Introduction
Anhydrous sulfates of alkali and/or transition metals are very sensitive to moist air. Due to this fact, the number of described anhydrous sulfate mineral species with transition and alkali metals is limited. A number of these minerals are known from active volcanic fumaroles with oxidising environments. Exhalative mineral assemblages from fumaroles of the First and Second scoria cones, Great Fissure Tolbachik eruption 1975–76 (Fedotov and Markhinin, Reference Fedotov and Markhinin1983) are very rich in anhydrous sulfate minerals of alkali and transition metals (mostly Cu2+) (Vergasova and Filatov, Reference Vergasova and Filatov2012).
Herein we report on the chemical composition, structure and properties of koryakite (Cyrillic: корякит), NaKMg2Al2(SO4)6. The mineral is named for the Koryaks, an ethnic group who are the original inhabitants living on the Kamchatka peninsula. Both the mineral and the mineral name were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2018-013, Nazarchuk et al., Reference Nazarchuk, Siidra, Zaitsev and Vlasenko2018). Type material is deposited at the Mineralogical Museum, St. Petersburg State University, St. Petersburg, Russia (catalogue no. 1/19688).
Occurrence and association
Koryakite occurs as a product of fumarolic activity. It was found in June 2016 in the Yadovitaya fumarole, Second scoria cone, North Breach, Great Fissure eruption, Tolbachik volcano, Kamchatka, Russia (55°49′59″N, 160°19′59″E). The Second Scoria Cone is located ~18 km SSW of the active shield volcano Ploskiy Tolbachik (Fedotov and Markhinin, Reference Fedotov and Markhinin1983). Koryakite closely associates with euchlorine (Fig. 1) and langbeinite. Koryakite is a fumarolic mineral that is deposited directly from volcanic gas emissions as a sublimate. The temperature of gases at the sampling location was ~300°C. All the recovered samples were packed immediately and isolated when collected to avoid any contact with the external atmosphere.

Fig. 1. Abundant elongated white transparent crystals of koryakite in association with green euchlorine in the voids of basaltic scoria.
Physical properties
Koryakite is colourless (Fig. 1), has a white streak and vitreous lustre. The mineral is brittle with uneven fracture. Cleavage or parting has not been observed. Hardness corresponds to 2–3 on the Mohs’ scale. The density could not be measured due to the lack of an appropriate sample, but it has been calculated as 2.892 g cm–3 using structural data and the empirical formula. No fluorescence is detected.
Koryakite is optically uniaxial (–), ω = 1.546(2) and ɛ = 1.535(2). Microscopically, it is colourless and non-pleochroic. Compatibility [1 – (K P/K C) = 0.030] is excellent (Mandarino, Reference Mandarino1981).
Chemical composition
One crystal (110 μm × 55 μm in size) was mounted in epoxy resin and polished with oil suspension. Compositional data from energy-dispersive spectroscopy (EDS) analyses (N = 8) (Table 1) were obtained using a Hitachi S-3400N scanning electron microscope equipped with an Oxford Instruments X-Max 20 Energy Dispersive Spectrometer. The electron beam accelerating voltage was 20 kV and the current 1.8 nA; a defocused beam (10 μm spot size) was used and the X-ray acquisition time was 30 s. The mineral is stable under the electron beam; no surface damage was observed after analyses.
Table 1. Compositional data (wt.%) for koryakite.

S.D. – standard deviation
The empirical formula calculated on the basis of 24 O atoms per formula unit (apfu) is Na1.03K0.93(Mg1.89Cu0.07Ca0.04Zn0.03)Σ2.03(Al1.68Fe3+0.26)Σ1.94(S6.02Si0.02)Σ6.04O24. The simplified formula is NaKMg2Al2(SO4)6, which requires Na2O 4.18, K2O 6.36, MgO 10.88, Al2O3 13.76, SO3 64.83, total 100.00 (wt.%).
Koryakite is soluble in warm H2O.
X-ray crystallography
Experiment
Powder X-ray diffraction data were collected using a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with cylindrical image plate detector using Debye–Scherrer geometry (CoKα radiation, d = 127.4 mm). For the powder-diffraction study, a Gandolfi-like motion was used to randomise the sample. Data (in Å) are given in Table 2. Unit-cell parameters refined from the powder data processed using osc2xrd software (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017) are as follows: rhombohedral, space group R ![]() $\bar{3}$, a = 8.1129(2) Å, c = 22.7064(9) Å, V = 1294.28(8) Å3 and Z = 3.
$\bar{3}$, a = 8.1129(2) Å, c = 22.7064(9) Å, V = 1294.28(8) Å3 and Z = 3.
Table 2. Powder X-ray diffraction data (d in Å) for koryakite.

The strongest lines are given in bold.
A transparent platy crystal fragment of koryakite was mounted on a thin glass fibre for X-ray diffraction analysis using a Bruker APEX II DUO X-ray diffractometer with a micro-focus X-ray tube operated with MoKα radiation at 50 kV and 40 mA. The data were integrated and corrected for absorption using a multi-scan type model implemented in the Bruker programs APEX and SADABS (Bruker-AXS, 2014). More than a hemisphere of X-ray diffraction data was collected. The structure was solved by direct methods and refinement by means of the SHELX program (Sheldrick, Reference Sheldrick2015) in the R ![]() $\bar{3}$ space group converged to R 1 = 0.072 (Table 3). The twinning matrix [0
$\bar{3}$ space group converged to R 1 = 0.072 (Table 3). The twinning matrix [0 ![]() $\bar{1}$ 0/
$\bar{1}$ 0/ ![]() $\bar{1}$ 0 0/ 0 0 1] was employed during the crystal-structure refinement. The refined twin ratio of the two components was 0.199(1):0.801(1). The M2 site was modelled on an Mg scattering curve (Mg being the major component) and the resulting site occupancy factor of 1.07(2) is supporting the proposed mixed composition for the M2 site based on EDS analysis (Table 1). The final model included anisotropic displacement parameters for all atoms. The final atomic coordinates and anisotropic displacement parameters are given in Table 4 and selected interatomic distances in Table 5. Bond-valence sums are given in Table 4. All bond-valence parameters were taken from Brese and O'Keeffe (Reference Brese and O'Keeffe1991). All of the bond-valence sums are in good agreement with the expected oxidation states for all atoms. Lists of observed and calculated structure factors have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
$\bar{1}$ 0 0/ 0 0 1] was employed during the crystal-structure refinement. The refined twin ratio of the two components was 0.199(1):0.801(1). The M2 site was modelled on an Mg scattering curve (Mg being the major component) and the resulting site occupancy factor of 1.07(2) is supporting the proposed mixed composition for the M2 site based on EDS analysis (Table 1). The final model included anisotropic displacement parameters for all atoms. The final atomic coordinates and anisotropic displacement parameters are given in Table 4 and selected interatomic distances in Table 5. Bond-valence sums are given in Table 4. All bond-valence parameters were taken from Brese and O'Keeffe (Reference Brese and O'Keeffe1991). All of the bond-valence sums are in good agreement with the expected oxidation states for all atoms. Lists of observed and calculated structure factors have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
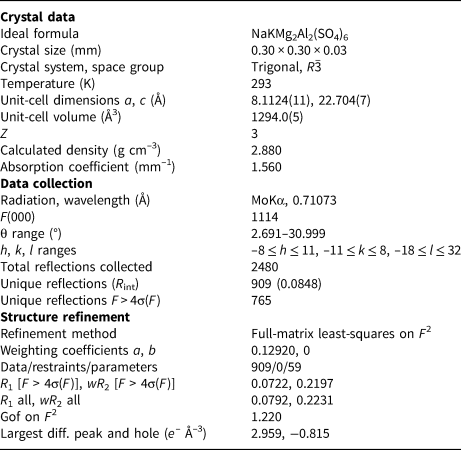
Table 3. Crystallographic data and refinement parameters for koryakite.

Table 4. Bond-valence sums (BVS), coordinates, anisotropic and isotropic displacement parameters (Å) of atoms in koryakite.

*Al0.88(2)Fe0.12(2); **Rounded and fixed in agreement with microprobe data as Mg0.93Cu0.035Ca0.02Zn0.015.
Bond-valence sums for mixed M1 and M2 sites were calculated using parameters for Al–O and Mg–O bonds from Brese and O'Keeffe (Reference Brese and O'Keeffe1991), respectively.
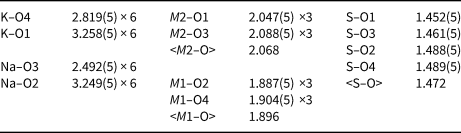
Table 5. Selected interatomic distances (Å) in koryakite.
Crystal structure
The crystal structure of koryakite contains five symmetrically independent cationic sites (Fig. 2, Table 4). K and Na sites are occupied exclusively by K+ and Na+ cations, respectively. The K site is symmetrically coordinated by six bonds K–O4 = 2.819(5) Å and by six K–O1 = 3.258(5) Å. This KO46 polyhedron is a trigonal antiprism. Similar coordination environments are observed for the Na site with six Na–O3 = 2.492(5) Å and six Na–O2 = 3.249(5) Å bonds.

Fig. 2. Coordination polyhedra of cations in the structure of koryakite. Displacement ellipsoids are drawn at 50% probability level.
There are two octahedrally coordinated M sites. An octahedral M1 site is occupied exclusively by Al3+ and Fe3+ cations. The refined ratio of Al:Fe (Table 4) is close to that obtained by microprobe analysis. The M2 site is predominantly occupied by Mg2+ cations and its full occupation by various divalent cations was fixed in agreement with microprobe data. M1–O bonds in the M 3+1O6 octahedron are shorter than M2–O bonds in M 2+2O6 (Table 5). Both of the octahedra are slightly distorted.
One S site is compatible with occupancy by S6+ and tetrahedrally coordinated by O anions. The <S–O> distance is 1.472 Å, which is in an excellent agreement with the <S–O> distance of 1.473 Å reported for sulfate minerals by Hawthorne et al. (Reference Hawthorne, Krivovichev, Burns, Alpers, Jambor and Nordstrom2000).
M 3+1O6 and M 2+2O6 octahedra are isolated from each other in the structure of koryakite. The SO4 tetrahedron shares each of its vertices with M 3+1O6 and M 2+2O6 octahedra and each M 3+1O6 and M 2+2O6 octahedron is linked to six SO4 tetrahedra. Thus a three-dimensional [M 2+2M 3+2(SO4)6]2– heteropolyhedral framework is formed (Fig. 3a). The channels run parallel to [001], where Na+ and K+ cations are located (Fig. 3b,c). The channel walls are lined by sulfate tetrahedra both in the case of Na and K. Na+ cations are segregated in the layers containing the smaller M 3+1O6 octahedra (Fig. 3b), whereas K+ cations are segregated in the layers with the relatively larger M 2+2O6 octahedra (Fig. 3c).

Fig. 3. General projection of the crystal structure of (a) koryakite (M 3+1O6 = green; M 2+2O6 = blue; and SO4 = yellow) with the channels filled by Na+ and K+ cations. The framework can be split into layers (b,c). The channels are empty in (d,e) millosevichite Al2(SO4)3 (after Dahmen and Gruehn, Reference Dahmen and Gruehn1993) (AlO6 = green) and filled by Na+ cations in (f,g) synthetic NaMgFe3+(SO4)3 (after Slater and Greaves, Reference Slater and Greaves1994) ((Mg0.5Fe3+0.5)O6 = turquoise).
Discussion
No natural or synthetic chemical analogues of koryakite are known to date. The topology of the [M 2+2M 3+2(SO4)6]2– framework in koryakite is very similar to that in millosevichite Al2(SO4)3 (Dahmen and Gruehn, Reference Dahmen and Gruehn1993) (Fig. 3b,d) and mikasaite Fe3+2(SO4)3 (Christidis and Rentzeperis, Reference Christidis and Rentzeperis1976) (Table 6). Replacement of the part of the trivalent cations in the [M 3+2(SO4)3]0 framework (Fig. 3b) by divalent cations provides the negative charge and allows the incorporation of the alkali species in the channels, which are empty in millosevichite and mikasaite. This structural mechanism is reminiscent of the concept of stuffed derivative structures first proposed by Buerger (Reference Buerger1954). Thus, the distant analogy of observed millosevichite–koryakite structural relationships can be found in silicates, as e.g. nepheline with a crystal structure derived from tridymite (Abbott, Reference Abbott1984). Regardless of the complex substitutions observed in koryakite, its general structural motif and the symmetry remain the same as in a parent Al2(SO4)3 crystal structure (Dahmen and Gruehn, Reference Dahmen and Gruehn1993).
Table 6. Crystal chemical data of koryakite, millosevichite, mikasaite and synthetic NaMgFe3+(SO4)3.

Koryakite is related structurally to NaMgFe3+(SO4)3 (Table 6) (Slater and Greaves, Reference Slater and Greaves1994) and, as well as this compound, to the broader family of NASICON-related phases (Anantharamulu et al., Reference Anantharamulu, Koteswara Rao, Rambabu, Kumar, Radha and Vithal2011). The (Mg0.5Fe3+0.5)O6 octahedra have a mixed occupancy in NaMgFe3+(SO4)3 (Fig. 3f) and Na+ cations are located in the channels similar to koryakite.
Acknowledgements
We are grateful to Tonči Balić-Žunić, one anonymous reviewer and Peter Leverett for valuable comments. This work was supported financially by the Russian Science Foundation, grant no. 16-17-10085. Technical support by the SPbSU X-ray Diffraction and Geomodel Resource Centres is gratefully acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.69