Introduction
Leaf weevils of the genera Phyllobius and Polydrusus (Coleoptera Curculionidae) are frequently associated with defoliation of young broadleaved trees (Savic, Reference Savic1963; Axelsson et al., Reference Axelsson, Larsson, Lohm and Persson1973; Witter & Fields, Reference Witter and Fields1977; Annila, Reference Annila1979). The adults of several species feed during the spring on the foliage of a wide variety of tree species, and high population densities can cause considerable damage to new plantings, especially in farm woodlands (Bevan, Reference Bevan1987). The larvae, in contrast, live below ground and are rhizophagous (Bevan, Reference Bevan1962; Ioannisiani et al., Reference Ioannisiani, Lavrova and Birg1970a; Axelsson et al., Reference Axelsson, Larsson, Lohm and Persson1973; Kula, Reference Kula, McManus and Liebhold2003). All are univoltine and over-winter either as larvae or pupae (Morris, Reference Morris1997).
One of the most common and widespread of these leaf weevils in the UK is Phyllobius pyri (L.) (Bevan, Reference Bevan1987; Morris, Reference Morris1997). This species is regularly reported as causing damage to young trees. Out of 133 enquiries concerning damage by Phyllobius received by the advisory services of UK Forestry Commission between 1953 and 2008, 92 enquiries (69%) related to P. pyri. The main tree species affected were oak, cherry, beech and birchFootnote 1. Reports of damage typically involved trees 1–10 years old, especially those planted on previously arable land or in grass leys or pasture. Defoliation in these situations raises particular concerns, because of the interest in planting broadleaved woodlands on farmland to diversify land use and to meet conservation and environmental objectives (Hibberd, Reference Hibberd and Hibberd1988; Williamson, Reference Williamson1992).
Even though adult Phyllobius are polyphagous, they are suspected of having particular host preferences, which are likely to be reflected in some tree species being more susceptible to damage than other species. However, the feeding preferences of most Phyllobius species are unknown. Phyllobius pyri has been observed feeding on many different broadleaved tree and shrub species, and occasionally on herbaceous species (Roginskaya, Reference Roginskaya1966; Axelsson et al., Reference Axelsson, Larsson, Lohm and Persson1973; Nielsen, Reference Nielsen1978; Annila, Reference Annila1979; Phillips, Reference Phillips1992; Marko et al., Reference Marko, Merkl, Podlussany, Vig, Kutasi and Bogya1995; Rougon et al., Reference Rougon, Roques, Rougon and Levieux1995; Morris, Reference Morris1997), but its preferences and performance on different hosts have not been established.
The host plants and habitat requirements of the larvae of most Phyllobius species, especially those associated with trees, are also largely unknown. Larvae of P. pyri have been found in open grassland, sometimes causing extensive damage to the grass sward, and it seems that they feed on the roots of grasses and also perhaps on the roots of herbaceous plants (Bevan, Reference Bevan1962; Axelsson et al., Reference Axelsson, Larsson, Lohm and Persson1973; Hill, Reference Hill1973; Nielsen, Reference Nielsen1978; Annila, Reference Annila1979; Lerenius & Jansson, Reference Lerenius and Jansson1995). However, larvae of other Phyllobius species (P. arborator Herbst, P. argentatus, P. maculicornis and P. oblongus) probably feed on the fine roots of trees (Vollman, Reference Vollman1954; Savic, Reference Savic1963; Ioannisiani et al., Reference Ioannisiani, Birg and Lavrova1970b).
The limited information on feeding preferences and performance of adult P. pyri, and on larval food plants and habitats, means that it is currently very difficult to predict when and where this species is most likely to cause damage. This study was undertaken to clarify the host preferences and habitat associations of adult P. pyri to indicate which tree species are most at risk and the situations where trees are most likely to be defoliated. The study was carried out over three years and included assessments of adult activity, population densities and parasitism in the field, and laboratory experiments on feeding preference, longevity and fecundity.
Materials and methods
Field activity and population densities
Field studies were carried out at the Forestry Commission research station at Alice Holt, near Farnham, Surrey, UK (Latitude: 51°11'N; Longitude: 0°51'W). The field station and the area immediately surrounding it provide a range of grassland and woodland habitats, from formal lawns to parkland, small grass fields and other enclosures set within a larger area of mixed woodland. The soils at Alice Holt comprise slowly permeable, seasonally waterlogged gravelly or relatively stone free sandy loams overlying Cretaceous sands and clays, at around 120 m above sea level (Jarvis et al., Reference Jarvis, Allen, Fordham, Hazelden, Moffat and Sturdy1984). These soils develop naturally a neutral to acidic grassland community dominated by Agrostis capillaris L., Holcus lanatus L., Anthoxanthum odoratum L., Festuca rubra L. and Arrhenatherum elatius (L.) P. Beauv.
Adult P. pyri were captured using emergence traps, flight interception traps and by beating foliage. Emergence traps provided an absolute estimate of adult population density in terms of numbers per m2 and indicated larval habitat requirements. They consisted of an up-turned dark green, plastic bowl, measuring 30×32 cm (0.096 m2), secured firmly to the ground with four metal pegs, with a clear plastic collecting pot (8.2×6.5 cm) attached toward the top on one side (Billiald, Reference Billiald2005). Adult weevils emerging from the soil beneath the trap congregated in the collecting pot as they moved toward the light.
Flight interception traps provided information on adult activity and, to some extent, larval habitat requirements, although information on the latter was less precise because of the free-ranging nature of adult dispersal. The traps were made from two pieces of transparent, 2-mm thick styrene sheet, measuring 30×16 cm, fixed together vertically along their centre line to form two upright panels at 90° to each other. A plastic funnel and screw-top pot containing 70% industrial methylated spirits (IMS) were attached below the panels to catch flying insects that dropped after they hit the interception surface (Billiald, Reference Billiald2005). A transparent, conical roof, made from 1-mm thick styrene and 30 cm in diameter, was fixed to the top of the trap to exclude rainwater. Traps were suspended from a horizontal bar attached to the top of an upright wooden stake, with the interception panels positioned 1.0–1.3 m above the ground.
Beating was used to collect P. pyri adults from the foliage of various tree species. Sampling was restricted to foliage growing at a reachable height (0.5–1.5 m above ground level), and samples were taken by striking a branch once with an 80 cm wooden stick over an 80×80 cm white tray. Weevils falling onto the tray were collected and either stored in 70% IMS or kept alive for use in laboratory and nursery experiments.
In 2002, emergence traps and flight interception traps were used to sample adult P. pyri at 12 sites representing closed canopy woodland with no ground flora (sites 1–4), young tree plantations with grass between and beneath the canopy (sites 5–8) and open grassland (sites 9–12). Sites 7–12 were also sampled in 2003 and 2004, along with six additional grassland sites (sites 13–18). Twelve emergence traps and four flight interception traps were used at each site. The emergence traps were placed 2.5 m apart in a 4×3 grid, and the flight interception traps were positioned 5 m apart in a line running through the centre of the emergence trap grid. Both types of trap were emptied every three days from early April to the end of June.
The species composition and percentage cover of the ground vegetation at each site was determined using point quadrat sampling (Southwood & Henderson, Reference Southwood and Henderson2000). A metal pin, 1.5 m tall and 5 mm diameter, was positioned vertically within a 10×10 m sampling area adjacent to the trap grid, using random numbers to generate a reference point. Every plant species touching the pin was recorded, with multiple touches by the same species recorded only once. Ninety samples were taken at each site. Grass species were identified using Hubbard (Reference Hubbard1968) and herbs using Stace (Reference Stace2001).
Beating foliage provided information on adult phenology and relative abundance on different tree species. Beating samples were taken every week from the beginning of April to the end of June, with 20 branches (each from a different tree) sampled per tree species on each date. In 2002, samples were taken from beech, birch, cherry, hawthorn and oak. In 2003 and 2004, the number of sites was reduced to just those with beech, birch and hawthorn.
Flight capacity and reproductive development
All field-caught P. pyri were measured and dissected to determine the condition of the flight muscles, the sex of the adult and reproductive development. Flight muscle development was measured in terms of the length, width and appearance of the thoracic dorsal longitudinal muscle (DLM). This muscle is the main wing depressor and full development is essential for sustained flight (Danthanarayana, Reference Danthanarayana1970; Wigglesworth, Reference Wigglesworth1974).
The reproductive system of male and female P. pyri is very similar to that of Phyllobius oblongus, which is described in detail by Vollman (Reference Vollman1954). Development of the reproductive organs was assessed by measuring the length and diameter of the germaria and vitellarium of the ovaries in females, and the length, width and follicle diameter of the testes in males. Variation in the size of the reproductive organs amongst adults caught in the emergence and flight traps was analysed using general linear regression.
Adult longevity and fecundity
Longevity and fecundity were determined by rearing adult P. pyri in the laboratory. Newly emerged adults were obtained from pupae produced during nursery experiments that were being used to investigate larval host plants (Billiald, Reference Billiald2005).
For the first seven days, adults were reared together in clear polystyrene boxes lined with damp filter paper and were provided with an ample supply of fresh birch shoots. At the end of this period, which allowed mating, 41 adults were placed separately into individual 60 ml polypropylene pots and provided with two birch leaves that were replaced every 48 h. A strip of filter paper was placed in each pot and kept damp with distilled water to maintain humidity, and a small piece of folded black card was added as an oviposition substrate (Pinski et al., Reference Pinski, Mattson and Raffa2005a). The pots were maintained in a controlled environment room at 17°C and 16 h light:8 h dark. Eggs were removed and counted every 24 h and the date of adult death was recorded. The sex of each adult was confirmed after death by dissection. Eggs were placed in small clear styrene boxes lined with moistened filter paper and were kept in an incubator at either 15°C or 20°C. The eggs were checked daily, and the numbers hatching and date of hatch were recorded.
Host plant preference: multiple-choice experiments
Feeding preferences of adult P. pyri for different broadleaved tree species were assessed through multiple-choice experiments conducted in the laboratory. The adults, collected from oak, were presented with leaves of 14 different tree species in an experimental arena, which consisted of a large plastic container 13 cm deep and 27 cm in diameter. Five adult P. pyri were placed in the arena and left to feed for 48 h. Leaf areas were determined at the beginning and end of the feeding period using a Hewlett Packard ScanJet 6100C image analyser and DT Scan (release v2) software, and the amount of each tree species consumed by the weevils was recorded (Billiald, Reference Billiald2005). Twenty replicates of the experiment were carried out on 21 May 2004 and a further 20 replicates were completed on 24 May 2004.
Foliage for the experiments was collected from a mixed planting of young trees, except for hawthorn, for which foliage from an adjacent hedgerow was used. Prior to the experiments, single, undamaged leaves (the fifth back from the shoot tip) were collected from 20 trees of each species. The leaves were stored at 5°C in plastic bags and were used within two hours of collection. Great care was taken to ensure that the area of leaf material offered to the adult weevils was the same for each tree species. This was achieved by attaching the leaves to the lid of a 15 cm diameter Petri dish so that, when covered by the base of the dish, a similar area of leaf tip was left exposed (Billiald, Reference Billiald2005).
Statistical analysis of multiple-choice tests is problematic because of the lack of independence between samples (Peterson & Renaud, Reference Peterson and Renaud1989; Roa, Reference Roa1992; Manly, Reference Manly1993; Lockwood, Reference Lockwood1998). Consequently, host plant preferences were analysed using Hotelling's T-squared statistic (Roa, Reference Roa1992). This indicates whether the mean differences between the amounts eaten for each tree species (i.e. preference) are significantly different from zero (Roa, Reference Roa1992; Manly, Reference Manly1993). An F value is used to assess the significance of the Hotelling's T-squared value:

where n=replicates and p=species.
Hotelling's T-squared is a parametric measure and assumes multivariate normality. This assumption was not met by the experimental data and, therefore, a distribution of T-squared was generated by randomly permuting species preferences 5000 times, and the original T-squared F value was then compared with this generated distribution.
Adult performance on different tree species: no-choice experiments
Groups of 35 newly emerged adult P. pyri were placed into clear polystyrene containers lined with damp filter paper and fed with leaves from one of 14 different broadleaved tree species. After four days, when the adults had mated, the adults were transferred to 60 ml pots and reared individually on leaves from the same tree species. Two leaves were placed in each pot and these were refreshed every 48 h. Pots were checked daily and the date of adult death was recorded. Eggs were removed and counted at the end of each week. Dead adults were preserved in 70% IMS and later measured and dissected to confirm their sex and to check for unlaid eggs.
Relationships between longevity and adult sex, size and the numbers of eggs laid were analysed by linear regression. The oviposition data (eggs laid per female), however, contained large numbers of zero counts and were analysed instead by comparing the percentage of females that laid eggs on each diet using a binomial regression model with a log link function.
Parasitism
During 2003 and 2004, 30 adult P. pyri were collected each week from April to the end of June by beating the foliage of birch at sites 7 and 8, sites 11 and 12, and from a young Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) plantation (site 19). The adults were kept individually in 60 ml pots at 17°C and 16 h light:8 h dark and were provided with fresh birch leaves every two days. The pots were checked daily for signs of parasitoid emergence and/or adult death. Adults that died were preserved in 70% IMS and later dissected.
Percentage parasitism was calculated using the total numbers of adult P. pyri caught between 19 May and 10 June (which was when the majority (90%) of adults and parasitoids were collected) and the total numbers of parasitoids emerging from these adults. Relationships between parasitism and host sex, host size, collection site and collection year were analysed using binomial regression with a log link function. All statistical analyses were carried out using GenStat (release 9.1) (Payne et al., Reference Payne, Harding, Murray, Soutar, Baird, Welham, Kane, Gilmour, Thompson, Webster and Tunnicliffe Wilson2006).
Results
Field activity and population densities
Adult P. pyri were found in the emergence traps from 16 April to 10 May (fig. 1a). Higher numbers were collected in 2002 compared with 2003 and 2004, and peak emergence occurred earlier, on 21 April (fig. 1a). The earlier emergence in 2002 may have been related to warmer spring temperatures. Mean daily temperatures in March and April 2002 (7.5°C and 9.4°C, respectively) were rather higher than in 2003 (7.4°C and 9.1°C) and 2004 (5.9°C and 8.6°C).

Fig. 1. Total numbers of adult P. pyri caught (a) in emergence traps, (b) by beating foliage and (c) in flight traps during April–July 2002, 2003 and 2004 (–•–, 2002; – -□- -, 2003; –▵–, 2004).
Adults appeared on tree foliage very soon after emergence and were obtained by beating throughout May and up to the end of June (fig. 1b). The highest numbers of adults in the beating samples occurred between 25 April and 21 May in 2002 and 2003, and on 4 May in 2004. Peak numbers in 2004 corresponded to a population density of 4.9 adults per branch. This density did not cause any noticeable defoliation. The majority of the adult P. pyri were beaten from birch, but adults were also common on beech and hawthorn (table 1). Very few were obtained from oak and none were collected from cherry. Males and females were caught in similar numbers in the emergence traps and by beating foliage, although there was a tendency for females to outnumber males (table 2).
Table 1. Numbers of adult P. pyri obtained by beating the foliage of different tree species. The mean number of adults per sample (±SE) for beating samples collected between 20 April and 29 JuneFootnote 1.

1 Samples were collected weekly and consisted of the total number of P. pyri obtained from 20 branches (beats) on a single tree species.
2 Birch and oak were sampled at two sites.
Table 2. The total number of female and male P. pyri collected in emergence traps, by beating foliage and in flight traps. Numbers within years tested for departure from 1:1 expectation using Chi-square. Data from all sites combined.

The first P. pyri in the flight traps were caught at the end of April. These were, presumably, adults dispersing from emergence sites to tree foliage. Peak numbers of adults in the flight traps occurred later, however, between 15 and 25 May, at the time when adult numbers on tree foliage were decreasing (fig. 1c). The majority of P. pyri caught in the flight traps were females (table 2), and it seems that the peak in flight activity at this time represented females travelling between trees and oviposition sites. Smaller numbers of adult P. pyri were caught in the flight traps up to 15 June (fig. 1c).
P. pyri were caught in the emergence traps in a young Corsican pine (Pinus nigra ssp. laricio Maire) plantation, where there was a mix of grass and small birch trees (sites 7 and 8), in one of the meadow areas (site 9), in two areas of rough grassland (sites 11 and 12) and in sheep-grazed pasture (sites 13 and 14) (table 3). No adults emerged in the mature woodland (sites 1–4) or in young plantations of oak and cherry (sites 5 and 6). The flight traps also caught adults at sites 7 and 8, and above the rough grassland at sites 11 and 12, but they did not catch any adults in the sheep-grazed field (table 3). In contrast, a small number of adults were caught in the flight traps in the grass meadow that was managed by annual cutting (sites 9 and 10), even though only one adult was collected in the emergence traps at these sites.
Table 3. The mean number (±SE) of P. pyri adults emerging per m2 and caught in the flight traps, 2002–2004.
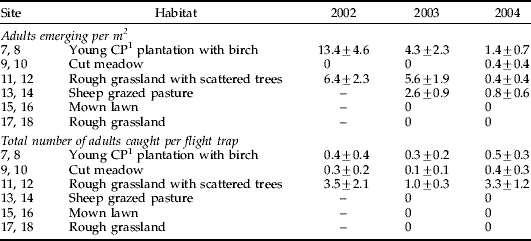
1 Corsican pine. Sites 13–18 were not sampled in 2002.
There were no particular herb or grass species exclusive to or especially dominant at sites where P. pyri were caught in the emergence traps, compared with where there was no emergence; and, therefore, no clear indication of what might have been the larval host plants. The grasses Agrostis capillaris and Holcus lanatus were abundant at the grassland sites where P. pyri emerged, and occurred in the young Corsican pine plantation, but these species were also common at sites where no P. pyri were caught in the emergence traps. Other grass species either did not occur at all sites where P. pyri emerged or were equally abundant at some of the sites with and without P. pyri emergence. The only exception was Deschampsia flexuosa, which was dominant at sites 7 and 8 in the young Corsican pine plantation and which was almost exclusive to these sites.
Flight capacity and reproductive development
Body length of the adult P. pyri varied from 4.8 to 7.7 mm. Females (6.42±0.04 mm) were significantly larger than males (6.16±0.05 mm) (F 1,274=18.2, P<0.001), but there was considerable overlap in size; and, therefore, body length could not be used to distinguish the sexes. Sex determination relied on dissection and examination of the reproductive organs.
The flight muscles of both sexes were fully developed at emergence, indicating that adults could fly immediately. There was no change in DLM length or width between individuals caught in the emergence and flight traps.
Females were reproductively immature at emergence, but their ovaries developed rapidly; and, by the time they were caught in the flight traps, both vitellarium length and diameter, and germarium diameter had increased significantly (table 4). In contrast, germarium length decreased (table 4). The ovaries were white or pale cream coloured when young, but darkened to yellow as the female matured.
Table 4. The size of the ovarioles (mm±SE) of female P. pyri caught in the emergence and flight traps.

In contrast, male P. pyri were reproductively mature at emergence. There was no significant difference in testis size between males caught in the emergence traps and flight traps. Mean testes length was 1.81±0.03 mm, diameter was 0.89±0.01 mm, and maximum follicle diameter was 1.01±0.02 mm. Testes were fleshy and cream to pale pink in colour at emergence but became light brown as the weevil aged.
Adult longevity and fecundity
The group of adults used to assess longevity and fecundity contained 13 males and 28 females. Mean longevity was 33.3±1.5 days (range: 11–46 days), with no significant difference between males and females. Females laid 191.9±34.5 eggs, on average, during their life-time (maximum=589). Eggs were laid in batches and gaps of several days often occurred between ovipositions. On days when eggs were laid, the average number of eggs laid per 24 h was 28.3±2.5 (maximum=86). The females preferred to deposit eggs in crevices, e.g. between the folds of the black card placed in the pots or in a leaf axil. In the absence of crevices, the females laid eggs onto any available surface.
The total number of eggs laid by a female was positively related to female body size (elytral length) (dev. ratio1,26=16.5, P<0.001). There was also a significant positive relationship between female longevity and total egg number (dev. ratio1,26=12.0, P=0.002). Females did not lay eggs immediately after emergence, but showed a pre-oviposition period averaging 15.9±0.9 days before the first batch of eggs was laid. The total number of eggs laid over the female's life-span was inversely related to the length of the pre-oviposition period (F 1,23=25.33, P<0.001). The egg-laying period of females lasted 14.7±1.9 days, on average, but only 6.3±0.9 of these days were egg laying days.
Eggs measured 0.717±0.004 mm by 0.385±0.002 mm (n=100). They were white when freshly laid but turned brown within 72 h. Shortly before hatching, the dark mandibles of the first instar larvae were clearly visible through the chorion. The larvae used a circular rotation of their heads to break through the shell and exit the egg. They did not consume the empty shell. Eggs kept at 15°C hatched after 20 days, whereas those kept at 20°C hatched after 16 days. Newly emerged, first instar larvae were highly mobile and were capable of climbing up the sides of Petri dishes and other containers.
Feeding preference and adult performance on different tree species
In the multiple-choice feeding experiment, adult P. pyri fed readily on oak, birch, alder, hornbeam, sycamore and cherry but consumed only small quantities of beech, lime, poplar, hawthorn, rowan and willow (fig. 2). The adults did not feed on ash or field maple. The amount of leaf tissue consumed was highly variable; and, as a result, in the first experiment (21 May), differences between tree species in the amount consumed were not significant (T=106.4, F=4.58, P=0.063). However, in the second experiment (27 May), the differences between tree species were significant (T=618.0, F=21.7, P=0.016).

Fig. 2. Mean leaf area (±SE) consumed by adult P. pyri for different tree species used in the multiple-choice feeding experiments. Scientific names of the tree species: alder, Alnus glutinosa (L.) Gaertn.; ash, Fraxinus excelsior L.; beech, Fagus sylvatica L.; birch, Betula pendula Roth.; cherry, Prunus avium (L.) L.; field maple, Acer campestre L.; hawthorn, Crataegus monogyna Jacq.; hornbeam, Carpinus betulus L.; lime, Tilia x europea L.; oak, Quercus robur L.; poplar, Populus nigra L.; rowan, Sorbus aucuparia L.; sycamore, Acer pseudoplatanus L.; willow, Salix alba L (
![]() , 21-May; ▪, 27-May).
, 21-May; ▪, 27-May).
Adult longevity in the no-choice feeding experiments showed significant variation between tree species (F 13,435=17.4, P<0.001) (fig. 3). Adults survived for significantly longer on cherry (20.6±1.0 days) and poplar (17.0±1.0 days) than on all of the other tree species (t>2.52, df=435, P<0.012) (fig. 3). In contrast, adults reared on ash lived for only 4.0±1.0 days, which was a significantly shorter period than on all of the other tree species, except lime (t>2.49, df=435, P<0.013) (fig. 3).

Fig. 3. Mean longevity of adult P. pyri when reared separately on different tree species. Horizontal bars indicate groups of tree species on which longevity was not significantly different (P>0.05).
The number of females reared on the different diets in the no-choice experiments varied from 17 to 26. The percentage of these females that laid eggs differed significantly between tree species (dev. ratio12,254=5.1, P<0.001) (fig. 4). (Ash was not included in this analysis as none of the females that were fed on this diet laid eggs.) A higher percentage of females laid eggs on cherry (82%) and poplar (72%) than on all of the other tree species (t>2.21, df=254, P<0.03). The percentage of females laying eggs on hornbeam, birch and oak was significantly higher than the percentage laying eggs on hawthorn and lime (t>1.96, df=254, P<0.05) (fig. 4). The total number of eggs laid per female on each diet was closely related to the percentage of females laying eggs, and ranged from 96.2±20.7 on poplar, 51.9±9.8 on cherry, 21.5±10.7 on birch, 20.0±7.8 on hornbeam to zero on ash (mean±SE; untransformed data).

Fig. 4. The percentage of females laying eggs when reared on different broadleaved tree species. Horizontal bars indicate groups of tree species on which the percentage of egg laying females was not significantly different (P>0.05).
Parasitism
Two parasitoids emerged from the adult P. pyri collected at Alice Holt: Pygostolus falcatus (Nees) (Hymenoptera: Braconidae) and Rondania fasciata (Maquart) (Diptera: Tachinidae). They were identified in the adult stage using von Haeselbarth (Reference von Haeselbarth1971), Shaw & Huddleston (Reference Shaw and Huddleston1991) and Belshaw (Reference Belshaw1993). Both were solitary endoparasitoids, with only one parasitoid larva emerging per host.
Final instar larvae of P. falcatus emerged from their adult hosts between 2 June and 28 June. Each larva spun a cocoon in which to pupate within 24 h of emerging; and, at 17°C, the adult wasp emerged after 8–10 days. Forty-eight P. falcatus larvae were obtained during the two years of the study; and, of the 29 of these that were reared through to the adult stage, 15 were females and 14 were males, giving a sex ratio of 1:0.93. The probability of P. falcatus being female was positively related to host body size. A higher proportion of males emerged from smaller P. pyri (dev. ratio1,24=4.21, P<0.04).
Larvae of R. fasciata exited from field-caught adult P. pyri between 26 May and 27 June. The larvae pupated within 24 h of leaving the host but then over-wintered and did not emerge as adults until the following spring. Pupae were over-wintered in an outdoor insectary and adults emerged from 22 April to 13 May. Over-winter mortality of R. fasciata pupae was high (54%), but of the 39 that were reared through to the adult stage, 21 were female and 18 were male, giving a sex ratio of 1:0.86. The probability of R. fasciata being male or female was not related to host size.
Total percentage parasitism of adult P. pyri by both parasitoid species and across all sites was 45% in 2003 and 30% in 2004 (table 5). The average rate of parasitism by P. falcatus (16% in 2003 and 11% in 2004) did not vary significantly between years, whereas the average rate of parasitism by R. fasciata was significantly lower in 2004 (19%) than in 2003 (29%) (dev. ratio1,485=6.0, P=0.015) (table 5). Percent parasitism by either species may originally have been higher, only for a larva to be out-competed by the other parasitoid species. However, the extent to which multiple parasitism might have occurred was not known.
Table 5. Percentage parasitism of adult P. pyri in 2003 and 2004.

1 Young Douglas-fir plantation.
Percentage parasitism differed significantly between collection areas and between male and female P. pyri. Pygostolus falcatus parasitised fewer P. pyri at site 19 than at sites 7 and 8 and sites 11 and 12 (dev. ratio2,486=12.1, P<0.001) (table 5) and parasitised only 1.6% of male P. pyri compared with 17.1% of females (dev. ratio1,486=23.0, P<0.001). Rondania fasciata parasitised a greater percentage of P. pyri at sites 11 and 12 (dev. ratio2,485=3.9, P=0.02) (table 5) and parasitised 0.9% of male P. pyri and 10.1% of females (dev. ratio1,485=23.4, P<0.001).
Adults of a second leaf weevil species, Polydrusus cervinus (L.), also occurred on birch at Alice Holt at the same time as P. pyri. Out of a total of 260 adult P. cervinus collected in 2004, 2.3% were parasitised by P. falcatus and 3.8% were parasitised by R. fasciata.
Discussion
Habitat associations and population densities
The sampling sites at Alice Holt were chosen to represent a range of possible P. pyri habitats, where emergence traps could be used to identify specific larval habitat associations. Adults emerged from an annually cut meadow, rough grassland with scattered broadleaved trees and a sheep-grazed field but not from mature woodland or young tree plantations that had a relatively closed canopy and shaded ground flora. This distribution indicates that the larvae of P. pyri feed on either the roots of grasses or herbaceous plants in relatively open situations, and is consistent with previous studies that have observed adult P. pyri ovipositing in open grass fields or have found larvae in the soil of grasslands, sometimes causing damage to the grass sward (Bevan, Reference Bevan1962; Axelsson et al., Reference Axelsson, Larsson, Lohm and Persson1973; Hill, Reference Hill1973; Larsson & Lohm, Reference Larsson and Lohm1975; Nielsen, Reference Nielsen1978; Annila, Reference Annila1979; Lerenius & Jansson, Reference Lerenius and Jansson1995).
The highest numbers of adult P. pyri in the current study, however, emerged from patches of grassy habitat within the young Corsican pine plantation where there were many young, naturally regenerating birch trees. The dominant grass species in this area was Deschampsia flexuosa, which seems likely to have been the larval food plant, but it is possible that the larvae were also feeding on roots of the birch trees or other woody vegetation. Recent experiments, however, have shown that the larvae of P. pyri feed and complete their development on the roots of a number of grass species, including D. flexuosa and A. capillaris, but not on the roots of birch (Billiald, Reference Billiald2005).
Adult P. pyri did not emerge from all of the grassland sites, even though the sward at some of these sites looked very similar to that at sites where adults were caught in the emergence traps. This suggests that the larvae might have other habitat requirements in addition to the presence of suitable food plants. Alternatively, larvae might have a clumped distribution within grassland, at a scale where the limited array of emergence traps used in the study might sometimes have missed adults emerging from the soil. The larvae of P. oblongus have been shown to have a strongly clumped distribution within plum orchards (Pinski et al., Reference Pinski, Mattson and Raffa2005b).
Densities of P. pyri in the young Corsican pine plantation reached 1.8 and 25.2 adults per m2 in 2002 (sites 7 and 8, respectively), but declined to 0.9–1.7 per m2 in 2004. These densities, and those recorded at the grassland sites, are similar to the mean population densities of 4.1, 9.3 and 1.8 adults per m2 recorded by Kula (Reference Kula, McManus and Liebhold2003) for P. arborator, P. argentatus and P. glaucus (Scop.) (=calcaratus (Fab.)) in birch stands in the Czech Republic. Larval populations appear to be capable of reaching much higher densities. Bevan (Reference Bevan1962) reported densities of up to 800–1350 larvae per m2 in grass leys in northern England and Axelsson et al. (Reference Axelsson, Larsson, Lohm and Persson1973), Larsson & Lohm (Reference Larsson and Lohm1975) and Nielsen (Reference Nielsen1978) recorded up to 500 larvae per m2 in grassland in Sweden and Denmark. Annila (Reference Annila1979) recorded combined densities of P. pyri and P. maculicornis larvae of 20–36 per m2 in a young birch plantation in Finland. Estimates of larval density for other Phyllobius species range from 18 to 250 larvae per m2 (Vollman, Reference Vollman1954; Ioannisiani et al., Reference Ioannisiani, Birg and Lavrova1970b; Axelsson et al., Reference Axelsson, Larsson, Lohm and Persson1973). Most of these estimates relate to outbreak situations where damage to grassland or trees was visible. Endemic population densities are likely to be much lower.
Adult P. pyri appeared on tree foliage immediately after emergence, but the peak in flight activity, as recorded by the numbers of adults caught in the flight traps, occurred later as peak numbers on foliage were declining. Most adults caught in the flight traps were females, and dissections showed that these included females with mature eggs and females without eggs but which had laid eggs recently. In the laboratory, adult females continued to feed between laying batches of eggs and feeding on foliage was essential to mature the full complement of eggs. Therefore, the flight activity observed throughout May and June probably involved females moving to-and-fro between tree foliage and oviposition sites. Males and females also mate on tree foliage, and individuals were observed to mate repeatedly during their lifetime. Multiple mating, however, was not necessary for females to continue to lay fertilised eggs, as found by Vollman (Reference Vollman1954) for P. oblongus; and, therefore, female flight activity was less likely to be associated with a need to find mates, compared with the requirement to continue feeding to mature eggs.
The highest numbers of P. pyri caught in the flight traps occurred in the rough grassland area, where there were a moderate number of widely spaced broadleaved trees. Adults also emerged at these sites, indicating that larvae occurred in the grassland; and the relatively high level of flight activity probably reflected small, local movements between the trees and oviposition sites, necessitated by the wide spacing of the trees. At the other grassland sites, there were no trees and there was little flight activity, even where adults were known to have emerged.
Surprisingly, the numbers of adult P. pyri caught in the flight traps in the Corsican pine plantation were lower than the numbers caught in the rough grassland area with scattered trees, even though the numbers of adults emerging in the plantation were higher or similar to those emerging in the grassland. Large numbers of adult P. pyri were present on the young birch trees in the pine plantation and were collected by beating the foliage, but this was not reflected in a high rate of captures in the flight traps. It appears that, in this situation, the close proximity of grassy patches and young trees provided ideal conditions for P. pyri, in which the adults did not need to disperse far by flight to oviposit and continue feeding and where large numbers of eggs could be laid, leading to relatively high population densities. A similar small-scale mix of grass and young broadleaved trees occurs in young farm woodlands, between three and ten years after planting. Young woodlands at this stage are likely, therefore, also to provide an optimum habitat for P. pyri, where adult and larval food resources occur close together. Under these conditions, population densities will build up rapidly and may occasionally cause defoliation. Within a few years, however, as the tree canopies develop and the grass sward becomes shaded, the suitability of the habitat for P. pyri larvae will decline, and adults will become less common and feeding damage will reduce.
Adult longevity and fecundity
The mean longevity of 33.3 days for adult P. pyri falls within the range of 21–40 days, recorded for adults of other tree-feeding Phyllobius species (Vollman, Reference Vollman1954; Ioannisiani et al., Reference Ioannisiani, Birg and Lavrova1970b; Schauermann, Reference Schauermann1973; Urban, Reference Urban1998). The mean fecundity of 191.9 eggs per female was higher than the maximum number of eggs laid per female (127) recorded by Bevan (Reference Bevan1962), although the conditions under which the latter figure was obtained are unclear. For other Phyllobius species, Ioannisiani et al. (Reference Ioannisiani, Birg and Lavrova1970b) estimated mean life-time fecundity of P. argentatus, P. maculicornis and P. arborator to be 95, 202 and 122 eggs per female, respectively; and Vollman (Reference Vollman1954) estimated mean fecundity in P. oblongus to be 123 eggs per female (range 25–361). Urban (Reference Urban1998) gives an estimate of mean fecundity for P. argentatus of 138 eggs per female.
In the current study, eggs of P. pyri hatched after 16–20 days, which is similar to the 14–16 days between oviposition and egg hatch observed by Bevan (Reference Bevan1962) and Nielsen (Reference Nielsen1978). Eggs of P. oblongus have been reported to hatch after 3–4 weeks (Nielsen, Reference Nielsen1997), 24 days (Witter & Fields, Reference Witter and Fields1977), 17–18 days at 15°C (Savic, Reference Savic1963) and 16 days at 18°C (Vollman, Reference Vollman1954).
Feeding preferences and performance on different tree species
In the multiple-choice experiment, where adult P. pyri were offered leaves from a range of broadleaved tree species, special care was taken to ensure that the weevils were presented with a similar amount of leaf material, and approximately the same amount of leaf edge, from each tree species. Adult P. pyri typically feed around the leaf margins. If whole leaves had been used, then species with larger leaves would have been encountered more often within the experimental arena, increasing the probability that these species would have been consumed and leading to bias in the detection of preference (Roa, Reference Roa1992; Manly, Reference Manly1993). Despite these precautions, there was still considerable variation in the amount of leaf material that was eaten. The experiment indicated that P. pyri preferred to feed on oak, birch, alder, hornbeam, sycamore and cherry, and did not feed to any large extent on poplar, beech, lime, hawthorn, rowan or willow (fig. 2). The adults did not feed on field maple or ash.
The adults used in the multiple-choice experiment were collected from oak and this may have had some influence on the results. Feeding preferences can be conditioned by previous diet (Dethier, Reference Dethier1982; Cunningham et al., Reference Cunningham, Zalucki and West1999), and preliminary laboratory experiments showed that adult P. pyri tended to prefer the tree species on which they had previously been reared (Billiald, Reference Billiald2005). Therefore, the ranking for oak in the multiple-choice experiments might be rather high, compared with if newly emerged adults, without any prior feeding experience, had been used in the experiment.
The distribution of adult P. pyri in the field differed from what might have been expected given the results of the multiple-choice experiment. Although large numbers of adults were beaten from birch, and a moderate number from beech, very few were obtained from oak and cherry, even though the latter were amongst the preferred group of tree species. Adult numbers on particular groups of trees, however, will also be influenced by other factors; and the low numbers on oak and cherry at Alice Holt might have reflected a lack of suitable larval habitats in the immediate area, proximity to other suitable or unsuitable tree species, or differences in the timing of bud burst and leaf development compared with other hosts in the years when sampling took place.
Oak and cherry, however, are the two species most frequently recorded as defoliated by P. pyri in enquiries to the UK Forestry Commission, followed by birch and beech, which indicates that P. pyri is often very abundant on these tree species. However, the number of enquiries also reflects that these tree species, along with ash, are also the most widely planted species in farm woodlands.
Performance (longevity and oviposition) of adult P. pyri in the no-choice experiments, where adults were reared on the leaves of a single tree species, was significantly higher on cherry and poplar compared with all of the other tree species. Cherry was a preferred species in the multiple-choice experiment, but poplar was not. Amongst the other tree species for which P. pyri showed a feeding preference (oak, birch, alder, hornbeam and sycamore), there were no significant differences in longevity or the percentage of females laying eggs; but performance on these species was generally higher than on those tree species that were avoided in the multiple-choice trial (i.e. rowan, field maple, hawthorn, lime and ash). Longevity and the percentage of females laying eggs were significantly higher on oak, birch and hornbeam than any of these less preferred species. P. pyri performed particularly poorly on lime and ash. On ash, the adults only lived for four days (they hardly fed) and none of the females laid eggs.
Performance on beech and willow was higher than expected given the results of the multiple-choice experiment, where these species were not eaten very frequently. These results, and the good performance on poplar, demonstrate a discontinuity between adult feeding preferences and potential adult performance, which has been observed in other studies on herbivorous insects (Thompson, Reference Thompson1988; Leather, Reference Leather and Bernays1994; Orians et al., Reference Orians, Huang, Wild, Dorfman, Zee, Dao and Fritz1997).
The experiments indicate that cherry, oak, birch and hornbeam are the most susceptible species to damage by P. pyri; whereas alder, sycamore, beech, poplar and willow, although accepted hosts, are less likely to be damaged because they were not preferred as food plants or because adults reared on these species performed relatively poorly. Field maple, rowan, hawthorn and lime were poor hosts and ash was especially resistant. These latter species are unlikely to be damaged in the field; and, consequently, they would be a better choice for planting in grasslands that have a history of damage by P. pyri. This rank ordering of tree species, however, relates specifically to susceptibility to P. pyri. Other Phyllobius species may show different host preferences and different performance on the same tree species.
Parasitism
P. falcatus has not been reported previously as parasitising P. pyri or Polydrusus cervinus, although Ioannisiani et al. (Reference Ioannisiani, Lavrova and Birg1970a) recorded it from adult P. maculicornis and P. argentatus, and Diaz (Reference Diaz1923) reared it from Polydrusus pilosulus Chevrolat. It, generally, appears to be a parasite of adult Curculionidae, particularly of species associated with grasslands. Loan & Holdaway (Reference Loan and Holdaway1961) describe it as an important parasitoid of Sitona lineatus, the larvae of which also live below ground in grassland; and Grossheim (Reference Grossheim1928), Thompson (Reference Thompson1953) and Loan (Reference Loan1961) list several other Sitona species as hosts, along with the weevil Brachyderes incanus.
Studies on parasitism in Sitona indicate that P. falcatus is bivoltine and overwinters as a first instar larva inside an adult host (Loan, Reference Loan1961; Loan & Holdaway, Reference Loan and Holdaway1961; Milbrath & Weiss, Reference Milbrath and Weiss1998; Phillips et al., Reference Phillips, Goldson, Reimer and Kuhlmann2000). In the spring, the mature fifth instar larva exits the host and spins a cocoon. The adult wasp emerges after 7–8 days (Loan & Holdaway, Reference Loan and Holdaway1961) and immediately begins to search for new hosts and to lay eggs. The second generation of adults emerges later in the summer, and these also search out adult weevils and lay eggs. The larvae of this second generation then enter diapause and overwinter, and complete their development in the host in the spring (Milbrath & Weiss, Reference Milbrath and Weiss1998). Brudea (Reference Brudea1984), working in North Moldavia, noted three P. falcatus generations in some years and two in other years.
Adult P. pyri were active for several weeks in the spring and, hence, were available to the first generation of P. falcatus; but other weevil species must have acted as hosts for the second generation later in the summer. The most likely alternative hosts at Alice Holt were Sitona species that over-wintered as adults (Loan & Holdaway, Reference Loan and Holdaway1961; Milbrath & Weiss, Reference Milbrath and Weiss1998; Phillips et al., Reference Phillips, Goldson, Reimer and Kuhlmann2000). Sitona lineatus, which passes the winter in the adult stage, was caught in the emergence and flight traps at some of the sites with P. pyri.
Loan & Holdaway (Reference Loan and Holdaway1961) and Milbrath & Weiss (Reference Milbrath and Weiss1998) describe reproduction in P. falcatus as being purely parthenogenetic and they failed to find any males. However, Jackson (Reference Jackson1928) recorded males as well as females, as found in the current study, and von Haeselbarth (Reference von Haeselbarth1971) also mentions both male and female P. falcatus.
Pygostylus falcatus parasitised 11–16% of the adult P. pyri at Alice Holt, which is similar to the 10% parasitism of P. maculicornis and P. argentatus by P. falcatus recorded by Ioannisiani et al. (Reference Ioannisiani, Lavrova and Birg1970a). Loan & Holdaway (Reference Loan and Holdaway1961), in contrast, record P. falcatus as parasitising up to 73% of S. lineatus. The tachinid Rondania fasciata parasitised a higher percentage of the adult P. pyri at Alice Holt, on average 19–21% of the adults collected. This species is also known as a solitary endoparasitoid, but it is univoltine and there is only a single flight period each year. Belshaw (Reference Belshaw1993) gives a single host record of it being reared from adult P. argentatus, whereas the UK Tachinid Recording Scheme (2002) records it as parasitising Strophosomus spp. (Curculionidae). Both Belshaw (Reference Belshaw1993) and the recording scheme indicate that adult R. fasciata are usually found in woodland habitats or along forest edges. It is regarded as a Nationally Notable (Nationally Scarce) dipteran species (Falk, Reference Falk1991).
Acknowledgements
We thank David Williams, Martin Jukes and Gill Green of Forest Research for technical help and support, Jane Poole for providing advice on statistics and three anonymous reviewers for their constructive comments on the manuscript.











