Introduction
Functional neurological disorder (FND), also known as conversion disorder, is a heterogeneous group of syndromes that commonly present to neurologists, primary care clinicians, psychiatrists, and emergency departments. The most common variants are functional seizure disorder (psychogenic non-epileptic seizures [PNES]), functional movement disorder (FMD), and functional cognitive disorder. Patients with FND tend to be high utilizers of health care resources due to diagnostic challenges, problems with access to treatment, and care coordination. Evidence-based treatments for FND exist, and more are being developed and need to be developed, that are seeking symptom resolution and improvement in quality of life.Reference Espay, Aybek and Carson1 While effective treatments are available, lack of awareness of FND among health care professionals and in the community remain significant problems and a barrier towards treatment.Reference Barnett, Davis, Mitchell and Tyson2 Many times, FND is persistent and chronic, and factors determining long-term outcomes are incompletely understood.Reference Perez, Aybek and Popkirov3 Given the prevalent and disabling nature of this common disorder, the rationale of this paper is to describe current approaches to FND treatment for the non-specialist, and to define a research agenda to advance available treatment modalities for the clinical and research communities. Didactic and explanatory illustrations of treatment approaches are described and provided in figures in this article.
Approach to FND Treatment: Delivery of Diagnosis as the First Step
Accurate diagnosis is the first step in the treatment of FND and relies predominantly on a clinician obtaining a comprehensive medical, neurological, and psychiatric history and examination. Identification of “positive signs,” that is, recognition of neurological examination findings that are incongruent with other neurologic disorders have been emphasized in DSM-5, has shifted clinician’s abilities from a hedging a diagnosis of exclusion, now making FND a diagnosis of inclusion.Reference Stone, LaFrance, Brown, Spiegel, Levenson and Sharpe4 Positive exam signs, such as Hoover’s sign, entrainment, and tubular vision defect are well described in other reviewsReference Espay, Aybek and Carson1 and are covered elsewhere in this issue.
Delivery of the diagnosis should occur in a positive, non-judgmental, non-pejorative, and clear manner.Reference Adams, Anderson, Madva, LaFrance and Perez5 FND needs to be understood within a biopsychosocial, spiritual, and cultural framework that informs the treatment (an approach to which is described below). Although FND is not associated with classical structural brain lesions, evolving research is demonstrating evidence for neural circuitry dysfunction in limbic and sensorimotor pathways. Providing an explanatory model to patients that addresses individual risk factors and triggers and outlining an individualized treatment path with emphasis on the potential reversibility of symptoms will aid in forming a therapeutic partnership. Motivational interviewing was recently studied and found to improve treatment adherence and outcomes in patients with PNES.Reference Tolchin, Baslet and Suzuki6 Explaining that the good news is that treatments exist for the symptoms may provide hope to patients with FND and their families, many of whom have been discouraged by the lack of resources and receptive practices for their condition.
Application of Treatment Models
Different treatment modalities may have variable benefits in different patient groups, depending on individually contributing factors toward pathophysiology. Clear communication in planning and treatment delivery and continuity of care among healthcare professionals is key to treatment success. Assessment of patients in specialized FND and neuropsychiatric clinics allows for dedicated patient education and treatment planning.Reference Aybek, Lidstone and Nielsen7, Reference Jacob, Kaelin, Roach, Ziegler and LaFaver8 In addition to addressing the “core symptoms” of FND, integrating addressing common comorbidities in the treatment plan, including chronic pain and fatigue is important. Patients with predominant pain may be advised to undergo a pain rehabilitation program along with, or prior to, focusing on other FND symptoms, and other medical and neurological comorbidities may need to be addressed separately, as well. Important goals of treating patients with FND are maximizing functional independence, regaining control, and self-agency. Components of treatment may include classic psychotherapeutic modalities, rehabilitation interventions, and addressing comorbidities. Regardless of the specific intervention, development of a trusting clinician/patient rapport is crucial in order for patients to feel heard and understood and not alone in the process, and help diminish feelings of shame, guilt, and distress.
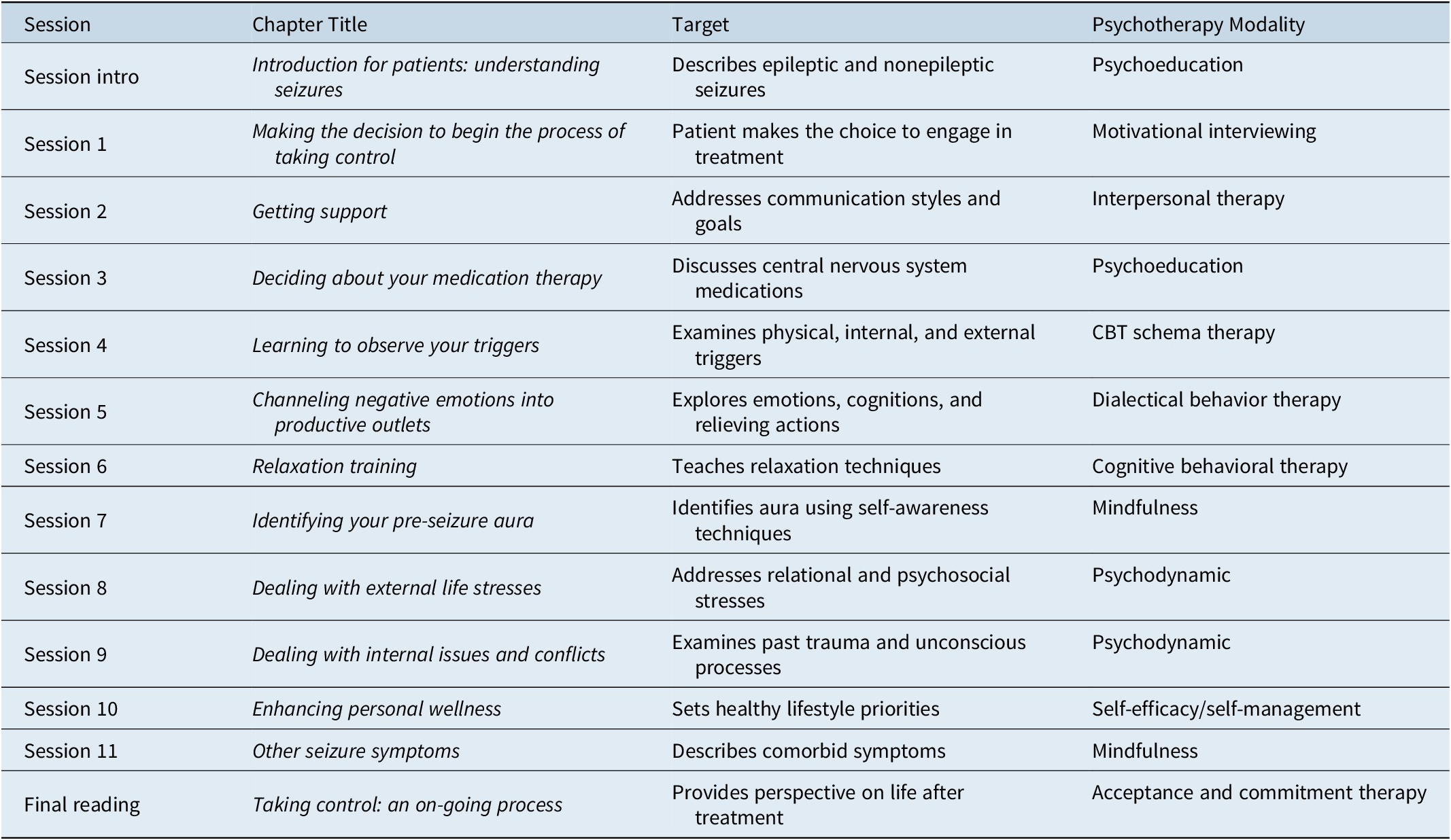
Within a stepped model of care as initially proposed by Carson and Stone,9 individual treatment is tailored to the needs of each patient based on symptom severity (Figure 1) and complexity to optimize resource allocation. Patients with mild or transient symptoms are provided education and advice by the neurologist making the diagnosis and referred back to primary care (Step 1). Patients with more severe symptoms but without complex psychiatric comorbidities may benefit from a brief psychotherapeutic or rehabilitation intervention with a physical, occupational, or speech therapist (Step 2). Patients with complex symptom presentations, a high level of disability and comorbidities and those who have failed prior treatment interventions are likely to benefit from specialized multidisciplinary FND therapy delivered in an outpatient, day hospital, or inpatient setting (Step 3). Depending on individual symptoms and comorbidities, the treatment team may involve a neurologist, psychiatrist, (neuro)psychologist, physiatrist, physical, occupational, and speech therapist, clinical social worker, mental health clinician, and others (Figure 2). Involvement of the patient’s family in psychoeducation and therapy is important to ensure transfer of treatment success to the home environment and prevention of relapses. We will review some of the evidence-based treatments from the literature for PNES and FMD, as examples of multi-modal therapeutic approaches.

Figure 1. Proposed model of stepped care for FND (adapted from Carson and Stone, NHS Scotland, 2014).9

Figure 2. Patient-centered systems model for PNES (modified from LaFrance and Devinsky, Reference LaFrance and Devinsky2004, with permission from John Wiley and Sons).Reference LaFrance and Devinsky10
Treatment of Functional Seizures/PNES
General treatment principles for PNES include psychoeducation to facilitate understanding and acceptance of the diagnosis, addressing predisposing, precipitating, and perpetuating factorsReference LaFrance and Devinsky11 within a biopsychosocial spiritual (BPSS) framework, which are queried in the developmental and social history, as well as addressing medical and psychiatric comorbidities. Patients will learn to identify seizure triggers and apply techniques such as action planning, mindfulness, and grounding to reduce seizure frequency and change maladaptive behavioral patterns. Many therapists work with methods based on conventional cognitive behavioral therapy (CBT),Reference Goldstein, Mellers and Landau12 psychoeducation,Reference Chen, Maheshwari, Franks, Trolley, Robinson and Hrachovy13 acceptance commitment therapy,Reference Barrett-Naylor, Gresswell and Dawson14 dialectical behavior therapy,Reference Bullock, Mirza, Forte and Trockel15 psychodynamic,Reference Howlett and Reuber16 and narrative therapyReference Myers, Vaidya-Mathur and Lancman17 to address current stressors, past trauma, and relational issues. Involvement of family in treatment, addressing relationships, work life, and social connectedness are important for treatment success.Reference LaFrance, Bjønaes, LaFrance and Schachter18
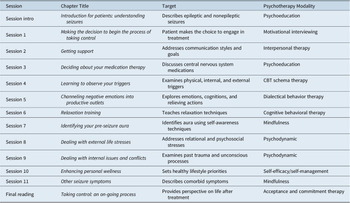
LaFrance and colleagues have designed, tested, and implemented a multi-modality, Beckian-based,Reference Beck19, Reference Beck20 CBT-informed psychotherapy,Reference Reiter, Andrews, Reiter and LaFrance21 described as Neurobehavioral Therapy (NBT), which is structured, time-limited, short-term, and present-oriented. The intervention is built on the assumption that life experiences and trauma in patients with PNES result in maladaptive core beliefs (negative schemas), cognitive distortions, and somatic symptoms. The 12, 1 hour long, individual therapy sessions are specifically tailored for known pathologies in patients with PNES in order to address both the seizures and the comorbidities that commonly occur in this disorder, and promote behavioral change, self-control, and self-efficacy (see Table 1 for sessions, targets, and modalities). The goal of treatment is to equip patients to assume control over their seizures; contextualizing the individual’s environment; identifying moods, situations, and thoughts; training healthy communication and support seeking; understanding central nervous system medications and seizures; conducting a functional behavioral analysis; learning relaxation techniques; examining external stressors and internal triggers; and preparing for life after completing the time-limited intervention.
Table 1. Therapeutic Topics, Targets, and Modalities for Neurobehavioral Therapy Treatment of PNES.
Abbreviation: PNES, Psychogenic Non-Epileptic Seizures.
Summary of psychotherapeutic modalities in each chapter of Reiter et alReference Reiter, Andrews, Reiter and LaFrance21 (reproduced with permission of The Licensor through PLSclear; in LaFrance Jr WC, Schachter SC, eds., Gates and Rowan’s Nonepileptic Seizures, New York: Cambridge University Press, 2018, p. 302).
Case illustrations of how to conceptualize the BPSS and cultural aspects in the patient formulation are given in clinical vignettes and clinician interactions in the therapy training manual,Reference LaFrance and Wincze22 which is used to facilitate the standardized, manual-based delivery.Reference Reiter, Andrews, Reiter and LaFrance21 An example of the formulation would include relevant data on birth, developmental, trauma/abuse, education, relationships in family of origin and extended family, work, avocation, military, legal, religion/faith history, derived from the patient and from accompanying family member/significant other, if present.
In the open labelReference LaFrance, Miller and Ryan23 and pilot randomized controlled trial (RCT) studies,Reference LaFrance, Baird and Barry24 therapy was administered according to the manual-based protocol devised and modified by LaFrance’s research group from the original Epilepsy Workbook.Reference Reiter, Andrews and Janis25 Given that NBT has shown significant reductions in seizures and co-morbidities in two civilian pilot trials, as cited above, and in a study with Veterans with documented PNES,Reference LaFrance, Ho, Bhatla, Baird, Altalib and Godleski26 it is being used in civilian and Veterans hospital and clinic facilities across the country by trained clinicians, including neurologists/epileptologists, psychiatrists, neuropsychiatrists, psychologists, neuropsychologists, and social workers.Reference Jung, Chen, Bullock, LaFrance and Schachter27 Clinicians at several VA Epilepsy Centers of Excellence have been trained to deliver NBT, in person and via clinical video telehealth, and service agreements have been established to provide telehealth treatment at VAs around the country.Reference LaFrance, Ho, Bhatla, Baird, Altalib and Godleski28 As shown in Figure 2, this intervention involves medical, allied health, community, and family resources for management,Reference LaFrance and Devinsky10 and addresses the gap of clinician frustration regarding lack of treatments, and provides hope to Veterans with PNES for improvement in seizures, comorbid symptoms, and quality of life.Reference McMillan, Pugh and Hamid29
Treatment of FMD
Treatment planning in FMD needs to be individualized, taking symptom severity and comorbidities into account. Members of the treatment team may include a neurologist, physiatrist, (neuro)psychologist, psychiatrist, physical/occupational/speech therapist, and a clinical social worker (see Figure 3). Many patients with FMD can benefit from focused interventions, that may be delivered by a psychologist, physical, occupational, or speech therapist, with the goal of regaining control over movements, identifying trigger factors, and facilitating behavioral changes. One randomized controlled trial showed modest benefit from a guided self-help manual based on CBT principles in a mixed FND patient group.Reference Sharpe, Walker and Williams30 A randomized clinical trial tested hypnosis compared to a wait list control in 44 patients with motor conversion disorder, which showed improvement in the hypnosis group.Reference Moene, Spinhoven, Hoogduin and van Dyck31
For patients without significant psychiatric comorbidities or chronic pain, a 5-day specialized physical therapy (PT) treatment protocol led to 72% symptoms improvement at 6 month follow-up in patients with functional motor disorders, compared to only 18% improvements in the control group.Reference Nielsen, Ricciardi, Demartini, Hunter, Joyce and Edwards32 The goal of PT for FMD is re-establishing normal movement patterns and learning control over involuntary movements. After education about the diagnosis and establishing a shared treatment plan therapy focuses on goal-directed rehabilitation, using strategies to facilitate automated movement patterns and controlling unwanted movements. Recommendations for PT in FMD have been summarized in a consensus statement in the UK.Reference Nielsen, Stone and Matthews33 FMD specific therapy principles include limited “hands-on” treatment, facilitating rather than supporting movements, encouraging early weight bearing, goal-directed rehabilitation focused on function and automatic movement, minimized reinforcement of maladaptive movement patterns and postures, and avoidance of adaptive equipment and mobility aids. Therapy is delivered within a “psychologically-informed” framework, and relaxation and mindfulness training are incorporated into treatment sessions.
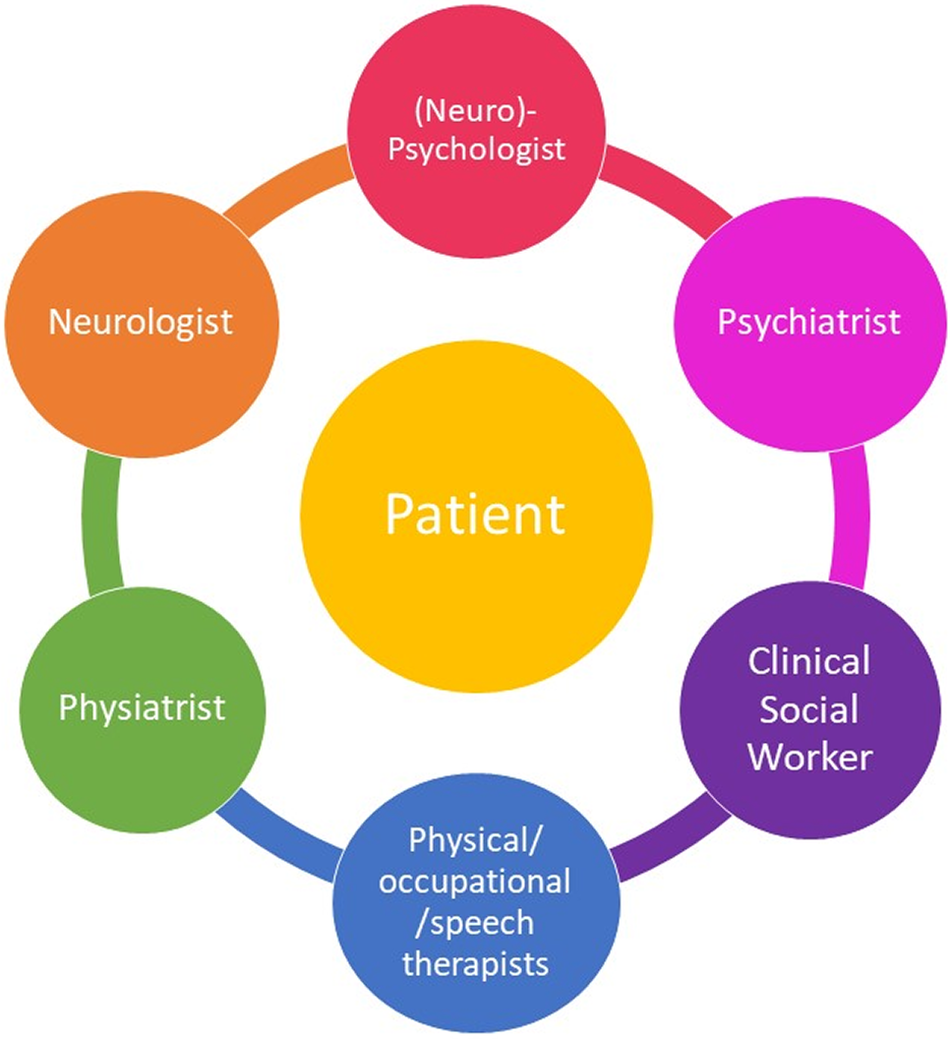
Figure 3. Multidisciplinary treatment for FMD.
For patients with a high degree of disability, treatment in a day hospital or inpatient setting can provide the benefits of an intensive multidisciplinary treatment approach by a specialized team under close monitoring and temporary withdrawal from a potentially maladaptive home environment. In a randomized-controlled trial by Jordbru et al., patients with functional gait disorder admitted to a three-week inpatient rehabilitation program had significant improvement in their ability to walk, higher functional independence and mobility and improved quality of life compared with an untreated control group, and sustained improvements at follow-up after 1 year.Reference Jordbru, Smedstad, Klungsoyr and Martinsen34 There is currently no standardized approach to provide inpatient treatment for patients with FMD, although several other retrospective case series have been encouraging.Reference Jacob, Kaelin, Roach, Ziegler and LaFaver8, Reference Saifee, Kassavetis and Parees35 In the motor retraining program developed by LaFaver and colleagues, patients were admitted to a one-week stay in a rehabilitation hospital, undergoing daily physical, occupational and speech therapy, a total of 5 hours of CBT, guided meditation, and motor imaging training.Reference Jacob, Kaelin, Roach, Ziegler and LaFaver8 Initial and 6-month outcomes showed significant improvements in motor symptoms, with more modest gains in overall quality of life.Reference Jacob, Smith and Jablonski36 Other treatment programs in the UK take place in psychiatric facilities with longer stays depending on patients’ needs.Reference McCormack, Moriarty and Mellers37 Well-designed randomized controlled treatment studies with larger number of patients are needed to optimize treatment allocation and understand factors determining long-term outcomes.
Unmet Needs in FND Treatment
Research agenda
Although many patients achieve treatment benefits with therapies outlined in the previous section, complete symptomatic remissions are rare, and some patients are refractory to currently available therapies despite good motivation and full engagement. The purpose of this section is to outline a research agenda to advance treatment for FND. Clinical experience and studies show that treatment needs to be interdisciplinary and unify experts from many disparate disciplines. A unique challenge is the design and conduct of adequately powered trial for current and future therapies. These challenges are universal, whether we are looking at psychotherapy, neuromodulation trials, or medication trials. In the past, there has been a siloed approach between neurology and psychiatry that might have been prevented more research in this area.
Current and future intervention to be studied include neuromodulation (eg, transcranial magnetic stimulation,Reference Taib, Ory-Magne and Brefel-Courbon38, Reference Pick, Hodsoll and Stanton39), hypnosis, pharmacological agents (including psychotropicsReference LaFrance, Blumer, LaFrance and Schachter40 and psychedelicsReference Butler, Seynaeve and Nicholson41), rehabilitation medicine, placebo interventions,Reference Kaas, Humbyrd and Pantelyat42 and different milieu, such as other individual and group therapies.Reference Bullock, LaFrance and Schachter43 In addition to the intervention modality, research questions revolve around frequency and duration, treatment setting (inpatient vs outpatient), individual vs group therapy, combined modalities, and durability of treatment effect and maintenance. There is also a need to explore the value of web-based and telehealth interventions, and the combination of remote and in-person treatments. These challenges are described further below and listed in Table 2.
Table 2. Proposed Research Agenda for FND Treatment Studies.
Abbreviation: FND, functional neurological disorder; RCTs, randomized clinical trials.
Future Treatment Interventions: Challenges and Opportunities
Study design challenges
One of the major challenges of designing trials for FND/CD is the comparison arm. Some of the potential options for comparator arms are treatment as usual, weightless control, active comparator arms, placebo-sham control. Elements of the control that must be addressed include time and attention. One unique approach to an innovative control study may involve dismantling an effective treatment into components. This approach allows investigators to determine which components of the accepted treatment are active and whether there is synergy between various components of the intervention.
Methodological challenges in developing trials for FND/CD
One of the major challenges in this area is how one conceptualizes FND—whether one is a “splitter or lumper.” From a “splitter” perspective, semiological and comorbidity heterogeneity is a potential problem for research in this population. Patients with FND have a protean range of symptoms and presentations, along with a plethora of psychiatric and somatic comorbidities. In contrast, the “lumper” conceptualization identifies conversion disorder as a unifying factor in most patients with FND, and the phenotypic variations and comorbidities are expected in a symptomatic continuum along a spectrum in this complex population.
Some of the other characteristics that can impact both the adherence and retention in trials include acceptance of the diagnosis, the motivation and readiness to change of the subject, the subject’s perception of expectancy, and credibility of the proposed interventions in the trial, and the ability of the patient being able to attend sessions regularly (whether due to transportation or insurance issues).
Characteristics of potential participants that need to be addressed in the inclusion/exclusion impact are dissociation, avoidance behavior, substance use, and disability application/litigation. Proximity to the site and support system can be integral to compliance.
Challenges in outcome measures
The choice of outcome measures depends on the main hypothesis and the intention of the trial. The study may be addressing efficacy or effectiveness of an acute-phase intervention, long-term effects, or examining cost and utilization. Thus, monitoring the desired outcome may use different measures to determine the treatment impact.
Defining best outcome measures is an area of active research. Common outcome domains and measures in FND intervention studies include: core symptoms, other physical symptoms, psychiatric symptoms, life impact (eg, QOL/disability, general functioning), and health economics/cost-utility, illness beliefs, and attributions.Reference Pick, Anderson and Asadi-Pooya44 The importance of the choice of primary and secondary outcome issue was recently demonstrated in the successful completion of the first fully powered psychotherapy RCT for PNES, with benefit in many secondary measures, but a negative primary outcome of difference between conventional CBT and enhanced standard medical care at 12 month follow up,Reference Goldstein, Robinson and Mellers45 which may not have been the most representative outcome measure representation of the benefit of CBT for PNES observed in this trial.
Other outcomes may include physiological outcomes, which could be monitored with serologic, genetic, neuroimaging, autonomic measures, and actigraphy. Integration of wearable devices and other technology-assisted devices may facilitate the subjective and objective response in patients’ natural environment.
Some other important components of trial design relate to the trial duration and impact, which includes whether the purpose of the trial is to assess acute treatment response, the durability of treatment, or the need for maintenance treatment. Factors to be taken into account include: the length of the acute treatment trial, the frequency of assessment within the trial, the frequency of the intervention delivery, the schedule of follow-up assessments administration, the length of follow-up, and the mechanism of collection of follow up data (eg, pen and paper, electronic survey).
Whether or not to allow for other treatments during and with the active intervention is another consideration for designing efficacy or effectiveness of trials. The potential use of naturalistic follow-up in the untreated population patients using registries or biorepository is another potential comparison group.
Along with the specific issues noted above, other general issues that may impact trial design and outcome involve patient-related factors These elements include: temporal variability of symptoms and the impact of attention beliefs and expectancy, the use of patient-rated outcome measures, which is important in assessing patient population, and discrepancy in patients rated outcomes and clinician observed ratings, which may provide insights into the mechanisms underlying symptoms and treatment responses.
In summary, evidence-based medicine determination is established based on a research hypothesis, supportive evidence, and conclusions based on data that yield treatment recommendations. Ultimately, the desired outcome of the research agenda is the conduct of Class I studies (fully powered, masked outcome, randomized, controlled trials) leading to Level A recommendations (evidence for established care) for applied practice parametersReference Gronseth, Cox and Gloss46 for evaluation and management of patients with FND.
Conclusion
Effective treatments for FND are available, however, one size does not fit all. Psychotherapy, rehabilitation-based approaches, and multidisciplinary treatment programs all have been shown to offer benefit for specific aspects of FND, but need to be studied further to replicate outcomes, aid in treatment stratification, and understand factors related to long-term treatment success. Additional treatment modalities need to be explored and studied to better serve this diverse population, especially for patients refractory to current therapies. In terms of a research agenda, it is imperative that studies are conducted to address challenges in trial design and best practices for the implementation and dissemination of treatment programs. As new evidence-based treatments for FND emerge, dissemination of knowledge and improving access to care for this underserved patient population remain important areas to address.
Acknowledgments
The authors would like to thank Karen Rommelfanger, PhD and Mark Rapaport, MD, for organizing the project, Janice Dell, who coordinated the project, and Dr. Maureen Michael, for facilitating the exercises for the writing retreat.
Funding
The authors did not receive any payment for writing this manuscript. This work was supported by the Klein Mind–Body Writing Retreat, 6–8 March 2020, Emory University, which provided travel and lodging support for the co-authors for the initial draft preparation.
Disclosures
The authors report the following disclosures: Kathrin LaFaver has received research support from the Ayers Foundation, serves on the Functional Neurological Disorder Society Board of Directors, has received honoraria by the American Academy of Neurology and the International Movement Disorder Society and reports honoraria for advisory board activities for Acorda. William LaFrance, Jr. has served on the editorial boards of Epilepsia, Epilepsy & Behavior; Journal of Neurology, Neurosurgery and Psychiatry, and Journal of Neuropsychiatry and Clinical Neurosciences; receives editor’s royalties from the publication of Gates and Rowan’s Nonepileptic Seizures, 3rd ed. (Cambridge University Press, 2010) and 4th ed. (2018); author’s royalties for Taking Control of Your Seizures: Workbook and Therapist Guide (Oxford University Press, 2015); has received research support from the Department of Defense (DoD W81XWH-17-0169), NIH (NINDS 5K23NS45902 [PI]), Providence VAMC, Center for Neurorestoration and Neurorehabilitation, Rhode Island Hospital, the American Epilepsy Society (AES), the Epilepsy Foundation (EF), Brown University, and the Siravo Foundation; serves on the Epilepsy Foundation New England Professional Advisory Board, the Functional Neurological Disorder Society Board of Directors, the American Neuropsychiatric Association Advisory Council; has received honoraria for the AES Annual Meeting; has served as a clinic development consultant at University of Colorado Denver, Cleveland Clinic, Spectrum Health, Emory University, and Oregon Health Sciences University; and has provided medico legal expert testimony. Mark Rapaport, Phyllis Rosen, and Michele Price do not have any relevant disclosures.