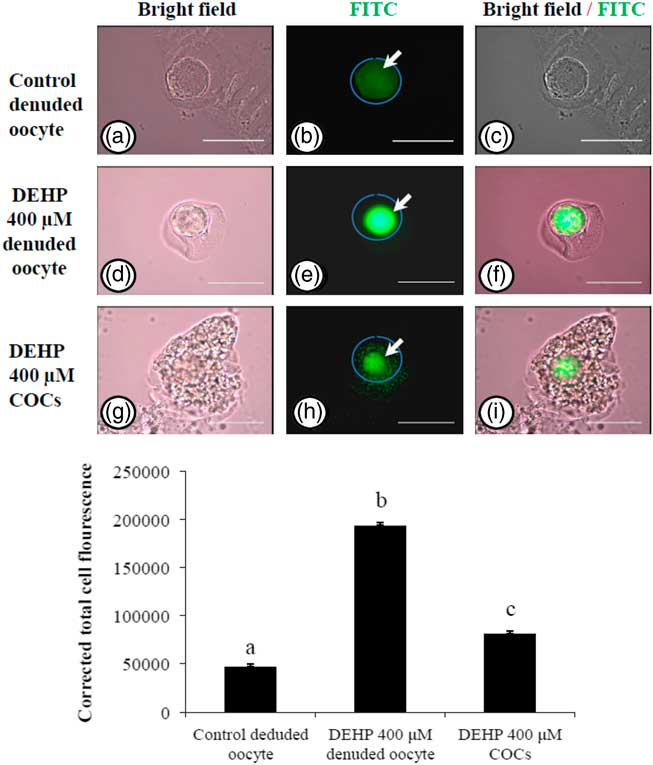
Encircling granulosa cells protect against DEHP-induced oxidative stress
In order to explore if encircling granulosa cells protected against DEHP-induced OS, COCs and denuded oocytes were exposed to DEHP (400 µM) and the OS level analyzed. As shown in Fig. 4e, treatment with DEHP (400 µM) was significantly (P<0.05) associated with increased immunofluorescence intensity in the denuded oocyte compared with the control denuded oocyte (Fig. 4b). Furthermore, as shown in Fig. 4h, encircling granulosa cells protected oocytes from DEHP induced OS, as evidenced by low fluorescence intensity compared with DEHP-treated denuded oocytes. CTCF analysis for measurement of OS level was carried out using 6–8 denuded oocytes/COCs from three independent experiments.
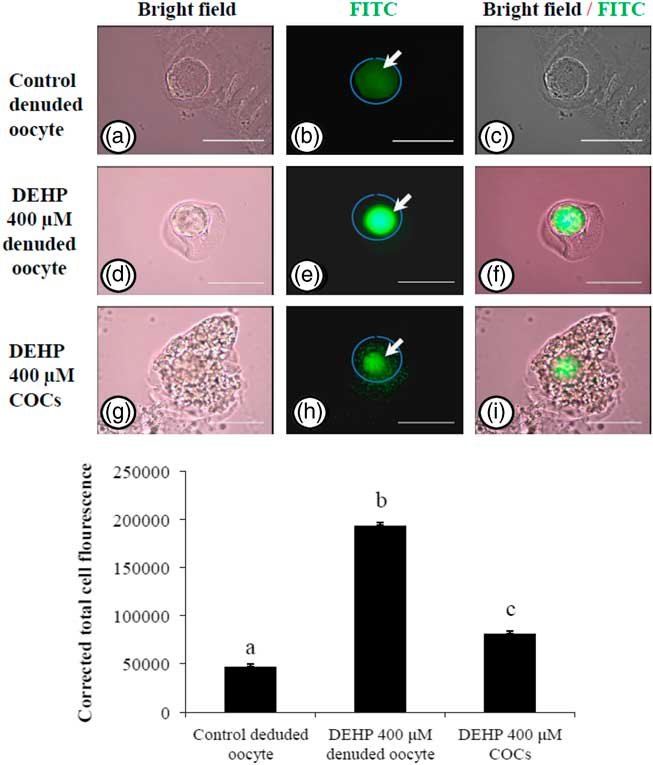
Figure 4 Representative photographs showing total oxidative stress level in COCs as well as in denuded oocyte cultured in vitro. (Upper) DEHP (400 µM) significantly increased oxidative stress levels in denuded oocyte (d–f) as evidenced by an increased CTCF value compared with the control denuded oocyte (a–c). The presence of encircling granulosa cells protected the oocytes against DEHP-induced increased oxidative stress level in treated COCs (g–i). (Lower) The level of oxidative stress is measured using oxidative stress detection kit. Data are presented as mean ± standard error of the mean (SEM) of three independent experiments. Bar represents 200 µm. a–cDifferent letters of superscript showing significant difference (P<0.05) (denuded oocyte vs. COCs).
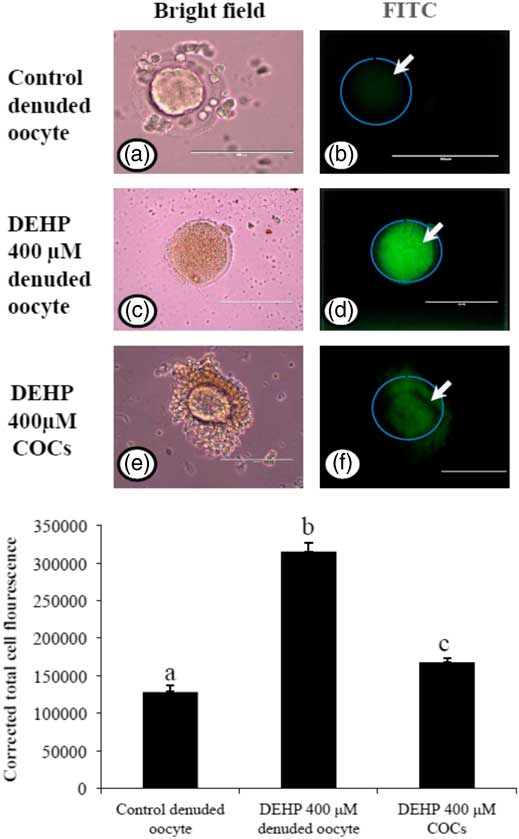
Encircling granulosa cells protect against DEHP-induced generation of ROS
As we measured OS levels in DEHP-treated in denuded oocytes and COCs, we were interested to find out whether OS could be associated with ROS generation. Therefore, we analyzed ROS levels in DEHP-treated (400 µM) denuded oocytes, as well as in COCs using the ROS-specific fluorescence probe DCFH-DA. As shown in Fig. 5d, DEHP treatment significantly (P<0.05) increased the ROS level in denuded oocytes compared with control denuded oocytes (Fig. 5b). However, encircling granulosa cells protected the oocyte from DEHP-induced increased ROS levels (Fig. 5f), as evidenced by low fluorescence intensity compared with DEHP-treated denuded oocytes. CTCF analysis for measurement of oxidative ROS levels was carried using 6–8 denuded oocytes/COCs from three independent experiments.
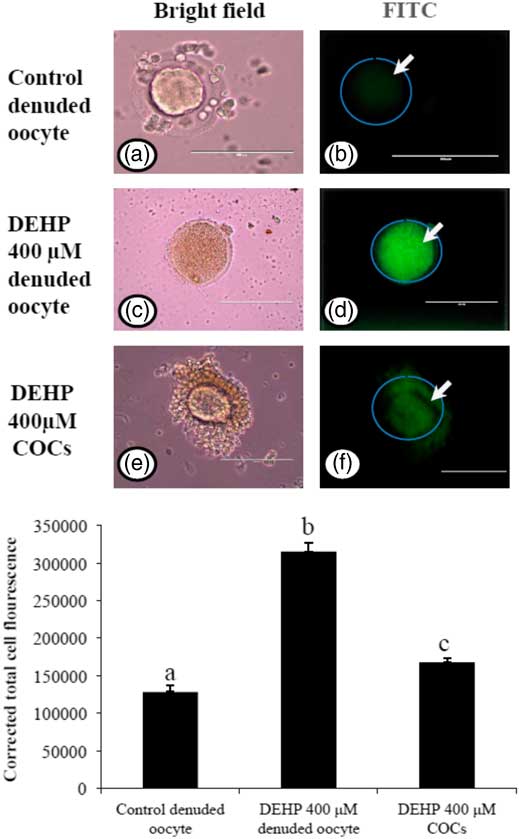
Figure 5 Representative photographs showing total intracellular ROS levels in COCs, as well as in denuded oocytes cultured in vitro. (Upper) DEHP (400 µM) significantly increased ROS levels in denuded oocyte (c, d) as evidenced by increased CTCF values compared with control denuded oocytes (a, b). The presence of encircling granulosa cells protected oocyte against DEHP-induced increased ROS level in treated COCs (e, f). (Lower) The level of ROS was measured using a DCFH-DA dye. Data are presented as mean ± standard error of the mean (SEM) of three independent experiments. Bar represents 100 µm. a–cDifferent superscript letters show significant differences (P<0.05) (denuded oocyte vs. COCs).
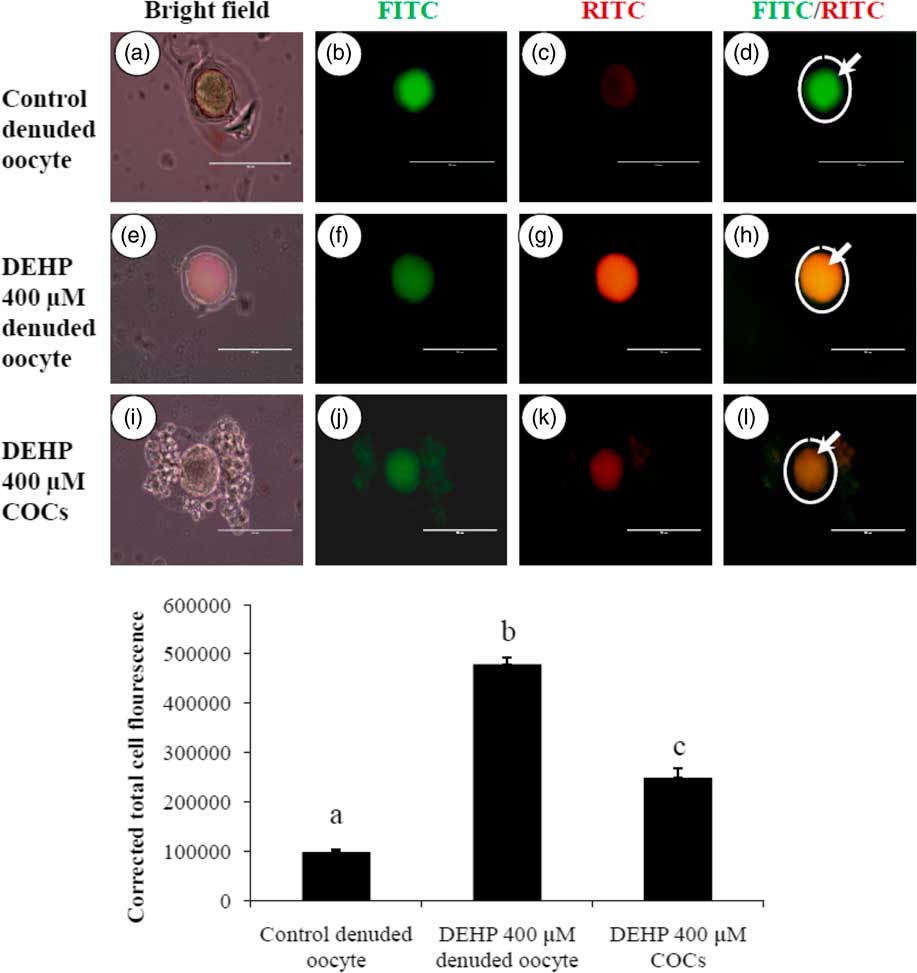
DEHP exposure induced mitochondrial activity in denuded oocytes
Active mitochondria produce a more red fluorescence light by readily accumulating JC1 dye in mitochondrial compared with less active mitochondria that produce a green fluorescence light; this ratio of red/green fluorescence is indicative of mitochondrial activity in a given cell (Reers et al., Reference Reers, Smiley, Mottola-Hartshorn, Chen, Lin and Chen1995). As shown in Fig. 6h, DEHP (400 µM) treatment significantly (P<0.05) increased mitochondrial activity in denuded oocytes compared with control denuded oocyte (Fig. 6d), as evidenced by the high ratio of red/green florescence. However, DEHP-treated COCs significantly reduced mitochondria activity, as evidenced by a low red/green fluorescence ratio (Fig. 6l) compared with DEHP-treated denuded oocytes, indicating a protective role for the encircling granulosa cells. CTCF analysis for measurement of mitochondrial activity levels was carried out using 6–8 denuded oocyte/COCs from three independent experiments.
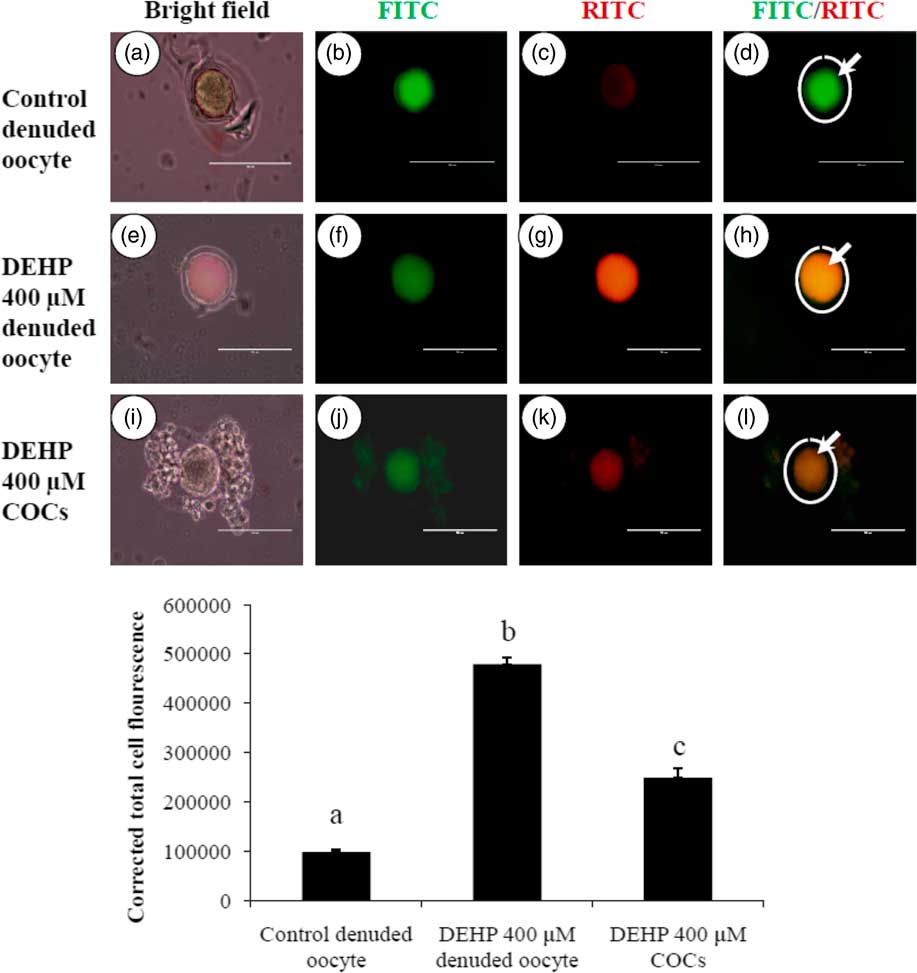
Figure 6 Representative photographs showing mitochondrial membrane potential in COCs as well as in denuded oocytes cultured in vitro. (Upper) DEHP (400 µM) significantly increased mitochondrial activity in denuded oocyte (e–h) as evidenced by increased CTCF values compared with control denuded oocytes (a–d). The presence of encircling granulosa cells protected oocytes against DEHP-induced increased activity of mitochondria in treated COCs (i–l). (Lower) Mitochondrial activity is measured using the mitochondrion-specific dye JC1. Data are presented as mean ± standard error of the mean (SEM) of three independent experiments. Bar represents 100 µm. a–cDifferent letters of superscript showing significant differences (P<0.05) (denuded oocyte vs. COCs).
Encircling granulosa cells protect against DEHP-induced apoptosis
We used an annexin V-FITC/PI apoptosis detection kit to further confirm our data on apoptosis obtained by AO/PI staining, As shown in Fig. 7g, DEHP treatment (400 µM) induced apoptosis in denuded oocytes, as evidenced by a more red PI fluorescence compared with control denuded oocytes, which produced very faint red fluorescence (Fig. 7c). However, encircling granulosa cells protected oocytes against DEHP-induced apoptosis, as evidenced by annexin-V-FITC green fluorescence, suggesting the protective role of granulosa cells that underwent apoptosis, evidenced by red fluorescence in encircling granulosa cells (Fig. 7k, l).
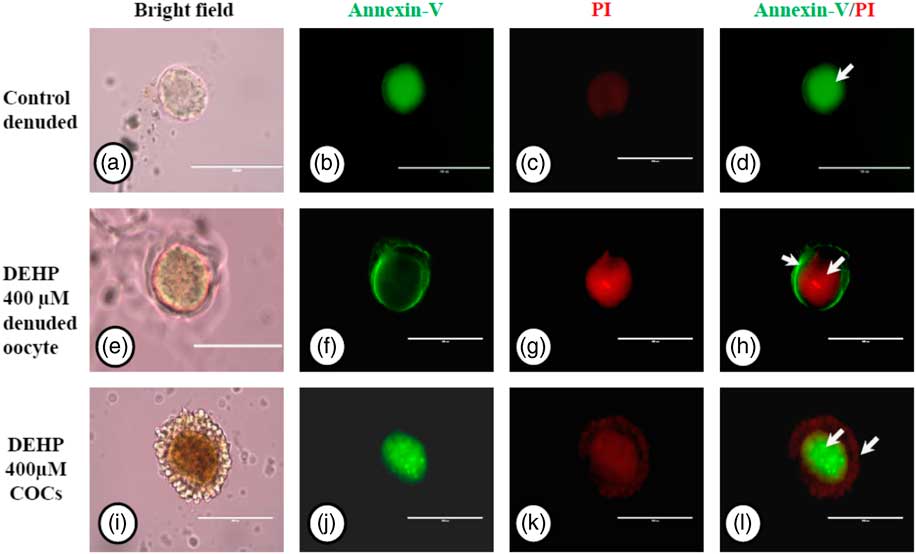
Figure 7 Representative photographs showing annexin-V/PI staining for analysis of apoptosis in DEHP-treated denuded oocytes and in COCs. DEHP treatment (400 µM) induced DNA fragmentation in denuded oocytes as evidenced by increased PI staining and reduced annexin-V staining (e–h) compared with control denuded oocytes (a–d). The presence of encircling granulosa cells protected the oocyte from DEHP-induced apoptotic changes (i–l). However, encircling granulosa cells showed apoptosis as evidenced by higher staining of PI (k, l). Three independent experiments were conducted to confirm the results. Bar represents 200 µm.
Encircling granulosa cells protect against DEHP-induced expression of apoptotic proteins
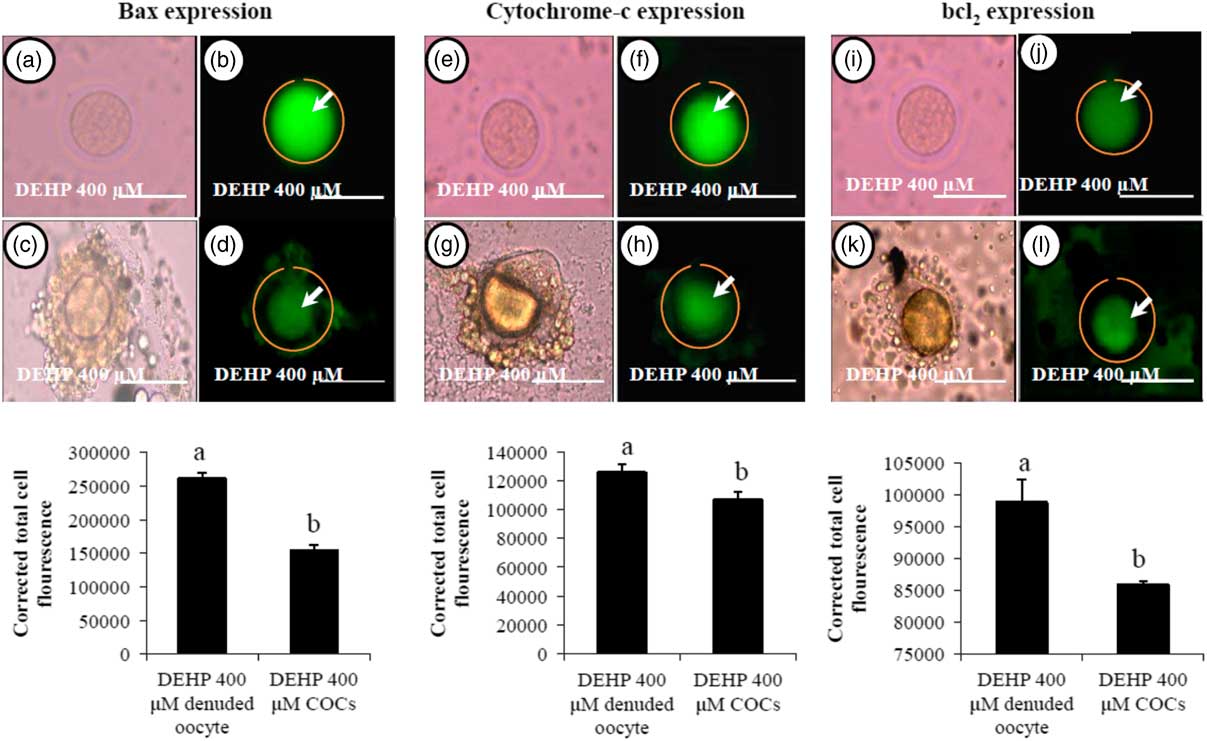
We were interested to find out whether DEHP-induced morphological apoptotic changes were associated with the altered expression of apoptotic proteins. Our data showed that DEHP treatment (400 µM) significantly increased Bax (Fig. 8b) and cytochrome c (Fig. 8f) expression levels in denuded oocyte, as evidenced by high fluorescence intensities compared with their respective COCs that had low green fluorescence (Fig. 8d, h). However, expression of the anti-apoptotic protein Bcl2 was reduced in DEHP-treated (400 µM) denuded oocyte (Fig. 8j), evidenced by low intensity green fluorescence and compared with a slightly increased intensity of green fluorescence in COCs (Fig. 8l). CTCF analysis for measurement of mitochondrial activity level was performed using 6–8 denuded oocyte/COCs from three independent experiments.
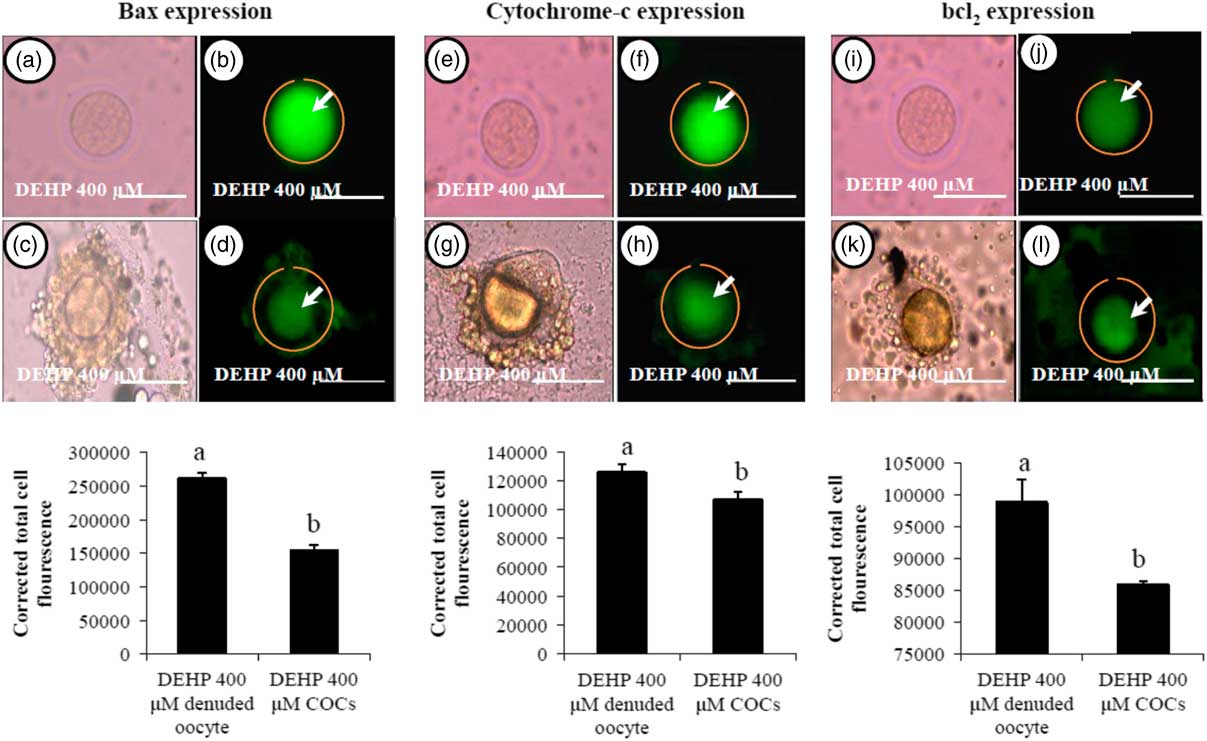
Figure 8 Representative photographs showing the expression level of apoptotic (Bax and cytochrome c) and anti-apoptotic (Bcl2) marker protein in DEHP-treated denuded oocyte and in COCs cultured in vitro. (Upper) DEHP significantly increased the expression of pro-apoptotic protein Bax (a, b) and cytochrome c (e, f) protein expression levels in treated denuded oocyte. Encircling granulosa cells protected the oocyte against DEHP-induced increase of Bax and cytochrome c expression in oocytes of COCs as evidenced by low corrected fluorescence intensity value (c, d) and (g, h) respectively. Conversely, DEHP treatment significantly reduced anti-apoptotic Bcl-2 expression level in COCs (i, j) compared with denuded oocytes (k, l). Encircling granulosa cells protected DEHP-induced increase of Bax and cytochrome c expression levels in COCs, as evidenced by low CTCF values, compared with denuded oocytes. (Lower) Three independent experiments were conducted to confirm the results. Bar represents 200 µm. a,bDifferent superscript letters showing significant difference (P<0.05) (denuded oocyte vs. COCs).
Analysis of mRNA expression of apoptotic genes
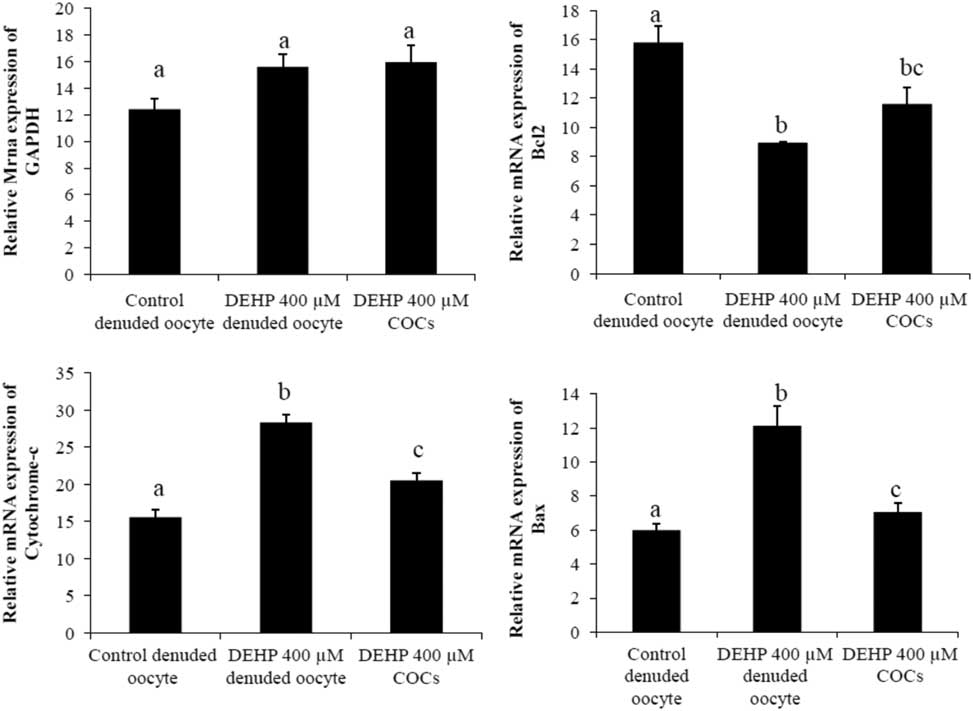
We performed semi-quantitative RT-PCR to evaluate the expression of apoptosis genes in DEHP-treated denuded oocytes and COCs. The results showed that mRNA expression levels of Bax and cytochrome c were higher in DEHP-treated (400 µM) denuded oocyte compared with control denuded oocytes (Fig. 9c, d). However, mRNA expression of Bax and cytochrome c was slightly reduced in DEHP-treated COCs (Fig. 9C, D), indicating the protective role of encircling granulosa cells. In addition, mRNA expression levels of anti-apoptotic gene bcl2 were reduced in DEHP-treated denuded oocyte compared with control denuded oocytes (Fig. 9b). However, the respective COCs showed slightly higher bcl2 mRNA expression levels, again suggesting the protective role of encircling granulosa cells (Fig. 9b). However, the expression level of GAPDH remained unchanged in all samples and did not differ significantly between samples (Fig. 9a).
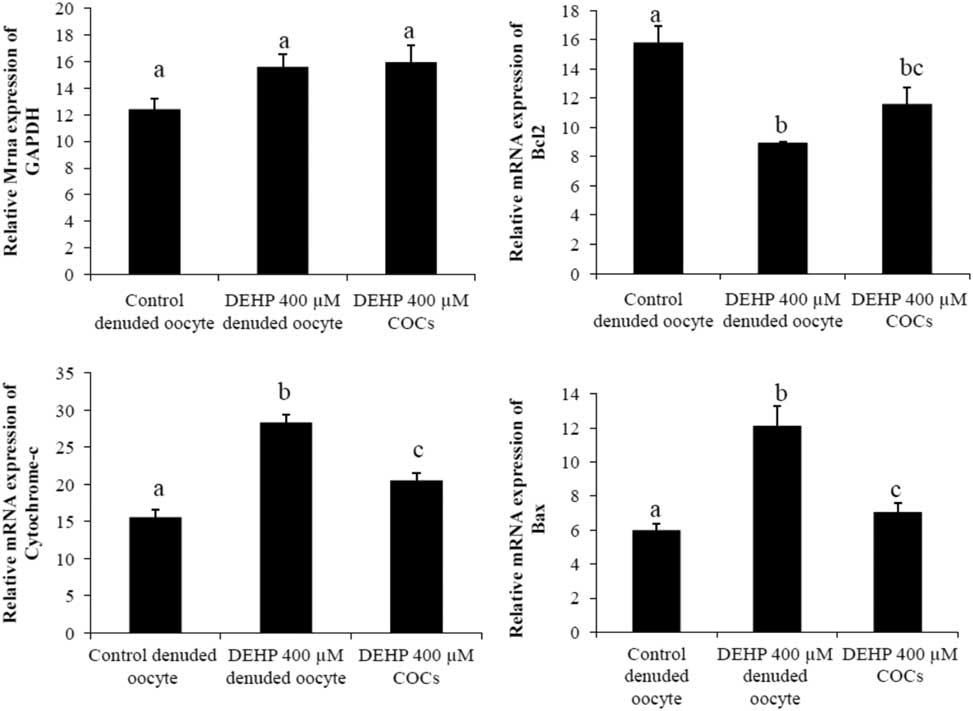
Figure 9 Representative photographs showing mRNA expression levels of apoptotic (Bax and cytochrome c) and anti-apoptotic (Bcl2) marker genes in DEHP-treated denuded oocytes and in COCs cultured in vitro. mRNA transcripts from control (denuded oocyte) and DEHP-treated groups (denuded oocyte and COCs) were converted into cDNA and amplified using pro-apoptotic (Bax and cytochrome c) and anti-apoptotic (Bcl2) primers. Treatment with DEHP significantly increased mRNA expression levels of Bax and cytochrome c in denuded oocytes. Encircling granulosa cells protected oocytes against any DEHP-induced increase in Bax and cytochrome c expression in oocytes and COCs. Furthermore, encircling granulosa cells prevented any decrease in Bcl2 expression levels in oocytes and COCs. Three independent experiments were conducted to confirm the results. Data are presented as mean ± standard error of the mean (SEM) of three independent experiments. Bar represents 200 µm. a–cDifferent superscript letters show significant difference (P<0.05) (denuded oocyte vs. COCs).
Discussion
In this study, we used an in vitro culture system to investigate whether DEHP induces OS and, if yes, whether the presence of encircling granulosa cells protects against DEHP-induced OS and apoptosis. DEHP is a ubiquitous endocrine-disrupting chemical that is known to induce cytotoxicity in many mammalian species including human (Heudorf et al., Reference Heudorf, Mersch-Sundermann and Angerer2007; Hogberg et al., Reference Hogberg, Hanberg, Berglund and Skerfving2008; Krotz et al., 2012; Li et al., Reference Li, Liu, Lai, Liu, Zhang and Dyce2016; Liu et al., Reference Liu, Lai, Li and Sun2017). Previous studies have indicated that MEHP (a metabolite of DEHP) inhibits maturation and the growth of antral follicles and induces atresia in mouse in vitro (Anas et al., Reference Anas, Suzuki, Yoshioka and Iwamura2003; Huang et al., Reference Huang, Kuo, Chou, Lin and Lee2009; Li et al., Reference Li, Liu, Lai, Liu, Zhang and Dyce2016). Furthermore, this result was confirmed in a subsequent study in which Eimani et al. (Reference Eimani, Dalman, Sepehri, Kazemi and Hassani2005) reported the inhibition of meiotic maturation in the mouse after in vivo oral DEHP administration. Our data also indicated that treatment with DEHP induced morphological apoptotic changes in a dose-dependent manner. Lower doses of DEHP (50 and 100 µM) induced the initiation of morphological apoptotic changes, while higher doses of DEHP (400 and 800 µM) induced apoptosis and degeneration. However, encircling granulosa cells protected the oocyte from DEHP-induced morphological apoptotic changes. Data in the present study are further strengthened by previous observations that the presence of encircling granulosa cells prevented oocytes from ROS-mediated OS damage for mouse (Li et al., Reference Li, Miao and Zhou2011; Zhou et al., Reference Zhou, Lian and Cui2012; Jiao et al., Reference Jiao, Cao and Cui2013), rat (Jiao et al., Reference Jiao, Cui and Yang2016), bovine (Morado et al., Reference Morado, Cetica, Beconi and Dalvit2009) and human (Goud et al., Reference Goud, Goud and Qian1998) oocytes cultured in vitro.
DEHP induced OS via ROS generation and affected oocyte physiology (Tatemoto et al., Reference Tatemoto, Sakurai and Muto2000; Suna et al., Reference Suna, Fuminori, Shoji, Masaaki and Fumihiko2007; Tripathi et al., Reference Tripathi, Shrivastav and Chaube2013). Our findings also suggested that DEHP treatment (400 µM) induced OS in denuded oocytes. Data from our findings are also supported by previous studies that suggested that DEHP induced OS (Tang et al., Reference Tang, Tong and Zhu2016; Franken et al., Reference Franken, Lambrechts, Govarts and Koppen2017). However, encircling granulosa cells protected oocytes from DEHP-induced OS. Our study corroborated findings that granulosa cells protected the oocyte from oxidative damage (Tiwari et al., Reference Tiwari, Tripathi and Chaube2017).
ROS are known to play important roles in many physiological processes (Ames, Reference Ames1999; Erkekoglu et al., Reference Erkekoglu, Rachidi, Yuzugullu, Giray and Favier2010). In this study, we assumed that DEHP exerted their toxic effect and potentially affected the physiology of oocytes by increasing ROS levels. Therefore, we assessed the ROS levels in DEHP-treated groups using a ROS-specific probe DCFH-DA. Our results showed that ROS levels were significantly higher in DEHP-treated denuded oocytes. DEHP might have induced ROS levels by increasing OS and, therefore, by induction of morphological apoptotic changes in treated denuded oocytes. Interestingly, we found that encircling granulosa cells protected the oocyte from DEHP-induced ROS generation. Granulosa cells encircling the oocyte somehow reduced the DEHP-induced OS and by lowering the level of intracellular ROS production. Taken together these data suggest the protective role of encircling granulosa cells towards DEHP-induced ROS-mediated apoptosis under in vitro culture conditions. These findings are further supported by previous studies in which encircling granulosa cells prevented an increase in ROS levels in oocytes under in vitro culture conditions (Huang & Wells, Reference Huang and Wells2010; Shaeib et al., Reference Shaeib, Banerjee, Maitra, Diamond and Abu-Soud2013).
Mitochondria respiration is the primary source of ATP generation. The balance between ROS generation and ATP synthesis in a healthy mitochondria is tightly regulated and any disturbance in this process can lead to mitochondrial membrane hyperpolarization in human and mouse embryos (Acton et al., Reference Acton, Jurisicova, Jurisica and Casper2004). Studies have used the fluorescent ΔΨm reporter dye JC-1 to determine metabolic activity and membrane potential (Ahn et al., Reference Ahn, Sohn and Kwon2002; Van Blerkom et al., Reference Van Blerkom, Davis and Alexander2003). In our study, we were interested to find out the mitochondrial membrane potential (hyperpolarize status of mitochondria) in DEHP-treated oocytes and COCs using the mitochondria-specific dye JC1. Our results showed that, in DEHP-treated oocytes, mitochondria were hyperpolarized and fluoresced red due to the formation of J-aggregates. However, for COCs, mitochondria were less hyperpolarized, as indicated by a lesser intensity of red fluorescence. Our result indicated that the encircling granulosa cells protected the oocyte from the adverse effects of DEHP. Our results were further strengthened by this previous observation (Goud et al., Reference Goud, Goud, Diamond, Gonik and Abu-Soud2008; Tripathi et al., Reference Tripathi, Shrivastav and Chaube2013; Tiwari et al., Reference Tiwari, Tripathi and Chaube2017).
It is well established that any change in mitochondrial membrane potential results in altered Bax/Bcl2 ratios and therefore cytochrome c is released into the cell, this leads to apoptosis (Tripathi & Chaube, Reference Tripathi and Chaube2012). We analyzed the mRNA and protein expression levels of Bax, Bcl2 and cytochrome c in DEHP-treated denuded oocytes, as well as in COCs. Our result showed that DEHP treatment increased mRNA and protein expression levels for Bax and cytochrome c (pro-apoptotic markers) and reduced the expression of anti-apoptotic Bcl2 protein. However, interestingly, we observed that encircling granulosa cells prevented an increase in mRNA and protein expression levels for these apoptotic markers. The expression levels of anti-apoptotic protein Bcl2 were only reduced slightly in the DEHP-treated denuded oocytes. Our findings are also supported by previous studies in that granulosa cells play a role in the prevention of ROS-induced apoptotic pathways in rat COCs cultured in vitro (Tripathi & Chaube, Reference Tripathi and Chaube2012; Tiwari et al., Reference Tiwari, Prasad and Tripathi2015, Reference Tiwari, Tripathi and Chaube2017).
To further confirm our results that encircling granulosa cells protected the oocyte from DEHP-induced apoptosis, we further analyzed cell survival and apoptosis using AO/PI and annexin-V/PI staining. Our result showed that DEHP induced oocyte death, as evidenced by PI-positive staining. However, the oocytes surrounded by granulosa cells did not take up the PI stain, although granulosa cells were found to be positive for PI staining. This finding indicates that granulosa cells protect the oocyte from apoptosis induced by DEHP and are the cells that first enter apoptosis, rendering the oocyte protected from damage (Tripathi & Chaube, Reference Tripathi and Chaube2012; Tripathi et al., Reference Tripathi, Shrivastav and Chaube2013).
Conclusions
Our data suggest that the presence of encircling granulosa cells acts as a potential protection barrier and effectively masks the oocyte against the adverse effects of DEHP, which exerts a dose-dependent deleterious effect on denuded oocytes. This effect was mediated by oxidative stress-induced ROS generation, which altered mitochondrial membrane potential and resulted in disturbance in the Bax/Bcl-2 ratio that ultimately led to oocyte apoptosis. Considering the extensive use of DEHP-containing plastic products, especially in rural areas because of the lack of adequate primary healthcare facilities, unhygienic conditions and direct exposure to various kinds of endocrine disruptors, the presence of encircling granulosa cells might be beneficial for protecting the oocyte during in vitro manipulation against DEHP-induced reproductive toxicity.
Financial support
This study was supported financially by the Science and Engineering Research Board, Ministry of Science and Technology, Government of India (grant no. SERB/YS/000819).
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical standards
Animal experimental work was carried out as according to regulations provided by the Animal Ethical Committee of the Banaras Hindu University, Varanasi, India. This study was approved by the Institutional Animal Ethical Committee of the University (see letter no. Dean/2015/CAEC/1517, dated 21 December 2015).