Introduction
Clinostomid metacercariae can be observed on the skin, muscle or internal organs of freshwater fish and amphibians (McAllister, Reference McAllister1990; Lemke et al., Reference Lemke, Dronen, Fox and Nambiar2008; Sutili et al., Reference Sutili, Tourem Gressler and Vilani de Pelegrini2014). The growth of adults in the buccal cavity and oesophagus of piscivorous birds, as well as the presence of members of the Nephrocephalinae subfamily in the oesophagus of reptiles, has been reported (Kanev et al., Reference Kanev, Radev, Fried, Gibson, Jones and Bray2002; Bullard & Overstreet, Reference Bullard, Overstreet, Eiras, Segner, Wahil and Kapoor2008; Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016). Until now, only Clinostomum complanatum (Rudolphi, 1809) Braun, 1899 has been recorded as zoonotic in Asian countries. Consumption of raw freshwater fish by humans can cause laryngeal or pharyngeal infections (Park et al., Reference Park, Kim, Joo and Kim2009; Hara et al., Reference Hara, Miyauchi, Tahara, Yamashita and Nagoya2014; Lee et al., Reference Lee, Park, Kim, Seo, You, Chung, Moon and Hong2017; Kim et al., Reference Kim, Cho, Oh and Byeon2019).
Fifteen species of Clinostomum Leidy, 1856 have been recognized so far on the basis of morphological and molecular descriptions (Locke et al., Reference Locke, Caffara, Marcogliese and Fioravanti2015, Reference Locke, Caffara, Barčák, Sonko, Tedesco, Fioravanti and Li2019; Rosser et al., Reference Rosser, Alberson, Woodyard, Cunningham and Pote2017; Sereno-Uribe et al., Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018). In Argentina, based only on morphology, Clinostomum detruncatum Braun, 1899, C. complanatum and Clinostomum marginatum (Rudolphi, 1819) Braun, 1899 have been reported (Boero & Led, Reference Boero and Led1971; Lunaschi et al., Reference Lunaschi, Cremonte and Drago2007; Lunaschi & Drago, Reference Lunaschi and Drago2009).
Matthews & Cribb (Reference Matthews and Cribb1998) and Caffara et al. (Reference Caffara, Bruni, Paoletti, Gustinelli and Fioravanti2014a, Reference Caffara, Davidovich, Falk, Smirnov, Ofek, Cummings, Gustinelli and Fioravantib), among others, agree that the validity of species within Clinostomum has been problematic. In recent years, molecular analysis has been used to confirm the diagnosis and, together with the morphological features, to help distinguish among species, revealing also a high biodiversity of lineages (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García Prieto and Mendoza-Garfías2007, Reference Pérez-Ponce de León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016; Gustinelli et al., Reference Gustinelli, Caffara, Florio, Otachi, Wathuta and Fioravanti2010; Caffara et al., Reference Caffara, Locke, Gustinelli, Marcogliese and Fioravanti2011, Reference Caffara, Bruni, Paoletti, Gustinelli and Fioravanti2014a, Reference Caffara, Davidovich, Falk, Smirnov, Ofek, Cummings, Gustinelli and Fioravantib, Reference Caffara, Locke, Echi, Halajian, Benini, Luus Powell, Tavakol and Fioravanti2017; Sereno-Uribe et al., Reference Sereno-Uribe, Pinacho-Pinacho, García-Varela and Pérez-Ponce de León2013; Locke et al., Reference Locke, Caffara, Marcogliese and Fioravanti2015, Reference Locke, Caffara, Barčák, Sonko, Tedesco, Fioravanti and Li2019; Pinto et al., Reference Pinto, Caffara, Fioravanti and Melo2015; Acosta et al., Reference Acosta, Caffara, Fioravanti, Utsunomia, Zago, Franceschini and da Silva2016; Briosio-Aguilar et al., Reference Briosio-Aguilar, Pinto, Rodríguez-Santiago, López-García, García-Varela and Pérez-Ponce de León2018; Sereno-Uribe et al., Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018).
Metacercariae parasitize a large variety of fish hosts, since they have been found in at least 12 families of freshwater fish: Cichlidae, Percidae, Centrarchidae, Symbranchidae, Eleotridae, Heptapteridae, Profundulidae, Poecilidae, Goodeidae, Characidae, Cyprinidae and Catostomidae (Szidat, Reference Szidat1969; Dias et al., Reference Dias, Eiras, Machado, Souza and Pavanelli2003; Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García Prieto and Mendoza-Garfías2007, Reference Pérez-Ponce de León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016; Gustinelli et al., Reference Gustinelli, Caffara, Florio, Otachi, Wathuta and Fioravanti2010; Caffara et al., Reference Caffara, Locke, Gustinelli, Marcogliese and Fioravanti2011, Reference Caffara, Bruni, Paoletti, Gustinelli and Fioravanti2014a, Reference Caffara, Davidovich, Falk, Smirnov, Ofek, Cummings, Gustinelli and Fioravantib, Reference Caffara, Locke, Echi, Halajian, Benini, Luus Powell, Tavakol and Fioravanti2017; Morais et al., Reference Morais, Varella, Fernandes and Malta2011; Sereno-Uribe et al., Reference Sereno-Uribe, Pinacho-Pinacho, García-Varela and Pérez-Ponce de León2013, Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018; Locke et al., Reference Locke, Caffara, Marcogliese and Fioravanti2015; Pinto et al., Reference Pinto, Caffara, Fioravanti and Melo2015; Acosta et al., Reference Acosta, Caffara, Fioravanti, Utsunomia, Zago, Franceschini and da Silva2016; Davies et al., Reference Davies, Ostrowski de Nuñez, Ramallo and Nieva2016; Briosio-Aguilar et al., Reference Briosio-Aguilar, Pinto, Rodríguez-Santiago, López-García, García-Varela and Pérez-Ponce de León2018).
During investigations on fish parasites from the Espinal and Esteros del Iberá, ecoregions of Argentina, three clinostomid metacercariae were found parasitizing Rachow's Darter Tetra (Characidium rachovii Regan, 1913 (Characiformes: Crenuchidae)), the pike cichlid (Crenicichla vittata Heckel, 1840 (Perciformes: Cichlidae)) and the Argentine humphead (Gymnogeophagus balzanii (Perugia, 1891) (Perciformes: Cichlidae)).
Rachow's Darter Tetra is a freshwater fish that feeds on small invertebrates (Bonetto et al., Reference Bonetto, Roldán and Oliver1978; Casciotta et al., Reference Casciotta, Almirón and Bechara2005; Teixeira de Mello et al., Reference Teixeira de Mello, González-Bergonzoni and Loureiro2011; Bastos et al., Reference Bastos, Miranda and Garcia2013; Ibarra-Polesel & Poi, Reference Ibarra-Polesel and Poi2016). This species is prey to larger fish, such as Hoplias malabaricus Bloch, 1794 and Crenicichla lepidota Heckel, 1840 (Ibarra-Polesel & Poi, Reference Ibarra-Polesel and Poi2016), and birds. The pike cichlid is a benthopelagic freshwater fish that feeds primarily on other fish species (Novakowski et al., Reference Novakowski, Cassemiro and Hahn2016), with a distribution in the Paraná River basin, Argentina (Lucena & Kullander, Reference Lucena and Kullander1992). The Argentine humphead inhabits the Río de la Plata River basin (Casciotta & Gomez, Reference Casciotta and Gomez2000) and feeds on invertebrates from sediments (Wantzen et al., Reference Wantzen, Arruda Machado, Voss, Boriss and Junk2002).
The objectives of this manuscript are to analyse the damage that the parasite inflicts on its hosts through the evaluation of histological sections and to identify Clinostomum metacercariae using a classical morphological approach together with DNA barcode analysis.
Materials and methods
Specimen sampling
Characidium rachovii (fig. 1a); C. vittata (fig. 1b) and G. balzanii (fig. 1c) were collected using different nets and a local fisherman. The Rachow's Darter Tetra was collected (N = 182, total length range 1.2–5 cm) from the coastal vegetation from Ayui River, Entre Ríos Province (fig. 2). Fish were transported alive to the laboratory, and were kept in aquariums. The pike cichlid (N = 10, total length range 25–30 cm) and the Argentine humphead (N = 10, total length range 10–15 cm) were collected from Iberá Lagoon, Corrientes Province (fig. 2). Both cichlid fish were transported to the laboratory after being euthanized and examined for digenean parasites in the field. The euthanasia of all specimens was performed quickly by decapitation following the guidelines of the American Fisheries Society (Nickum et al., Reference Nickum, Bart and Bowser2004).

Fig. 1. Fish host harbouring Clinostomum sp. metacercariae: (a) Characidium rachovii; (b) Crenicichla vittata; (c) Gymnogeophagus balzanii.
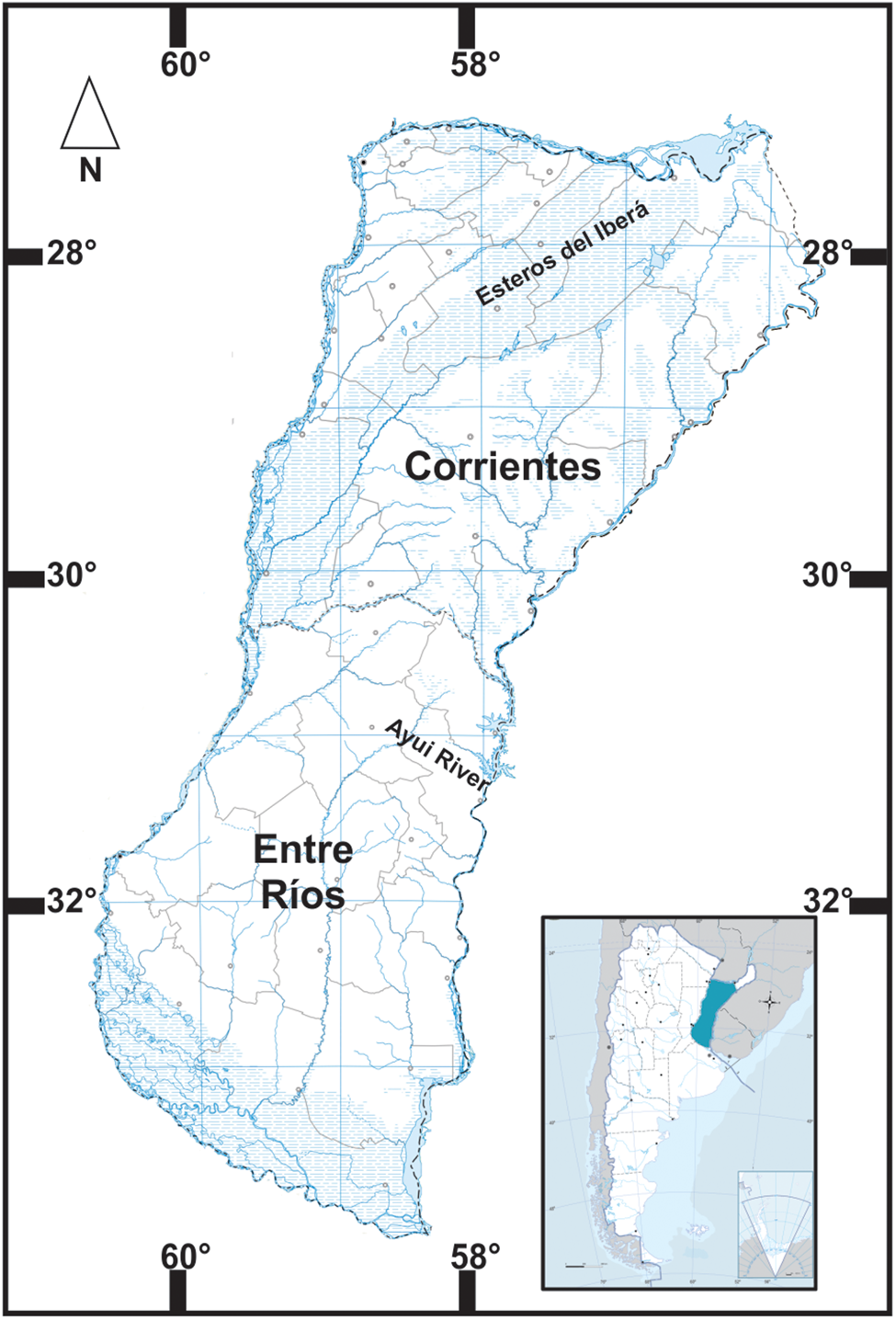
Fig. 2. Sites of collection from Argentina, Ayui River from Concordia (Entre Ríos province) and Iberá Lagoon (Corrientes province).
Morphological analysis
Metacercariae were excysted using dissecting needles under a stereomicroscope, and then fixed in 10% formalin. Metacercariae samples were stained using alcoholic hydrochloric acid-carmine (Pritchard & Kruse, Reference Pritchard and Kruse1982). Measurements and digital images of specimens were obtained using an Olympus BX51 microscope (Tokyo, Japan). Illustrations were made with the aid of a drawing tube. The structures were photographed with an AmScope MU 1000 10 MP digital camera (Tokyo, Japan) attached to an Olympus BX51 microscope and measured using ImageJ software (Schneider et al., Reference Schneider, Rasband and Eliceiri2012). The measurements are given in micrometres (μm). The type material was deposited in the invertebrate collection of Museo de La Plata, Argentina. The mean and the 2.5th and 97.5th percentiles of the distribution of each parameter were calculated with Bayesian statistics using WinBUGS (MRC Biostatistic Unit School of Clinical Medicine, Cambridge Institute of Public Health Forvie Site, Cambridge, UK). This interval was used to represent a 95% Bayesian credible interval. This program was used to generate 100,000 samples from the posterior distributions for each measurement after discarding the initial 10,000 samples as a ‘burn in’. The prior probability distribution used was non-informative. A significance level (α) of 5% or less was considered significant (P ≤ 0.05).
Histological methods
In order to evaluate the histopathological lesions of the infected tissues, sections with encysted metacercariae from the mesentery of C. vittata and muscle of C. rachovii and G. balzanii were fixed in 10% buffered formalin. After dehydration and paraffin embedding, the samples were sectioned at 5 μm and then stained with haematoxylin and eosin (H&E), Masson's trichrome and Giemsa techniques to study the histology and cellular infiltration at the site of encysted metacercariae. The stained slides were observed and photographed under an Olympus CX31 microscope equipped with an Olympus U-CMAD3 camera (Tokyo, Japan).
Molecular data
For the genetic analysis, the total genomic DNA of the whole metacercariae fixed in 96% alcohol was extracted using a Wizard® Genomic DNA Purification Kit (Promega, Madison, Wisconsin, USA) according to the manufacturer's protocol.
A fragment of the partial mitochondrial gene cytochrome oxidase subunit I (COI-mtDNA gene) was amplified from individual worms, and no hologenophore specimens were preserved. Amplification was conducted by polymerase chain reaction (PCR) on an Eppendorf Mastercycler thermal cycler (Hamburg, Germany) using forward primers DICE 1F (5′ –ATT AAC CCT CAC TAA ATT WCN TTR GAT CAT AAG- 3′) and DICE 14R (5′ –TAA TAC GAC TCA CTA TAC CHA CMR TAA ACA TAT GAT G- 3′) as proposed by Van Steenkiste et al. (Reference Van Steenkiste, Locke, Castelin, Marcogliese and Abbott2015). The reactions were made with GoTAQ Master Mix (Promega) according to the manufacturer's protocol. Thermocycling conditions were as follows: 94°C for 2 min; five cycles of 95°C for 30 s, 48°C for 40 s, 72°C for 1 min; followed by a re-amplification of 40 cycles of 94°C for 30 s, 56°C for 40 s, 72°C for 1 min; and a final extension at 72°C for 10 min.
The PCR products were sequenced using an ABI 3730XLs sequencer (Macrogen Inc., Seoul, Korea) and the primers DICE 1F/DICE 14R.
Phylogenetic analysis
Sequences were edited by eye, checking the nucleotide alignment for the presence of pseudogenes using the translated amino acid sequences based on the invertebrate mitochondrial genetic on the platform GENEIOUS 5.1.7 (Kearse et al., Reference Kearse, Moir and Wilson2012). According to the last works on clinostomids (Locke et al., Reference Locke, Caffara, Marcogliese and Fioravanti2015, Reference Locke, Caffara, Barčák, Sonko, Tedesco, Fioravanti and Li2019; Pinto et al., Reference Pinto, Caffara, Fioravanti and Melo2015; Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016; Caffara et al., Reference Caffara, Locke, Echi, Halajian, Benini, Luus Powell, Tavakol and Fioravanti2017; Rosser et al., Reference Rosser, Alberson, Woodyard, Cunningham and Pote2017, Reference Rosser, Baumgartner, Alberson, Noto, Woodyard, King, Wise and Griffin2018; Sereno-Uribe et al., Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018), one or two sequences representing each species or lineages deposited in the GenBank database were selected. Barcode fragment alignments were assembled using MAFFT version 7 (Katoh & Standley, Reference Katoh and Standley2013). The best partitioning scheme and substitution model for each DNA partition was chosen under the Bayesian information criterion (Schwarz, Reference Schwarz1978) using the ‘greedy' search strategy in Partition Finder version 1.1.1 (Lanfear et al., Reference Lanfear, Calcott, Ho and Guindon2012). The barcode fragment dataset was partitioned into first-, second- and third-codon positions with the appropriate nucleotide substitution model implemented for each codon position (TrN + I + G for the first and third codon position (Tamura & Nei, Reference Tamura and Nei1993); and K81uf for the second codon position (Kimura, Reference Kimura1981).
The phylogenetic reconstruction was carried out using Bayesian inference through MrBayes version 3.2.1 (Ronquist et al., Reference Ronquist, Teslenkovan and van der Mark2012). The phylogenetic trees were reconstructed using two parallel analyses of Metropolis-coupled Markov chain Monte Carlo for 20 × 106 generations each to estimate the posterior probability distribution. Topologies were sampled every 1000 generations. The first 25% of the sampled trees were discarded as ‘burn in’. Additionally, the proportion (p) of absolute nucleotide sites (p-distance) (Nei & Kumar, Reference Nei and Kumar2000) was obtained to compare the genetic distance among and between lineages, with and without outgroups. The P-value matrix was obtained using MEGA version 6.0 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013) with variance estimation, using the bootstrap method (500 replicates) and with a nucleotide substitution (transition + transversions) uniform rate. The final trees were visualized in FigTree software version 1.4.3 (Rambaut, Reference Rambaut2014).
The six sequences obtained were deposited in GenBank under the accession numbers MF673556–MF675361 (supplementary table S1).
Results
Morphological data
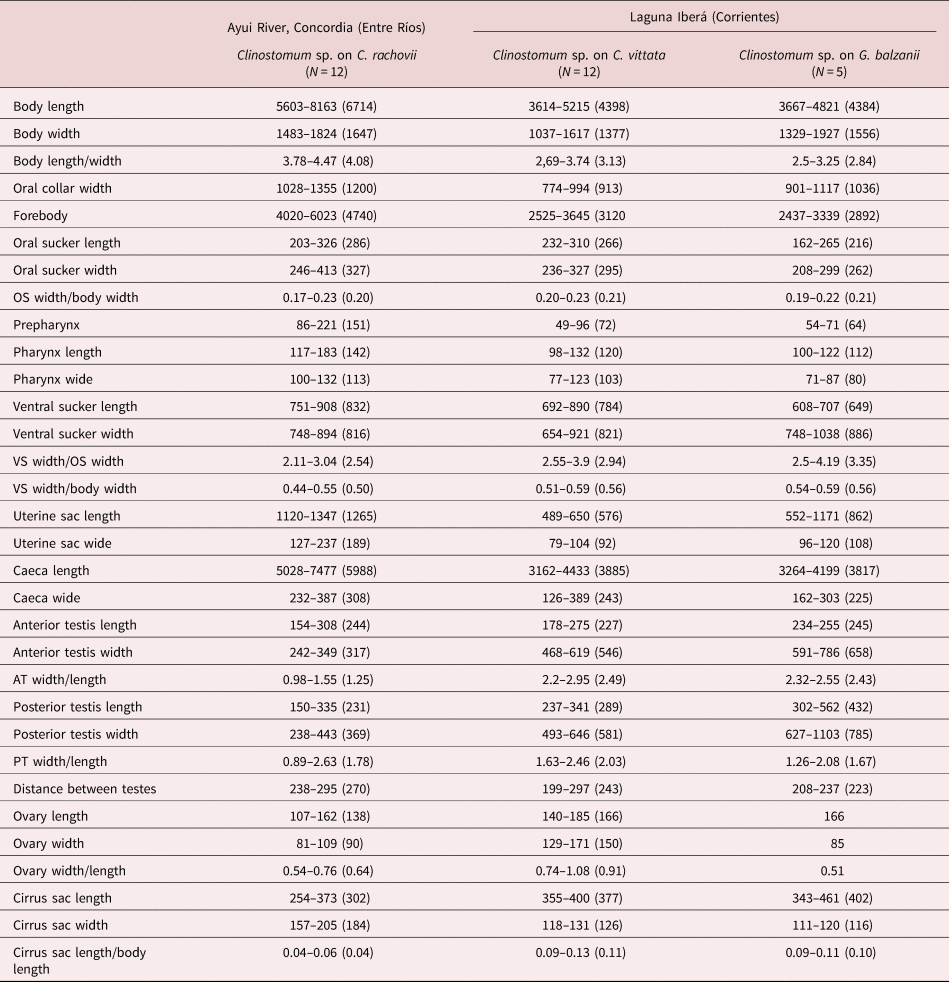
The morphological characteristics of the Clinostomum metacercariae on C. rachovii, C. vittata and G. balzanii (Clinostomum Cra, Cvi and Gba, respectively) (fig. 3 and supplementary fig. S1) were apparently similar. The measurements are presented in table 1.

Fig. 3. Clinostomid metacercaria on (a) Characidium rachovii; (b) Crenicichla vittata; (c) Gymnogeophagus balzanii. Abbreviations: At, Anterior testis; C, caecum; Cs, cirrus sac; O, ovary; Oc, oral collar; Oo, ootype; Os, oral sucker; P, pharynx; Pt, posterior testis; Vs, ventral sucker; Us, uterus.
Table 1. Measurements of Clinostomum sp. metacercariae parasites on Characidium rachovii, Crenicichla vittata and Gymnogeophagus balzanii.
Clinostomum sp. Cra
Taxonomic summary
Host. Characidium rachovii Regan, 1913.
Locality. Ayui River (Concordia), 31°16′38″S, 58°0′5″W, Entre Ríos Province, Argentina.
Specimens deposited. MLP-He 7623.
Representative DNA sequences. MF673556–MF673557.
Description (based on 12 specimens) (fig. 3a). Body elongated, devoid of spines, flattened anterior end with oral collar. Oral sucker subterminal, rounded, smaller than the ventral sucker. Pharynx short. Intestinal caeca lateral to ventral sucker and genital primordium to posterior end of body. Intestinal wall diverticulated. Ventral sucker 2–3 times larger than oral sucker, with rounded, almost triangular opening. Testes slightly triangular, with the base facing each other and smooth, concave. Anterior testis mostly rounded, almost oval, and posterior testis more elongated. Apex posterior testis directed to the posterior end, poorly defined and irregular margin. Cirrus sac, kidney-shaped, in right margin of anterior testis, opening into genital atrium. Ovary small, oval, intertesticular and dextrally. Uterine sac tubular between genital complex and ventral sucker.
Molecular data
The fragments of the partial COI mtDNA gene from Clinostomum sp. Cra measured 570 and 588 bp. The BLASTN analysis of those sequences shows a similarity with Clinostomum sp7 and Clinostomum L1 (94–96 and 84%, respectively).
In the phylogram (fig. 4), the sequences of Clinostomum Cra were grouped with Clinostomum L1 (reported by Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016) and Clinostomum sp7 reported by Pinto et al. (Reference Pinto, Caffara, Fioravanti and Melo2015). Clinostomum L1 was found in Honduras and Mexico (Middle America), and Clinostomum sp7 was recorded in Brazil (South America).

Fig. 4. Phylogenetic position of the new lineages of Clinostomum sp. Values above branches represent Bayesian posterior probavility values higher than 95%. Abbreviations: Clinostomum sp1–sp8 (sensu Locke et al., Reference Locke, Caffara, Marcogliese and Fioravanti2015), Clinostomum L1–L5 (sensu Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016), Clinostomum M1–M4 (sensu Caffara et al., Reference Caffara, Locke, Echi, Halajian, Benini, Luus Powell, Tavakol and Fioravanti2017). Clinostomum sp. Cra on Characidium rachovii; Clinostomum sp. Cvi on Crenicichla vittata; and Clinostomum sp. Gba on G. balzanii. Scale bar shows number of subtitutions per site.
The genetic distance between Clinostomum sp. Cra and the Clinostomum sp. from the Old World is (range, mean ± standard deviation) 16–19 (17 ± 1)%, and from the New World is 5–18 (15 ± 3)% (supplementary table S2). The closest species to Clinostomum Cra is Clinostomum L1, with a p-distance of 5% (table 2).
Table 2. Interspecific genetic distance (expressed in percentage) among Clinostomid metacercariae found in the present study against species and lineages of the world.

album, Clinostomum album; arq, Clinostomum arquus; AT, anterior testis; atte, Clinostomum attenuatum; brieni, Clinostomum brieni; cafa, Clinostomum caffarae; cf_marg, Clinostomum cf. marginatum; cichli, Clinostomum cichlidorum; comp, Clinostomum complanatum; Cra, Clinostomum sp. on Characidium rachovii; cuta, Clinostomum cutaneum; Cvi, Clinostomum sp. on Crenicichla vitatta; dent, Clinostomum detruncatum; E, Euclinostomum sp.; E_het, Euclinostomum heterostomum; Gba, Clinostomum sp. on Gymnogeophagus balzanii; heluans, Clinostomum heluans; L1 and L3, Clinostomum sp. sensu Pérez-Ponce de León et al. (Reference Pérez-Ponce de León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016); M1–M4, Clinostomum sp. sensu Caffara et al. (Reference Caffara, Locke, Echi, Halajian, Benini, Luus Powell, Tavakol and Fioravanti2017); marg, Clinostomum marginatum; OS, oral sucker; phala, Clinostomum phalacrocoracis; phil, Clinostomum philippinensis; potae, Clinostomum potae; PT, posterior testis; sp1, sp2, sp4, sp5, Clinostomum sensu Locke et al. (Reference Locke, Caffara, Marcogliese and Fioravanti2015); sp7, sensu Pinto et al. (Reference Pinto, Caffara, Fioravanti and Melo2015); sin, Clinostomum sinensis; tata, Clinostomum tataxumui; tila, Clinostomum tilapiae; VS, ventral sucker.
Taxonomic remarks
The Clinostomum sp. Cra can be compared with the genetically closest species, Clinostomum L1 (parasite on Rhamdia sp. Bleeker described by Sereno-Uribe et al. (Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018)) and Clinostomum sp7 (parasite on Poecilia reticulata Peters, 1859).
The Clinostomum sp. Cra specimens found have similar measurements to Clinostomum L1, but they differ in several features. The integument of Clinostomum L1 is covered with spines that are absent in Clinostomum sp. Cra. The oesophageal bulb is inconspicuous in Clinostomum L1, but conspicuous in Clinostomum sp. Cra. In Clinostomum L1, the intestinal caeca have irregular margins from the posterior edge of the ventral sucker to the posterior body end, whereas in Clinostomum sp. Cra the intestinal caeca have irregular margins anterior and posterior to the ventral sucker towards the anterior and posterior body ends. Compared to Clinostomum sp7, Clinostomum sp. Cra has greater body length, oral sucker width, ventral sucker length and width, anterior and posterior testes and cirrus sac is longer.
Davies et al. (Reference Davies, Ostrowski de Nuñez, Ramallo and Nieva2016) and Lunaschi & Drago (Reference Lunaschi and Drago2009) described Clinostomum sp. metacercariae and immature specimens, respectively, from Argentina only using morphological analysis. The body length, cirrus sac width and distance between the anterior and posterior testes are greater in Clinostomum sp. Cra than in the two Clinostomum sp. metacercariae found parasitizing Hoplosternum littorale Hanckock, 1828 (Siluriformes, Callichthyidae) and Trigonectes spp. Myers, 1925 (Cyprinodontiformes, Rivulidae). Clinostomum sp. Cra has a larger body, ventral sucker, greater ovary width and smaller anterior testis compared with the immature specimens identified by Lunaschi & Drago (Reference Lunaschi and Drago2009) as C. marginatum. Additionally, C. marginatum have long caeca with small lateral diverticula in the last quarter of the body, a feature not observed in our specimens.
Pathological analysis
Specimens of C. rachovii appeared in a lethargic state near the riverside; they showed macroscopic whitish-looking cysts localized mainly in the axial musculature and with raised scales around the cyst area. The histological analysis revealed that metacercariae were invading the muscle tissue (fig. 5a) and were encapsulated by a thin fibrotic tissue layer of fibroblast-like cells with abundant blood capillaries (fig. 5b). Although the underlying tissue appeared normal, an interruption of the muscle tissue was observed and its fibres had undergone a rearrangement around the capsule of the cyst. Also, in the muscle tissue, vascular congestion areas and the infiltration of numerous inflammatory cells, mainly lymphocytes (figs 5c, d), were observed.

Fig. 5. Musculature axial of Characidium rachovii infected with Clinostomum metacercariae. (a) General view of the cysts with metacercaria (asterisks) within the musculature axial, Masson's trichrome. (b) Capsule of a thin fibrotic tissue (arrow) with abundant blood capillaries (stars), Masson's trichrome. (c) Infiltration of inflammatory cells (arrow) in the musculature that surrounded to the cyst, H&E. (d) Detail of the vascular congestion areas (arrows) in the muscle tissue, H&E. Abbreviations: M, musculature; P, parasite.
Clinostomum sp. Cvi
Taxonomic summary
Host. Crenicichla vittata Heckel, 1840.
Locality. Iberá Lagoon, 28°32′12″S, 57°10′17″W, Corrientes Province, Argentina.
Specimens deposited. MLP-He 7624.
Representative DNA sequences. MF673558–MF673559.
Description (based on 12 specimens) (fig. 3b). Body elongated, flattened anterior end with oral collar. Integument smooth. Oral sucker subterminal, rounded, smaller than the ventral sucker. Pharynx short, close to oral sucker. Intestinal caeca, lateral to ventral sucker and genital complex, reaching the end of body, with small diverticula along the wall. Ventral sucker with triangular opening. Testes, slightly triangular, irregular, with the base facing each other and slightly concave. Cirrus sac, kidney-shaped, overlapping right margin of the anterior testis opening into genital atrium. Ovary oval, dextral, between testes. Uterine sac tubular and not reaching ventral sucker.
Molecular data
The fragments of the partial COI mtDNA gene from the Clinostomum sp. Cvi measured 561 and 570 bp. The BLASTN analysis of those sequences show similarity (98%) with Clinostomum sp2 and sp1 on Sicydium salvini Ogilvie-Grantand, 1884 and Rhamdia guatamensis from Mexico reported by Locke et al. (Reference Locke, Caffara, Marcogliese and Fioravanti2015).
In the phylogram (fig. 4), the sequences of Clinostomum sp. Cvi grouped with species from South and Middle America.
The genetic distance between Clinostomum sp. Cvi and the Clinostomum sp. from the Old World is 14–18 (16 ± 1)%, and from the New World is 2–18 (12 ± 5)% (supplementary table S2). The closest species to Clinostomum sp. Cvi are Clinostomum sp1, sp2 and L3, with a p-distance of 2, 3 and 5%, respectively. Between the new species reported here, the most similar to Clinostomun sp. Cvi is Clinostomum sp. Gba, with a genetic distance of 5% (table 2).
Taxonomic remarks
The Clinostomum sp. Cvi can be compared with the genetically closest species, Clinostomum arquus Sereno-Uribe et al., Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018, Clinostomum caffarae Sereno-Uribe et al., Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018, Clinostomum cichlidorum Sereno-Uribe et al., Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018, Clinostomum tataxumui Sereno-Uribe et al., Reference Sereno-Uribe, Pinacho-Pinacho, García-Varela and Pérez-Ponce de León2013, Clinostomum L3 sensu Sereno-Uribe et al. (Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018), Clinostomum sp1, sp2, sp4, sp5 sensu Locke et al. (Reference Locke, Caffara, Marcogliese and Fioravanti2015) and Clinostomum sp. Gba (reported below). Unfortunately, the metacercariae reported by Locke et al. (Reference Locke, Caffara, Marcogliese and Fioravanti2015) were sequenced, but a morphological approach was not applied.
Clinostomum sp. Cvi specimens have triangular testes, three-lobed with irregular margins, whereas in C. arquus the anterior and posterior testes are smooth and rounded, in C. caffarae the anterior testis has smooth margins with irregularly shape and in C. tataxumui the testes have smooth margins and the posterior testis appears triangular (according to the drawing provided by Sereno-Uribe et al. (Reference Sereno-Uribe, Pinacho-Pinacho, García-Varela and Pérez-Ponce de León2013)). Also, the anterior testis of C. cichlidorum metacercariae is displaced to the right side of the body, but is not displaced in Clinostomum sp. Cvi.
In the metacercariae of C. arquus and C. caffarae, the intestinal caeca are smooth, while in C. tataxumui they have slightly indented margins. Clinostomum L3 have diverticulated margins from the posterior margin of the ventral sucker to the posterior body end; whereas, in Clinostomum sp. Cvi, the intestinal caeca are highly irregular. In C. cichlidorum the intestinal caeca have diverticula more prominent from the ventral sucker to the posterior body end, but those are absent in Clinostomum sp. Cvi.
The two metacercariae described by Davies et al. (Reference Davies, Ostrowski de Nuñez, Ramallo and Nieva2016), despite the similarities they share, both have smaller cirrus sac length compared with Clinostomum sp. Cvi. The immature specimens of C. marginatum have a larger anterior testis, cirrus sac width and narrow ovary compared with Clinostomum sp. Cvi. In addition, the immature specimens of C. marginatum have long caeca with small lateral diverticula in the last quarter of the body, but the lateral diverticula in our specimens are in all the length of the body.
Pathological analysis
Specimens of C. vittata showed no obvious macroscopic signs. When performing the inspection in search of parasites, cysts with encapsulated metacercariae were found in two body sites. At the first site, in the palate, the digenean was found in the fascia of the muscle tissue (fig. 6a), and leukocyte infiltration was also observed (fig. 6b). The other cyst was found in the mesentery (fig. 6c); it was also surrounded by its capsule but, unlike in the other cases, leukocyte infiltration was not abundant, and in the connective tissue some desquamation of cells and the occurrence of pigment granules could be observed (fig. 6d).

Fig. 6. Cysts with Clinostomum metacercariae in Crenicichla vittata infected. (a) General view of the cyst with metacercaria (asterisk) encapsulated into the fascia of the muscle tissue, with abundant leukocyte infiltration and desquamation cells (arrow), H&E. (b) Leukocyte infiltration in the fascia, H&E. (c) Metacercaria cyst (asterisk) in the mesentery with infiltration of lymphocytes and desquamation cells (arrows), Giemsa. (d) Connective tissue with lymphocytic infiltration, abundant cellular detritus and pigment granules (stars), Masson's trichrome and H&E. M, musculature.
Clinostomum sp. Gba
Taxonomic summary
Host. Gymnogeophagus balzanii (Perugia, 1891).
Locality. Iberá Lagoon, 28°32′12″S, 57°10′17″W, Corrientes Province, Argentina.
Specimens deposited. MLP-He 7625 (one specimen).
Representative DNA sequences. MF673560– MF673561.
Description (based on four specimens) (fig. 3c). Body elongated, flattened anterior end with oral collar. Integument without spines. Oral sucker subterminal, rounded, smaller than the ventral sucker. Pharynx short, close to oral sucker. Intestinal caeca with diverticulated margins, evident in post acetabular regions. Caeca lateral to the ventral sucker and genital complex, reaching end of body. Ventral sucker with triangular opening. Testes slightly triangular, irregular margin, with the base facing each other and slightly concave. Cirrus sac, kidney-shaped, in overlapping right margin on the anterior testis, opening into genital atrium. Ovary small, oval, dextral and intertesticular. Uterine sac tubular, and overlapped with the posterior end of the ventral sucker.
Molecular data
The fragments of the partial COI mtDNA gene from the Clinostomum sp. Gba measured 564 and 579 bp. The BLASTN analysis of those sequences show a similarity with Clinostomum sp2 and sp1 (98%) of S. salvini and R. guatamensis from Mexico reported by Locke et al. (Reference Locke, Caffara, Marcogliese and Fioravanti2015).
In the phylogram (fig. 4), the sequences of Clinostomum sp. Gba were grouped with species from South and Middle America. The closest species are Clinosotmum sp1, sp2, sp4, L3 and Cvi.
The genetic distance between Clinostomum sp. Gba and the Clinostomum sp. from the Old World is 12–17 (16 ± 2)%, and from the New World is 5–18 (12 ± 4)% (supplementary table S2). Between the new species reported here, the most similar to Clinostomun sp. Gba is Clinostomum sp. Cvi, with a genetic distance of 5%. The other closest species to Clinostomum sp. Gba are Clinostomum sp1, sp2 and L3, with a p-distance of 5, 7 and 7%, respectively (table 2).
Taxonomic remarks
In our study, the closest metacercaria to Clinostomum sp. Gba is Clinostomum sp. Cvi, not only in the phylogram, but also in their morphology. The main differences between them are the size of the oral sucker, which is larger in Clinostomum sp. Cvi, and the posterior testis, which is larger in Clinostomum sp. Gba.
Pathological analysis
Specimens of G. balzanii also showed macroscopic cysts in the axial musculature of the trunk and tail. The inflammatory response showed characteristics similar to those described for C. rachovii, but leucocyte infiltration was more abundant (fig. 7a). The muscle tissue where the metacercariae were attached by the oral sucker showed signs of damage and was replaced by a thick layer of connective tissue arranged concentrically (fig. 7b). In areas near the cyst, dilated lymphatic vessels with infiltration of lymphocytes and disruption of the muscle tissue occurred (fig. 7c). In addition, in G. balzanii, an onset of melanosis was observed; numerous melanophores invaded the connective tissue (fig. 7d), possibly in response to the presence of the metacercariae.

Fig. 7. Musculature axial of Gymnogeophagus balzanii infected with Clinostomum metacercariae. (a) General view of muscle tissue showing a metacercaria with oral sucker (asterisks) and abundant leucocyte infiltration with numerous melanophores (arrows), H&E. (b) Detail of the oral sucker (asterisk) – note the thick layer of connective tissue arranged concentrically within the muscle tissue (arrow), H&E. (c) Dilated lymphatic vessels with infiltration of lymphocytes (arrow), H&E. (d) Leukocyte infiltration in the muscle tissue, mainly lymphocytes and numerous melanophores, H&E. M, musculature.
Discussion
The three metacercariae under study represent new lineages, different from the ones known until now in South America and the world (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García Prieto and Mendoza-Garfías2007, Reference Pérez-Ponce de León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016; Gustinelli et al., Reference Gustinelli, Caffara, Florio, Otachi, Wathuta and Fioravanti2010; Caffara et al., Reference Caffara, Locke, Gustinelli, Marcogliese and Fioravanti2011, Reference Caffara, Bruni, Paoletti, Gustinelli and Fioravanti2014a, Reference Caffara, Davidovich, Falk, Smirnov, Ofek, Cummings, Gustinelli and Fioravantib, Reference Caffara, Locke, Echi, Halajian, Benini, Luus Powell, Tavakol and Fioravanti2017; Morais et al., Reference Morais, Varella, Fernandes and Malta2011; Sereno-Uribe et al., Reference Sereno-Uribe, Pinacho-Pinacho, García-Varela and Pérez-Ponce de León2013, Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018; Locke et al., Reference Locke, Caffara, Marcogliese and Fioravanti2015; Pinto et al., Reference Pinto, Caffara, Fioravanti and Melo2015; Acosta et al., Reference Acosta, Caffara, Fioravanti, Utsunomia, Zago, Franceschini and da Silva2016; Davies et al., Reference Davies, Ostrowski de Nuñez, Ramallo and Nieva2016; Briosio-Aguilar et al., Reference Briosio-Aguilar, Pinto, Rodríguez-Santiago, López-García, García-Varela and Pérez-Ponce de León2018). The p-distance supports the independence from all the other sequences, confirming their status as new lineages or species.
In keeping with the previous works based on molecular analysis (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García Prieto and Mendoza-Garfías2007, Reference Pérez-Ponce de León, García-Varela, Pinacho-Pinacho, Sereno-Uribe and Poulin2016; Gustinelli et al., Reference Gustinelli, Caffara, Florio, Otachi, Wathuta and Fioravanti2010; Caffara et al., Reference Caffara, Locke, Gustinelli, Marcogliese and Fioravanti2011, Reference Caffara, Bruni, Paoletti, Gustinelli and Fioravanti2014a, Reference Caffara, Davidovich, Falk, Smirnov, Ofek, Cummings, Gustinelli and Fioravantib, Reference Caffara, Locke, Echi, Halajian, Benini, Luus Powell, Tavakol and Fioravanti2017; Sereno-Uribe et al., Reference Sereno-Uribe, Pinacho-Pinacho, García-Varela and Pérez-Ponce de León2013, Reference Sereno-Uribe, García-Varela, Pinacho-Pinacho and Pérez-Ponce de León2018; Locke et al., Reference Locke, Caffara, Marcogliese and Fioravanti2015, Reference Locke, Caffara, Barčák, Sonko, Tedesco, Fioravanti and Li2019; Pinto et al., Reference Pinto, Caffara, Fioravanti and Melo2015; Acosta et al., Reference Acosta, Caffara, Fioravanti, Utsunomia, Zago, Franceschini and da Silva2016; Briosio-Aguilar et al., Reference Briosio-Aguilar, Pinto, Rodríguez-Santiago, López-García, García-Varela and Pérez-Ponce de León2018), our results confirm the differentiation into two groups of clinostomids, one from the New World and another from the Old World. Our analysis complements the information about the clinostomids around the world, by reporting the presence of three new lineages from South America and the first molecular analysis of species from Argentina. But, further studies in South America must be encouraged. If we observe the phylogram, sub-nodes inside the New World start to differentiate: one from Middle America, another from North America and others in South America, with a few species occurring all throughout the Americas. We hope that in the future, new species and sequences could shed light on this incipient arrangement of the species and prove it or disprove it.
The identification of the Clinostomum sp. reported from Argentina until now was only based on morphology. The specimens reported by Lunaschi & Drago (Reference Lunaschi and Drago2009) as C. marginatum should be studied taking into consideration the new information and analysed based on an integrative analysis.
Clinostomum complanatum is a species found only in the Old World (Locke et al., Reference Locke, Caffara, Marcogliese and Fioravanti2015), and the record of this parasite by Lunaschi et al. (Reference Lunaschi, Cremonte and Drago2007) in specimens collected in La Balandra, Buenos Aires, and deposited in the helminthological collection of the Museo de La Plata could be a misidentification. Clinostomum detruncatum was found in Argentina (Buenos Aires Province) by Boero & Led (Reference Boero and Led1971), but only adult specimens were reported. These species were not included in the comparison because the metacercariae described by Acosta et al. (Reference Acosta, Caffara, Fioravanti, Utsunomia, Zago, Franceschini and da Silva2016) are genetically distant from the metacercariae under study.
In light of our discovery of three new metacercariae with two different lineages from the same collection site (Iberá Lagoon), we believe that perhaps the clinostomid metacercariae reported until now in Argentina that were identified only by morphology should be studied by molecular methods to be able to determine the real biodiversity of the Clinostomidae family in Argentina.
The results of the histopathological analysis of the metacercariae found in C. rachovii, C. vittata and G. balzanii were similar: in all three cases, the most affected tissue was the muscle. Several researchers have studied clinostomid metacercariae (Kalantan et al., Reference Kalantan, Arfin and Nizami1987; Adeyemo & Agbede, Reference Adeyemo and Agbede2008; Purivirojkul & Sumontha, Reference Purivirojkul and Sumontha2013) and they have reported that this parasite causes damage to the viscera and musculature. As in other infections by digeneans described by Bullard & Overstreet (Reference Bullard, Overstreet, Eiras, Segner, Wahil and Kapoor2008), clinostomid cysts were surrounded by a connective tissue capsule and an evidently severe mononuclear inflammatory response. This infiltration was mainly composed of lymphocytes and macrophages that expanded to the adjacent musculature, as has also been observed by Shamsi et al. (Reference Shamsi, Halajian, Tavakol, Mortazavi and Boulton2013) in infected piscivorous birds. According to Roca et al. (Reference Roca, Sepulcre, Lopez-Castejon, Sarropoulou and Kotoulas2007), the recruitment of lymphocytes suggests a role in the regulation of early immune responses to infection. Ferguson (Reference Ferguson1989) has observed that the presence of metacercariae may or may not induce a melanin response. In the cases presented in this study, only in G. balzanii an onset of melanosis was observed, with melanophores invading the connective tissue close to the musculature.
In conclusion, in the present work we propose the presence of three new lineages, different from those reported from Middle America or even the rest of the world. The only remaining doubts arise in the comparisons made with the clinostomid metacercariae reported from Argentina, as these lack genetic studies. This could lead to the discovery of a large number of lineages or species of Clinostomum from South America. Besides, despite the variety of organs infected by the clinostomid metacercariae, it is evident that the most affected one is the muscle tissue. It could be of interest to study and correlate the effect of the parasite load on the host's mobility, its response to predators or search for food.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X20000292
Acknowledgements
We want to thank the Facultad de Ciencias Naturales y Museo (UNLP), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Centro de Estudios Parasitológicos y Vectores for providing the infrastructure necessary to carry out this work; to the Cátedra de Zoología III Vertebrados and the Corrientes province for providing the sampling permissions; to Jorge Casciotta and Adriana Almiron for the photos of Crenicichla vittata and Gymnogeophagus balzanii; to Sebastián Preisz for the picture of Characidium rachovii; and to Marcia Montes for the line drawings. We are very grateful to the reviewers for their critical comments and thoughtful suggestions, and, last but not least, to Carlos Romero and Patricia Tedesco for correcting the English.
Financial support
This work was supported by CONICET-CCT La Plata (grant number PIP 0015) to SR Martorelli.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.











