INTRODUCTION
Long bemoaned in the literature, the magnitude of morphological variation among conspecific specimens of octopuses has rarely been quantified (Allcock et al., Reference Allcock, Strugnell and Johnson2008). Although net preservation-linked deformation is minimal in the uniformly muscular bodies of shallow-water octopodid specimens (Voight, Reference Voight1991), specimens of the deep-sea octopus genus Graneledone have a fluid-rich layer. Measurements of these specimens change during long-term storage in ethanol (Voight, Reference Voight2001). To minimize real and perceived problems with measurements, meristic characters or serial counts of body elements (O'Reilly & Horn, Reference O'Reilly and Horn2004), such as gill lamellae and arm suckers, have become standard in species descriptions. These meristic characters should be unaffected by preservation artefact, but the extent of their variation remains nearly unexplored in cephalopods and in molluscs generally.
González & Guerra, 1998 (in González et al., Reference González, Guerra, Pascual and Briand1998) described a distinctive species, Vulcanoctopus hydrothermalis, from the hydrothermal vent Genesis at 12°48.43′N 103°56.41′W on the East Pacific Rise. Male specimens that have received study were exclusively collected in this area (González et al., Reference González, Guerra, Rocha and Briand2002), although the species is documented at several areas of hydrothermal activity on the ridge crest and ranges from at least 13°N to 23°S (González et al., Reference González, Guerra and Segonzac2006). In its unusual habitat, the octopus feeds on swarming amphipods (Voight, Reference Voight2005) which often associate with giant tube worms of Riftia pachyptila Jones, 1981 that are sustained by hot, metal-rich anoxic fluids. Strugnell et al. (Reference Strugnell, Voight, Collins and Allcock2009) demonstrated the species to be nested within the monophyletic genus Benthoctopus. Taxonomic issues plaguing that genus name led Gleadall et al. (Reference Gleadall, Guerrero-Kommritz, Hochberg and Laptikhovsky2010) to extend the definition of Muusoctopus Gleadall, 2004 to include all species assigned to Benthoctopus. They, however, stated that Vulcanoctopus although a member of the clade should be retained as distinct, based on the criterion of sufficient difference. As taxonomy is intended to reflect phylogenetic relationships, the species is here assigned to the clade Muusoctopus.
To document variation in meristic characters in a single octopodid species, counts and measurements of specimens of M. hydrothermalis are considered. Data are from 13 specimens collected between 1994 and 2003 by the Deep Submergence Vehicle (DSV) ‘Alvin' from four hydrothermal vents between 8°38′N and 12°48′N on the East Pacific Rise. The variation found here remains enigmatic; it might be linked to genetic differences, or to environment, specifically temperature, following a generality in fish known as Jordan's rule.
MATERIALS AND METHODS
Octopuses of Muusoctopus hydrothermalis were collected using the suction sampler by the DSV 'Alvin' at from 2495 to 2620 m depths on the East Pacific Rise. One specimen collected at 8°38′ N104°13′W and three from 9°50′ N104°18′W form the 9°N group; the 13°N group contains one specimen from 10°46′ N103°39′W, a new lava flow habitat described by Voight et al. (Reference Voight, Zierenberg, McClain, Batson, Beers, Daly, Dushman, Gollner, Govenar, Haney, Hourdez, Liow, Parker, Von Damm, Zekely and Zelnio2004), four specimens from Genesis Chimney at 12°48.57′N 103°56.37′W and four from Parigou, a small vent at 12°48.64′N 103°56.42′W, a site described by Voight (Reference Voight2005). Slight differences in position between the site specified here and those in, e.g. González et al. (Reference González, Guerra, Rocha and Briand2002) are likely due to different navigational systems.
The Clipperton Fracture Zone forms an east–west offset near 10°N on the otherwise linear ridge, separating these areas (Figure 1). Intraspecific genetic differences in vent taxa from north and south of the fracture zone, including annelids and molluscs, have been sought using allozymes (see review by Jollivet, Reference Jollivet1996), DNA sequence data (Won et al., Reference Won, Young, Lutz and Vrijenhoek2003; Hurtado et al., Reference Hurtado, Lutz and Vrijenhoek2004; Matabos et al., Reference Matabos, Thiébaut, Le Guen, Sadosky, Jollivet and Bonhomme2008) and even microsatellite data (Fusaro, Reference Fusaro2008); no significant within-species differences have been detected. Despite the morphological differences reported here, considering these specimens to be conspecific is conservative.

Fig. 1. Map of collection areas (marked by dark oval) on the East Pacific Rise. The Clipperton Fracture Zone is visible as the east–west offset of the ridge at 10°15′N (map modified from Carbotte et al., Reference Carbotte, Arko, Chayes, Haxby, Lehnert, O'Hara, Ryan, Weissel, Shipley, Gahagan, Johnson and Shank2004).
By necessity, collections were ad hoc; therefore, the interval between collection of an octopus and its preservation on-board ship likely differed. Specimens were fixed in 8 to 10% buffered formalin in seawater and later transferred to 70% ethanol.
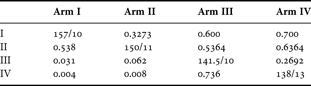
Standard octopodid measurements, as defined by Roper & Voss (Reference Roper and Voss1983), were recorded from preserved specimens. Abbreviations are: ML, mantle length; MW, mantle width; HW, head width; AL, arm length, with the roman numerals indicating arm number; AW, arm width (measured at the level of the twentieth sucker on the first right arm); WD, web depth, only the dorsal sector (A) and dorso-lateral (B) sectors are reported; SD, diameter of the largest sucker; FL, funnel length; FFL, free funnel length. Arm length and web depth were measured with a 25 cm rule, features of the mantle, sucker and funnel were measured with electronic callipers. Lengths <10 mm were rounded to the nearest 0.1 mm; others were rounded to the nearest mm. Weights of preserved specimens were determined to the nearest 0.1 g on an electronic scale. Mantle width was measured only after the mantle was opened. Arm suckers and gill lamellae, including the tiny terminal lamella, were counted with the aid of a dissecting microscope. Only the higher count from each arm pair is reported, except counts from both third arms are reported because hectocotylization modifies the third right arm. The mid-ventral funnel was slit longitudinally and the shape of the funnel organ and the state of contraction of the funnel recorded.
To assess whether parasitism by copepods of Genesis vulcanoctopusi López-González, Bresciani and Huys, 2000 impacted octopus morphology, the number of copepods in the outer mantle and the inner funnel, including what appear to be scars from previous infections following López-González et al. (Reference Lopez- González, Bresciani, Huys, González, Guerra and Pascual2000), from the level of the anus to the funnel opening, were counted in each octopus. The total counts were compared between the groups using a Mann–Whitney U test.
Data collection revealed that different arm pairs of an individual carry a different number of suckers (Table 1). Whether the arm pairs differ significantly in the number of suckers they carry was tested with a Kolmogorov–Smirnov test using pooled data from all specimens. The difference between the greatest and the fewest number of suckers on the arms of each individual were determined. This difference was plotted against the greatest number of suckers on an arm and, in a separate analysis, against arm length. Correlations were calculated to test whether the difference decreased as the number of suckers increased or as the arms lengthened, respectively.
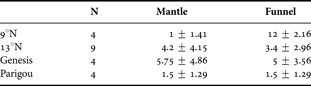
Table 1. Means and ranges for each weight, length and sucker count from specimens from 13°N and 9°N. Weight in g; measurements in mm.

N, number; L, length; diam, diameter; Gill lamellae, mean outer/mean inner counts.
These results are compared to those of González et al. (Reference González, Guerra, Rocha and Briand2002) who report the number of suckers per row, with a row being the longitudinal column on the arm for specimens from Genesis, at 12°48.7′N 103°56.43′W. Their counts were doubled prior to comparison.
Le Bris et al. (Reference Le Bris, Sarradin and Caprais2003) reported the chemical composition of hydrothermal vent fluids from Genesis, the collection site of four octopuses reported here (Table 1). The chemical analyses were performed in situ using the submersible-mounted chemical analyser Alchimist, during the ‘Nautile' cruise Hope '99 (Le Bris et al., Reference Le Bris, Sarradin and Caprais2003). Those and comparable analyses from diverse 9°N vents (Le Bris et al., Reference Le Bris, Govenar, LeGall and Fisher2006) serve as proxies of vent fluid toxicity.
RESULTS
The means and ranges of weights and measurements from the 9°N and 13°N groups are essentially the same (Table 1; original data, supplementary material online), as are the medians (unpublished data). The arms of the 9°N group of four specimens of Muusoctopus hydrothermalis, however, have between 11.7 and 22.8% fewer suckers (Figure 2; Table 1) than do the arms of the nine specimens in the 13°N group; the 9°N specimens also have slightly fewer gill lamellae. The differences are not statistically significant and are within the range González et al. (Reference González, Guerra, Rocha and Briand2002) report.

Fig. 2. Plot of arm sucker count for the first (dorsal) arm pair and the hectocotylus versus log head width. Because variation in arm length artificially enhanced the difference in sucker count, log head width is used here as the x-axis. Solid diamonds are the first arm pair of seven 13°N specimens; solid triangles are the hectocotylus of nine 13°N specimens. Open circles are sucker counts of the dorsal arm pair of four 9°N specimens; open squares are the sucker counts of the hectocotylus of four 9°N specimens.
In all individuals, different arms carry different numbers of suckers. The difference is significant between nonadjacent, but not between adjacent, arms (Table 2). The difference in the number of suckers carried by the different arms appears consistent regardless of size. The difference between the number of suckers on the arm with the most and with the least suckers is independent of both the greatest number of suckers on one arm of an individual (R2 = 0.0323) and the arm length (R2 = 0.0234).
Table 2. Results of the Kolmogorov–Smirnov test for differences in sucker counts on unmodified arms. On diagonal, median number of suckers/number of specimens for each arm, above the diagonal, D statistic; below the diagonal, probability that the difference is significant.
Variation in arm length relative to mantle size among these ‘Alvin'-collected specimens is apparent (Table 1). Specimens collected late in a dive may have been preserved relatively soon after collection, while those collected early in a dive could only have been preserved many hours later. Specimen handling regimes also differed within the 9° group.
González et al. (Reference González, Guerra, Pascual and Briand1998) were unable to describe the funnel organ from the type specimen, but the organs are clear in these specimens. The funnel organ of M. hydrothermalis is W-shaped with short lateral limbs. Octopuses from 9°N, however, have a thinner, more linear funnel organ than do most specimens from 13°N (Figure 3); variation is present within both groups. Neither the extent of funnel contraction nor mantle collar morphology can be identified as contributing to differences in funnel organ shape. Funnel retraction and blood pooled ventral to the funnel complicated measurements, but both funnel length and free funnel length are independent of head width in the narrow size-range available here (R2 = 0.00003).

Fig. 3. Funnel organs of (A) FMNH 307185 (#2) collected at Genesis (12°48.57′N 103°56.37′W) the species type locality; (B) FMNH 307183 collected at 10°46′N 103°39.41′W; (C) FMNH 287366, collected at 9°50′N 104°18′W.
Octopuses in the 9°N group carried more copepods and apparent copepod scars than did those in the 13°N group, but the difference was not significant (Table 3; U = 29, P = 0.106). Octopuses from 9°N have abundant copepods in the funnel but few on the outer mantle; the funnels and mantles of octopuses from 13°N carry about the same number of copepods. The difference in distribution is not significant (G = 5.88; 0.2> P > 0.10). The number of copepods and their apparent scars ranges in 13°N specimens from zero to 23 (supplementary material online).
Table 3. The number of parasitic copepods in the outer mantle and inner funnel of octopuses from 9°N and 13°N, the latter by site.
N, number.
DISCUSSION
Variation in meristic characters appears to be nearly unexplored in molluscs and in cephalopods. Sucker and gill lamellae counts of specimens of Muusoctopus hydrothermalis forming the 9°N group are lower by at least 12% than are those forming the 13°N group (Table 1); the funnel organs of the specimens also differ in shape (Figures 2 & 3). González et al. (Reference González, Guerra, Rocha and Briand2002) had reported mean sucker counts with standard deviations of up to 16% of the mean of each arm in 18 specimens of the species from Genesis (12°48.7′N 103°56.43′W). Their report of the number of suckers per row that is, the longitudinal column of suckers may have minimized the apparent variation.
The sucker count variation seen here in M. hydrothermalis appears to be comparable to the within-species variation shown graphically by Toll (Reference Toll1988) for five octopodid species. Toll (Reference Toll1988) cited differences in arm and hectocotylus sucker counts as characters distinguishing the Atlantic Scaeurgus unicirrhus (delle Chiaje, 1839) from the Hawaiian S. patagiatus Berry, 1913. Norman et al. (Reference Norman, Hochberg and Boucher-Rodoni2005), however, reported only what they perceived to be trivial differences in sucker count between Mediterranean and Pacific specimens of these species. They concluded that Toll examined western Atlantic specimens, and cited his graphical data as evidence that two species of Scaeurgus exist in the Atlantic Ocean. This study cautions that differences in arm sucker counts, even of over 15%, do not necessarily distinguish species.
Genetic variation might impact sucker number variation. In fish, however, subtle differences in meristic characters, such as the number of vertebrae, correlate with latitude and are attributed to differences in temperature. A generality termed ‘Jordan's rule' (Jordan, Reference Jordan1892; McDowall, Reference McDowall2008 and many others) states that lower temperatures, especially during development, increase counts of meristic features (e.g. Hubbs, Reference Hubbs1922; Tåning, Reference Tåning1952; Lindsey & Harrington, Reference Lindsey and Harrington1972).
Given the broad range of temperatures at hydrothermal vents, these mobile vent-endemic octopuses would presumably select an optimal temperature for egg development, if temperature were the primary environmental variable. Hydrothermal fluid analyses, however, demonstrate that chemically the fluids can differ substantially (Le Bris et al., Reference Le Bris, Govenar, LeGall and Fisher2006). Vent fluids at 13°N at Genesis have been shown to be anomalously low in iron, which results in abundant highly toxic hydrogen sulphide (Le Bris et al., Reference Le Bris, Sarradin and Caprais2003). The hydrothermal fluids at most 9°N vents carry more dissolved iron which binds to sulphide to form the much less toxic iron sulphide (Le Bris et al., Reference Le Bris, Sarradin and Caprais2003). If fluids high in hydrogen sulphide are toxic to the octopuses, and no available data suggest octopuses of M. hydrothermalis tolerate sulphide exposure, to survive in areas with low iron, eggs would have to develop away from vent fluids, at lower temperatures. In contrast, in areas such as 9°N where vent fluids generally carry more iron and are therefore less toxic (Le Bris et al., Reference Le Bris, Govenar, LeGall and Fisher2006), developing eggs might tolerate modest exposure to warm fluids. Jordan's rule predicts that if embryos at Genesis in the 13°N area develop in lower temperatures resultant individuals would have higher counts for both suckers and gill lamellae, consistent with the available data (Table 1).
Unique to this study is the demonstration that an octopus' four arm pairs carry different numbers of suckers (Table 2). All non-hectocotylized arms of shallow-water octopuses tend to carry the same number of suckers (unpublished data), so that the mean count from all intact arms conveys the same information as the count from any one intact arm (Toll, Reference Toll1988). Because performing counts is time-intensive and results in minimal variation, often only the suckers of one arm and the hectocotylus are reported; differences among arm pairs in other taxa may exist undiscovered.
Lopez-González et al. (2000) suggested that octopuses may encounter swarms of infective stages of copepods of Genesis vulcanoctopusi, consistent with the high variation in parasite incidence seen here. Why the copepods occur primarily inside the funnels of octopuses from 9°N and on the outer mantles of those from 13°N remains enigmatic. Although copepod infection elicits host tissue response (Lopez-González et al., 2000) the morphological differences in counts identified here cannot be attributed to the stress of exceedingly heavy parasite loads.
Variation in funnel organ morphology has been noted in several octopodids (five species of Bathypolypus by Muus (Reference Muus2002), nine species of Pareledone by Allcock (Reference Allcock2005) and Allcock et al. (Reference Allcock, Strugnell, Prodöhl, Piatkowski and Vecchione2007), two species of Graneledone by Voss & Pearcy (Reference Voss and Pearcy1990) and Allcock et al. (Reference Allcock, Collins and Vecchione2003), two species of Muusoctopus by Voss & Pearcy (Reference Voss and Pearcy1990) and Allcock et al. (Reference Allcock, Strugnell, Ruggiero and Collins2006) and in Ameloctopus litoralis by Norman (Reference Norman1992)); all of these except the species of Pareledone also lack the ink sac. Species of Pareledone occur in a seasonally dark Antarctic habitat that may limit the effectiveness of the ink sac. This pattern supports Norman's (1992) suggestion that the funnel organ might be reduced in species lacking an ink sac, and Young & Mangold's (Reference Young and Mangold2000) hypothesis that funnel organ secretions are important in forming ink masses that distract visual predators. Apparently without the ink sac, the funnel organ is freer to vary in shape, a potential liability for using this character to diagnose species of deep-sea octopodids. However, the differences in funnel-dwelling copepods may enhance any innate differences in this species.
The variation in arm sucker count seen in this unique hydrothermal vent octopus cautions against undue reliance on this character in species delineation. The parallel changes in gill lamellae and arm suckers suggest a single factor could be responsible for the variation seen. If the eggs that survived developed in different temperatures, these observations are consistent with Jordan's rule; additional data are required to test that hypothesis.
ACKNOWLEDGEMENTS
W.L. Smith generously provided helpful discussion and constructive comments on the manuscript, N. Le Bris provided literature and important insight, C.R. Fisher and R. Lutz each deposited a specimen at The Field Museum of Natural History for study. The science party of FIELD I (Focused Investigations of Environment and Life at Depth), the captain and crew of the RV ‘Atlantis' and the pilots of the DSV ‘Alvin' are thanked for their invaluable assistance at sea. In preparing the figures, C. Richardson demonstrated a keen eye for variation which enhanced this work. The National Science Foundation grant DEB-0072695 to the author funded specimen collection.
Supplementary materials and methods
The Supplementary material refered to in this article can be found online at journals.cambridge.org/mbi.








