Introduction
Septal surgery is one of the most common surgical procedures in otorhinolaryngological practice. It is performed either using an endonasal or an open technique. Both surgical approaches aim to remove and reconstruct the deviated parts, after mucoperichondrial flap elevation.
The nasal septum mucoperichondrium consists of four layers, as follows. The mucosal layer is outermost, and mostly consists of many stratified cells with or without cilia, goblet cells, and basal cells. The basal layer (also known as the submucosa-subepithelial connective tissue) consists of thick collagen fibres, and separates the epithelial layer from the lamina propria. The lamina propria (also known as the subepithelial connective tissue) consists of tubuloalveolar glands, capillary vessels and a venous plexus. The deepest layer is the perichondrium, which is composed of several layers of connective tissue fibres running parallel to the cartilage.Reference Fawcett, Jensh, Bloom and Fawcett1
During septal surgery, the most appropriate approach is to perform mucoperichondrial flap elevation by dissecting along the plane between the perichondrium (the innermost mucoperichondrial layer) and the cartilage. Dissection in this avascular area not only provides the surgeon with a bloodless surgical field with good visibility, but also reduces the risk of damage to the nasopalatine and incisive nerves and to specific structures such as the vomeronasal organ and submucosal organs.Reference Ozturan and Miman2
Particular difficulty is often experienced when elevating mucoperichondrial flaps from the anterior nasal septum, compared with the posterior nasal septum. To our knowledge, no previous reports have investigated the reasons for this difficulty.
The present study aimed to examine the histological structural characteristics of the nasal septum cartilage, mucoperichondrium and intervening layer, in both the anterior and posterior nasal septum, and to evaluate any differences, using light and electron microscopy.
Materials and methods
We enrolled in the study four patients scheduled for total septal reconstruction via open septoplasty.
Ethics committee approval was obtained. Subsequently, all patients were informed of the surgical procedure, and gave both verbal and written consent.
Decortication of the nasal tip and dorsum was performed under general anaesthesia, without local lidocaine-adrenaline infiltration into the nasal septum. Unilateral mucoperichondrial flap elevation was performed via a posterosuperior approach. The first full-thickness sample (5 × 5 mm in dimension, and including cartilage and mucoperichondrium) was removed in a caudal to cephalic direction. A cephalic sample (again 5 × 5 mm in dimension) was removed from the point at which the septal cartilage unites with the vomer. The origin of both samples was recorded.
The tissue samples were fixed in 2.5 per cent glutaraldehyde in 0.1 M sodium cacodylate buffer, before staining with haematoxylin and eosin (H&E) and Masson's trichrome stain, for light microscopy. Slides were examined under ×10, ×40, ×100 and ×400 magnification.
Samples were prepared for electron microscopy as follows. After post-fixation with 1 per cent osmium tetroxide in 0.1 M sodium cacodylate buffer, samples were dehydrated in a graded alcohol series and propylene oxide. Sections of 0.5 µm thickness were made, embedded in plastic blocks, and contrasted with lead citrate and uranile acetate. Samples were examined using a Jeol-1010 electron microscope (Jeol, Tokyo, Japan).
Results
Four patients (two men and two women) were enrolled in the study. Their mean age was 34 years.
Light microscopy
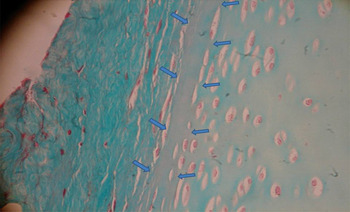
On examination of the H&E-stained anterior nasal septum specimens, the demarcation between the perichondrium and the cartilage matrix could not be clearly distinguished. In these samples, the lamina propria was rich in vascular and glandular structures (Figure 1).

Fig. 1 Photomicrograph of the anterior nasal septum. The demarcation between cartilage and perichondrium cannot be clearly distinguished. Arrows indicate the lamina propria, which is rich in vascular and glandular structures. (H&E; ×100)
Similarly, on examination of H&E-stained posterior septum specimens the demarcation between the perichondrium and the cartilage matrix could also not be clearly distinguished. In these specimens, the lamina propria showed fewer vascular and glandular structures, compared with anterior septum specimens (Figure 2).

Fig. 2 Photomicrograph of the posterior nasal septum. The demarcation between cartilage and perichondrium cannot be clearly distinguished. Arrows indicate the lamina propria, which contains few vascular and glandular structures. (H&E, ×100)
However, in the H&E-stained posterior nasal septum specimens the demarcation between the perichondrium and the cartilage matrix was clearer at higher magnifications (Figure 3).

Fig. 3 Photomicrograph of the posterior nasal septum. The demarcation between cartilage and perichondrium is more clearly distinguished at higher magnification. Arrows indicate the demarcation between cartilage and perichondrium. (H&E; ×400)
Light microscopy identified no difference between the anterior and posterior nasal septum samples in terms of perichondrial thickness and subperichondrial region cell density.
In the anterior nasal septum samples, although the connective tissue between the mucosa and perichondrium generally exhibited the characteristics of loose connective tissue, we observed focal, dense, irregular connective tissue in some areas. The demarcation between the lamina propria and the perichondrium could not be clearly distinguished.
On examination of the Masson's trichrome stained specimens from the anterior nasal septum, we observed that type II collagen, the most common collagen in human septal cartilage, was present at the demarcation between the perichondrium and the cartilage matrix and between the perichondrium and the lamina propria. Neither of these two demarcations could be clearly distinguished (Figure 4).

Fig. 4 Photomicrograph of the anterior nasal septum. The demarcation between cartilage and perichondrium cannot be clearly distinguished. Arrows indicate indentations between the perichondrium and the cartilage matrix. (Masson's trichrome; ×100)
In these same specimens, we also observed loose connective tissue and focal areas of dense, irregular connective tissue, together with a large number of vascular and glandular structures (Figure 5). The demarcation line between the perichondrium and the cartilage matrix was not clearly distinguishable (Figure 6).

Fig. 5 Photomicrograph of the anterior nasal septum. The demarcation between cartilage and perichondrium cannot be clearly distinguished. Arrows indicate the lamina propria, which is rich in vascular and glandular structures, as well as loose connective tissue and focal areas of dense, irregular connective tissue (Masson's trichrome; ×100)

Fig. 6 Photomicrograph of the anterior nasal septum. The demarcation between cartilage and perichondrium cannot be clearly distinguished. Arrows indicate indentations between the perichondrium and the cartilage matrix. (Masson's trichrome; ×400)
In Masson's trichrome stained specimens from the posterior nasal septum, we observed loose connective tissue within the lamina propria. The demarcation between the perichondrium and the cartilage matrix was relatively regular, compared with that observed in the anterior nasal septum specimens (Figure 7).

Fig. 7 Photomicrograph of the posterior nasal septum. The demarcation between cartilage and perichondrium is more clearly distinguished. Arrows indicate the demarcations between cartilage and perichondrium and between perichondrium and lamina propria. (Masson's trichrome; ×200)
Electron microscopy
In the specimens observed under electron microscopy, we observed no significant differences in chondroblast activity, comparing anterior and posterior nasal septum specimens.
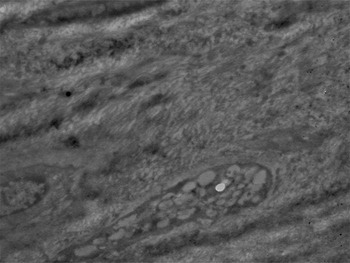
Anterior nasal septum specimens revealed irregular, dense connective tissue in the peripheral region of the perichondrium. The perichondrium contained irregular collagen fibre clusters (Figure 8).

Fig. 8 Electron micrograph of the anterior nasal septum, showing the components of dense, irregular connective tissue, within the peripheral region of the perichondrium. (×5000)
Posterior nasal septum specimens showed the components of regular, loose connective tissue (Figure 9).

Fig. 9 Electron micrograph of the posterior nasal septum, showing the components of regular, loose connective tissue. (×5000)
Discussion
Connective tissue is commonly observed around cartilage, and is composed of a limited number of live cells scattered within an extracellular matrix. Connective tissue may be loose or dense.
Loose connective tissue (also known as areolar tissue) is derived from mesenchyma. It supplies blood to the epithelial tissue, and is also a component of mucous membranes in the respiratory and digestive systems. Although loose connective tissue is mostly composed of fibroblasts, it also consists of macrophages differentiated from mesenchyma or migrated from blood, as well as mast cells, adipocytes and plasma cells. The commonest types of loose connective tissue fibre are collagen and elastin fibres, although reticular fibres are also present.Reference Kierszenbaum3
In contrast, in dense connective tissue fibres are more abundant than cells and extracellular matrix. The dense connective tissue is mainly composed of fibroblasts and usually does not contain wandering cells that are present in the connective tissue.
Dense connective tissue may be of two types: regular and irregular. Regular dense connective tissue has regular collagen fibres which are bundled in a parallel fashion. These regular fibre bundles make up a large part of the tissue. In contrast, irregular dense connective tissue contains collagen fibres arranged in thick bundles and extending out in different directions, giving an irregular appearance. Elastin fibres support this tissue by forming a reticular structure. The matrix found within irregular dense connective tissue is less compact compared with that of regular dense connective tissue.Reference Richmon, Sage, Shelton, Schumacher, Sah and Watson4
In the present study, light microscopy of anterior nasal septum specimens identified connective tissue displaying the characteristics of both loose connective tissue and focal irregular dense connective tissue. Electron microscopy of anterior nasal septum specimens revealed the components of irregular dense connective tissue. In the posterior nasal septum specimens, light microscopy revealed loose connective tissue, while electron microscopy showed loose connective tissue with a regular arrangement of fibres.
Cartilage grows by means of interstitial or appositional growth. In interstitial growth, cartilage grows ‘internally’ by means of chondrocyte mitosis and secretion of new matrix from sibling cells. In appositional growth, cells in the chondrogenic layer of the perichondrium secrete new matrix while differentiating into new chondrocytes. During active cartilage growth, the perichondrium is separated into two layers: an outer fibrillar layer and an inner cellular layer. The outer fibrillar layer provides mechanical support, protection and attachment to other structures, whereas the inner cellular layer produces new cartilage cells (hence, this layer has also been termed the chondrogenic layer). As the chondroblasts synthesise and secrete matrix, they push apart from the perichondrium and from each other. These cells then differentiate into mature chondrocytes.Reference Kaiser, Karam, Sepehr, Wong, Liaw and Vokes5
In the present study, electron microscopy showed no difference between the chondroblast activity of the anterior and posterior nasal septum. Furthermore, light microscopy identified no differences in perichondrial thickness and subperichondrial region cell density, comparing the anterior and posterior nasal septum specimens.
The septal cartilage matrix is mainly composed of type II collagen, although small amounts of type IX, X and XI collagen are also found. Type II collagen is produced by chondroblasts and is specific to cartilage tissue. It is arranged in fibres and degenerates with age.Reference Holden, Liaw and Wong6 In the present study, Masson's trichrome staining of anterior nasal septal specimens showed that type II collagen was present at the demarcation between perichondrium and cartilage matrix and between perichondrium and lamina propria. Neither of these two demarcations could be clearly distinguished.
The extracellular matrix of cartilage plays an important role in the maintenance of structural integrity, as well as in adhesion, proliferation and differentiation. Laminin and fibronectin are among the glycoproteins present in cartilage extracellular matrix. These glycoproteins are present in all types of connective tissue, and have both structural and metabolic roles. Their mechanical roles are to provide an intercellular connection, as well as a connection between the different components of the matrix.Reference Culav, Clark and Merrilees7 The laminin concentration has been found to gradually decrease as one moves from the peripheral to the central parts of the cartilage, while the fibronectin concentration gradually increases, moving from the periphery to the centre.Reference Ustünel, Cayli, Güney, Celik-Ozenci, Tanriöver and Sahin8 Fibronectin present on cell surfaces has a role in many processes, including cell–cell and cell–substrate adhesion, cell morphology, and cell motility. In the present study, we performed both light and electron microscopic evaluations of the tissue between the perichondrium and the lamina propria, and between the perichondrium and the cartilage matrix. Immunohistochemical examination of the molecules found in these structures would provide more information on their structural integrity.
• Elevation of mucoperichondrium is more difficult on the anterior nasal septum than the posterior nasal septum
• This is probably due to the presence of irregular structures between the cartilage matrix and the perichondrium
• In this study, electron microscopy showed no significant difference in chondroblast activity between the anterior and posterior nasal septum
• Light microscopy of anterior nasal septum tissue showed both loose connective tissue and focal areas of dense, irregular connective tissue
Yazıcı et al. examined human septal cartilage (from both cadaveric and living sources) under both light (Masson's trichrome stain) and electron microscopy, and reported a longitudinal fibrillar arrangement (i.e. a reticular arrangement) in the dorsal and caudal parts of the septal cartilage, whereas in the basal septum fibres ran perpendicular to the palate. However, fibres in the central part had no definite orientation. They concluded that the caudal septum (in particular) and the dorsal septum were stronger because of the longitudinal arrangement of their fibres.Reference Yazıcı, Yavuzer, Gözil, Erdoğan and Atabay9
Conclusion
Findings from the present study suggest the presence of an irregular interstructural connection between the perichondrium and the lamina propria, and between the perichondrium and the cartilage matrix, which may explain the greater difficulty encountered when elevating mucoperichondrium from the anterior nasal septum, compared with the posterior nasal septum.
Immunohistochemical examination of these tissues would improve our understanding of these interstructural connections.