Introduction
The rearing of egg parasitoids on factitious hosts is crucial for the success of many biological control programs because it reduces production costs and consequently increases the utility of the biocontrol agent for large-scale releases (Parra, Reference Parra, Parra and Zucchi1997). However, the release success of a parasitoid produced on a factitious host depends on detailed information on its bioecological characteristics and its interaction with the targeted host in the field (Bourchier & Smith, Reference Bourchier and Smith1996). A host switch might trigger changes in foraging behavior and parasitism capacity of the egg parasitoid (Jones et al., Reference Jones, Bilton, Mak and Sait2015). Therefore, for a successful control of target pests in the field a well-designed quality control procedure is required in order to ensure that the parasitism capacity of the laboratory-reared parasitoid is similar to that of the same parasitoid species found in nature or produced on its natural host (Clarke & McKenzie, Reference Clarke and McKenzie1992).
Among various egg parasitoid species with high potential to be used in augmentative biological control programs, Telenomus remus (Nixon) (Hymenoptera: Platygastridae) stands out for being effective against various pest species of the genus Spodoptera Guenée (Lepidoptera: Noctuidae) (Pomari et al., Reference Pomari, Bueno, Bueno and Menezes Junior2012), mainly due to its high reproductive capacity (Cave, Reference Cave2000; Bueno et al., Reference Bueno, Carneiro, Pratissoli, Bueno and Fernandes2008). However, due to the difficulties and costs of rearing T. remus on its natural host (Pomari-Fernandes et al., Reference Pomari-Fernandes, Bueno, Queiroz and De Bortoli2015), this parasitoid to date has only been used against Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) for experimental purposes or for releases in small areas (Ferrer, Reference Ferrer2001).
S. frugiperda rearing is time- and resource-consuming (Perkins, Reference Perkins1979), mainly because of larval cannibalism, which requires the rearing of larvae in individual vials to reduce pre-imaginal mortality (Chapman et al., Reference Chapman, Williams, Martinez, Cisneros, Caballero, Cave and Goulson2000). In this context, Kumar et al. (Reference Kumar, Pawar and Divakar1986) and, more recently, Pomari-Fernandes et al. (Reference Pomari-Fernandes, Bueno, Queiroz and De Bortoli2015) reported the successful development of T. remus in Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) eggs, suggesting it to be a promising factitious host, which is easily reared in the laboratory on a larger scale at lower costs compared with S. frugiperda (Bueno et al., Reference Bueno, Carneiro, Pratissoli, Bueno and Fernandes2008). However, to the best of our knowledge, quality control of T. remus reared on C. cephalonica for successive generations has never been examined.
According to van Lenteren (Reference van Lenteren1992), quality control aims to determine whether a parasitoid remains effective in controlling a target pest in the field after being reared for several generations in the laboratory. The development of each T. remus generation takes between 13 and 15 days at 25°C (Bueno et al., Reference Bueno, Carneiro, Pratissoli, Bueno and Fernandes2008; Pomari-Fernandes et al., Reference Pomari-Fernandes, Bueno, Queiroz and De Bortoli2015). It is unclear for how long a parasitoid colony can be kept in the laboratory while maintaining acceptable quality for releasing purposes, but longer time periods are desirable for a successful augmentative biological control programs (van Lenteren, Reference van Lenteren2003).
Quality assessment in the field can be excessively time- and labor-consuming, and as a result may not be sufficiently effective (Dias et al., Reference Dias, Parra and da Costa Lima2008). Therefore, laboratory procedures for a quick evaluation of laboratory-produced egg parasitoids are essential for the quality control of biocontrol agents (van Lenteren, Reference van Lenteren1992). The standardized quality control procedures established by the International Organization of Biological Control (Global IOBC Working Group: ‘Quality Control of Mass Reared Arthropods’) identify the number of emerged adults, sex ratio, fertility, longevity, adult size, flight activity, and performance in the field as the most important biological parameters to be evaluated (van Lenteren, Reference van Lenteren2003). Among these, parasitoid longevity, parasitism capacity, and flight activity are the main parameters when testing parasitoid quality for the use in augmentative biological control programs (Prezotti & Parra, Reference Prezotti, Parra, Parra, Botelho, Ferreira and Bento2002). We therefore aimed to evaluate the quality of T. remus reared on C. cephalonica eggs for successive generations (P35, P40, and P45) by recording parasitoid size, flight activity, and parasitism capacity on its natural host (S. frugiperda eggs). In order to represent a profitable time period for the commercial exploitation of this parasitoid species, we reared 35–45 successive generations of T. remus during 455–585 days.
Material and methods
Parasitoid and host colonies
C. cephalonica and S. frugiperda eggs as well as T. remus females used in the experiments originated from insect colonies kept at Embrapa Soybean, Londrina, State of Paraná, Brazil. S. frugiperda was originally collected from maize plants in Rio Verde, State of Goiás. This strain was kept in the laboratory for approximately 9 years during which new field insects were introduced on a yearly basis to maintain insect quality. Perkins (Reference Perkins1979) reported successful rearing of S. frugiperda in the laboratory for more than 18 years without any indication of degeneration.
Rearing in our study was carried out under controlled environmental conditions inside Biochemical Oxygen Demand (BOD) climate chambers (ELETROLab®, model EL 212, São Paulo, SP, Brazil) set at 80 ± 10% humidity, a temperature of 25 ± 2°C, and a 14/10 h photoperiod (L/D). Insects were fed on an artificial diet described by Greene et al. (Reference Greene, Leppla and Dickerson1976) and Parra (Reference Parra2001). C. cephalonica was collected from UNESP/Jaboticabal and had been kept in the laboratory for approximately 26 generations (3 years) prior to the experiment. It was reared on a diet composed of whole-wheat flour (97%) and yeast (3%), as described by Bernardi et al. (Reference Bernardi, Haddad and Parra2000), using an adapted rearing method for Anagasta kuehniella (Zeller) (Lepidoptera: Pyralidae) (Parra, Reference Parra, Parra and Zucchi1997).
T. remus was originally collected in Ecuador and grown at the parasitoid rearing facilities of ESALQ/USP (Luiz de Queiroz College of Agriculture/University of São Paulo), from where some specimens were transferred to Embrapa Soybean 9 years ago. In the laboratory, T. remus was reared using S. frugiperda egg masses (approximately 150 eggs each), which were glued onto a cardboard sheet (2 × 8 cm2) and introduced into tubes together with eggs previously parasitized by T. remus. Small drops of honey were placed inside these tubes to feed the adults as soon as they emerged. The tubes were then closed, and the eggs allowed to be parasitized for 24 h. Adults that emerged from these eggs were used for trials or colony maintenance.
Bioassays
Three independent experiments were carried out to study parasitoid size, flight activity, and parasitism capacity on S. frugiperda eggs of T. remus emerged from C. cephalonica eggs. All trials were carried out in controlled environmental conditions inside BODs as previously described for the parasitoid and host colonies.
Morphological characters of T. remus reared on C. cephalonica eggs for successive generations (bioassay 1)
The experiment was carried out in a 3 × 2 factorial completely randomized design; three parasitoid generations (P35, P40, and P45) × 2 parasitoid genders (female and male). Ten replicate adults of both genders were measured individually. Thus, ten males and ten females were measured for each parasitoid generation, totaling 60 adults in bioassay 1. Parasitoids reared on S. frugiperda eggs and exposed to parasitism on C. cephalonica eggs formed the P0 generation. Eggs of S. frugiperda are almost spherical in shape (length 454.9 mm and width 390.2 mm), with a volume of approximately 0.036 mm3. The chorion is about 2.50 mm thick, but thicker (up to 11.95 mm) where the exochorion forms a bridge or a ridge (Cônsoli et al., Reference Cônsoli, Kitajima and Parra1999). The P1 generation was the first generation of parasitoids reared on C. cephalonica eggs. In contrast to S. frugiperda eggs, C. cephalonica eggs are ellipsoid-shaped (length 573.5 mm and width 346.1 mm), but with the same volume of 0.036 mm3. Their chorion thickness ranges from 4.18 to 5.32 mm (Cônsoli et al., Reference Cônsoli, Kitajima and Parra1999). Length and width of the right anterior wing, length of the right hind tibia, and body length (head to tip of the abdomen) were measured in each replicate (adult insect). To measure the morphological characters, each specimen was photographed using a stereoscopic microscope (Leica Application Suite, Version 1.6.0). Images were used for morphometric analysis with the software Image J (Version 1.47).
Flight activity of T. remus reared on C. cephalonica eggs for successive generations (bioassay 2)
The experimental design was completely randomized, consisting of four treatments [three generations (P35, P40, and P45) of T. remus reared on C. cephalonica eggs, and T. remus from eggs of the natural host S. frugiperda (P0)] and ten replicates. Each replicate consisted of 100–150 T. remus pupae (100–150 eggs parasitized by T. remus). Shortly before emergence, T. remus pupae were positioned on a plastic plate of 2.5 cm diameter and 1 cm height, which was placed at the bottom of each replicate. Emergence was allowed for 48 h to ensure complete emergence of all parasitoids from the pupae. This protocol and setup of the test unit was originally proposed by Dutton & Bigler (Reference Dutton and Bigler1995) and adapted in ESALQ-USP (Prezotti et al., Reference Prezotti, Parra, Vencovsky, Dias, Cruz and Chagas2002) as described in the following: The test unit (fig. 1) consisted of a PVC cylinder (18 cm high and 11 cm in diameter). The top of the cylinder was closed using a clear Petri dish (diameter 13 cm) sprayed with entomological glue (composed of polybutene and synthetic silica) to trap flying T. remus (‘flyers’). In order to attract the insects toward the light source at the top of the cage, the interior was painted with black ink and the bottom was sealed with a flexible black plastic sheet. Entomological glue was spread on the walls of the cage (3.5 cm from the bottom) to serve as a trap for ‘walkers’ (parasitoids that were unable to fly, but could walk and had no visible deformation). Originally, this test unit was used to measure parasitoid flight initiation (Dutton & Bigler, Reference Dutton and Bigler1995). However, in addition to trapping deformed individuals, parasitoids were also caught inside the cage that did not have enough time to unfold their wings. Therefore, this test unit was modified by Prezotti et al. (Reference Prezotti, Parra, Vencovsky, Dias, Cruz and Chagas2002) by inserting a vial (12 mm Ø × 75 mm height) inside the cage into which the pupae were placed. This modification allowed sufficient time for the emerged non-deformed parasitoids to unfold their wings during their walk inside the vial toward the entomological glue (3.5 cm from the bottom) (Prezotti et al., Reference Prezotti, Parra, Vencovsky, Dias, Cruz and Chagas2002).
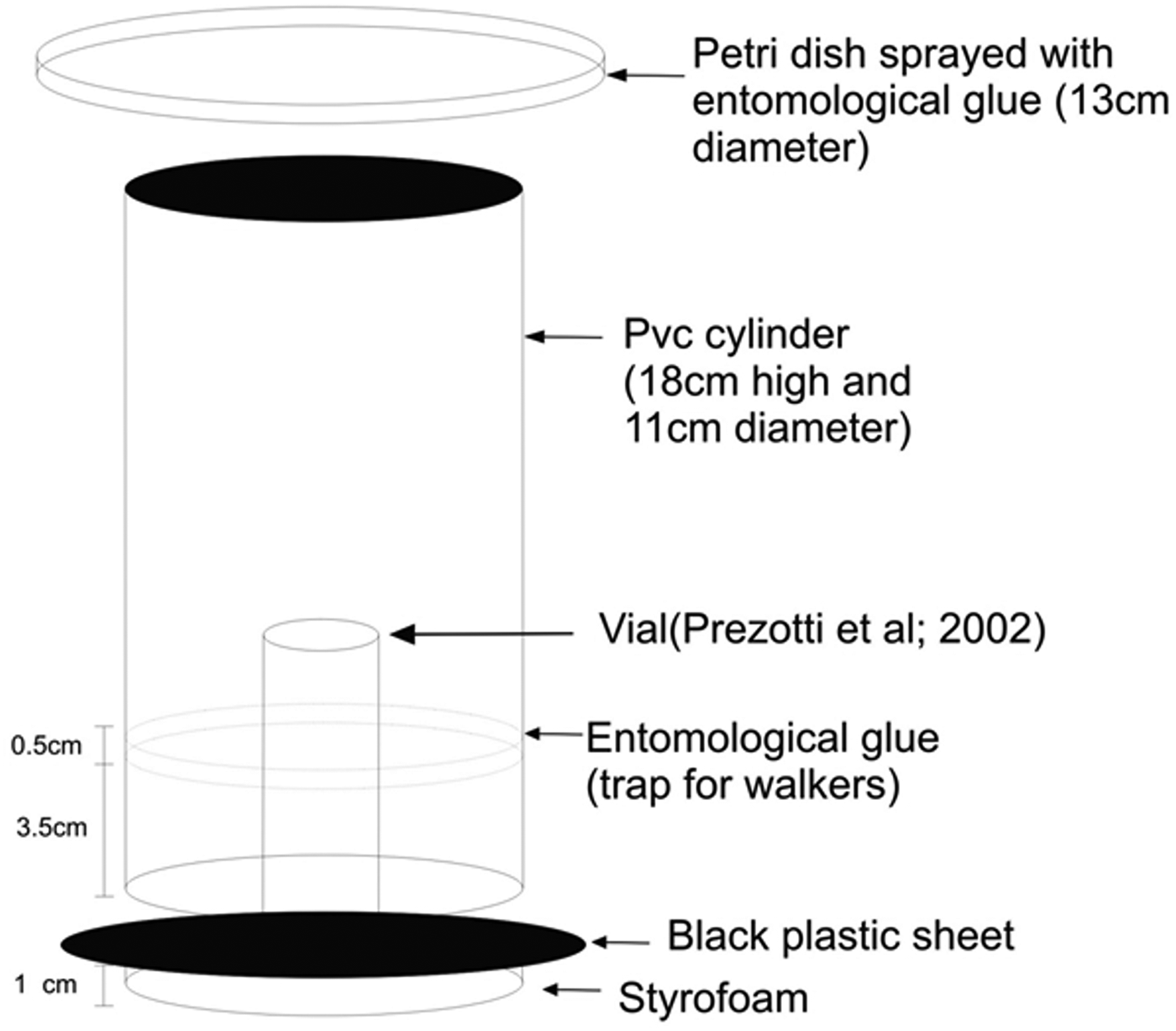
Fig. 1. Laboratory flight test unit developed by Dutton & Bigler (Reference Dutton and Bigler1995) and adapted in ESALQ-USP by Prezotti et al. (Reference Prezotti, Parra, Vencovsky, Dias, Cruz and Chagas2002).
The number of parasitoids in the adhesive ring (‘walkers’), in the Petri dish (‘flyers’), and the ‘deformed’ individuals were recorded and used to calculate the percentages of these three groups of the total number of emerged adults. The parasitoids considered ‘non-flyers’ were observed under a stereoscope to determine the percentage of individuals with wing deformities (‘deformed’) (Prezotti et al., Reference Prezotti, Parra, Vencovsky, Dias, Cruz and Chagas2002).
Parasitism capacity on S. frugiperda eggs of T. remus reared on C. cephalonica eggs for consecutive generations (bioassay 3)
The experiment was conducted in a completely randomized design with three treatments (T. remus reared on eggs of C. cephalonica for consecutive generations – P35, P40, and P45 parasitizing S. frugiperda eggs) and six replicates (each replicate consisting of five individualized females).
Mated T. remus individuals (newly emerged: ≤48 h old) were placed into separate glass tubes (12 mm Ø × 75 mm tall), which were then covered with the PVC film. Droplets (about 100 µl each) of pure honey were placed on the walls of the glass tubes to feed the females. Thirty glass tubes (six replicates of five females each) were prepared for each treatment. Approximately 100 eggs of S. frugiperda (≤24 h old) were glued onto cards made of white Bristol board paper (2.5 × 5 cm). Each paper was previously labeled with the respective treatments. Then, these cards were exposed to parasitism for 24 h. The cards were replaced daily until the death of the female parasitoid. Eggs removed from the glass tubes were maintained inside the same environmental chamber under controlled conditions until the emergence of parasitoids. Evaluated parameters were: parental T. remus female longevity (days), lifetime number of parasitized eggs/female, egg-to-adult duration (days), viability of parasitism (%) (percentage of parasitized eggs from which parasitoids emerged), progeny sex ratio, and the number of parasitized eggs per day (daily parasitism).
Statistical analysis
For the analysis of bioassays 1 and 3, prior to ANOVA, experimental results were subjected to exploratory analyses to assess the assumptions of normality of residuals (Shapiro & Wilk, Reference Shapiro and Wilk1965) and homogeneity of variance of the treatments (Burr & Foster, Reference Burr and Foster1972) and, if necessary, transformed for ANOVA. Treatment means were then compared by Tukey's test at the 5% probability level. For the analysis of bioassay 2, percentages of ‘flyers’, ‘walkers’, and ‘deformed’ individuals were compared using Chi-square statistics (SAS Institute, 2009).
Results
Morphological characters of T. remus reared on C. cephalonica eggs for successive generations
Factorial analysis did not detect a significant interaction between parasitoid generation and parasitoid gender regarding the morphological characters wing length (P generation × gender = 0.6366; F generation × gender = 0.46), wing width (P generation × gender = 0.6302; F generation × gender = 0.47), body length (P generation × gender = 0.7541; F generation × gender =0.28) and right hind tibia length (P generation × gender = 0.3548; F generation × gender = 1.06) (table 1).
Table 1. Morphological characters (mm) of Telenomus remus reared on Corcyra cephalonica eggs for successive generations (P35, P40, and P45) (bioassay 1) under controlled environmental conditions (25 ± 2°C, 80 ± 10% RH, and photoperiod of 14/10 h L/D) (N = 60).
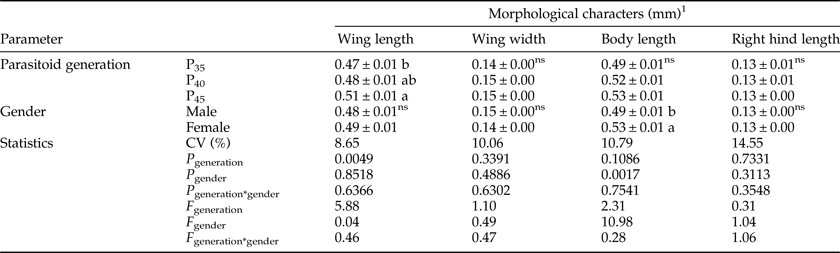
1 Means ± SE followed by the same letter in the column of each parameter are not significantly different from each other (Tukey's test, P > 0.05); nsANOVA non-significant.
Wing length differed between generations (P generation =0.0049; F generation = 5.88), with the longest wings observed in P45 (0.51 mm), followed by P40 (0.48 mm) and by P35 (0.47 mm). Wing length was similar between parasitoid genders (P gender = 0.8518; F gender = 0.04) (table 1).
Wing width did not differ between parasitoid generations (P generation = 0.3391; F generation = 1.10) or genders (P gender = 0.4886; F gender = 0.49) (table 1). Differently, body length did not differ between generations (P generation =0.1086; F generation = 2.31) but was higher for females than for males (P gender = 0.0017; F gender = 10.98) (table 1). Right hind tibia length did not differ between generations (P generation = 0.7331; F generation = 0.31) or genders (P gender = 0.3113; F gender = 1.04) (table 1).
Flight ability of T. remus reared on C. cephalonica eggs for successive generations
The percentage of ‘flyers’ that emerged from C. cephalonica eggs in generation P35 was similar to the percentages in P40 (table 2, χ2 = 28.77, P = 0.0696, df = 19) and P45 (table 2, χ2 = 12.30, P = 0.8726, df = 19), and to the percentage of ‘flyers’ that emerged from S. frugiperda eggs (P0) (table 2, χ2 = 12.21, P = 0.8765, df = 19). In contrast, the percentage of ‘flyers’ in P40 was lower than in P45 (table 2, χ2 = 148.65, P < 0.0001, df = 19) and P0 (table 2, χ2 = 156.03, P < 0.0001, df = 19). Percentages of ‘flyers’ in P45 and P0 were similar (table 2, χ2 = 4.75, P = 0.9996, df = 19).
Table 2. Percentages of ‘flyers’, ‘walkers’, and ‘deformed’ of Telenomus remus when reared on different hosts for various generations (bioassay 2) under controlled environmental conditions (25 ± 2°C, 80 ± 10% RH, and photoperiod of 14/10 h L/D) (N = 20).
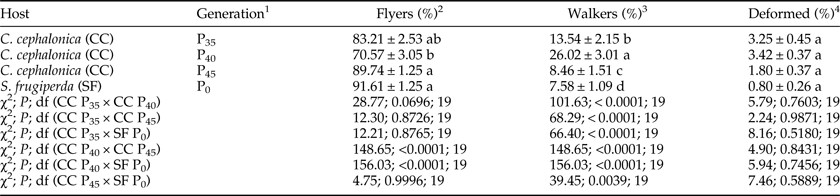
Means ± SE followed by the same letter in the column are not significantly different from each other (χ2 test, P > 0.05).
1 Generation of parasitoids used in the treatment [T. remus was reared on eggs of S. frugiperda for approximately 350 generations (P0) and on eggs of C. cephalonica for 35 (P35), 40 (P40), and 45 (P45) generations].
2 Percentage of parasitoids able to fly.
3 Percentage of parasitoids that did not fly but had no visible deformation.
4 Percentage of parasitoids with visible deformation.
The percentage of ‘walkers’ in generation P35 was lower than in P40 (table 2, χ2 = 101.63, P < 0.0001, df = 19) but higher than in P45 (table 2, χ2 = 68.29 P < 0.0001, df = 19) and P0 (table 2, χ2 = 66.40, P < 0.0001, df = 19). The percentage of ‘walkers’ in generation P40 was higher in both P45 (table 2, χ2 = 148.65, P < 0.0001, df = 19) and P0 (table 2, χ2 = 156.03, p < 0.0001, df = 19) and it was higher in generation P45 than in P0 (table 2, χ2 = 39.45, P = 0.0039, df = 19).
The fraction of ‘deformed’ individuals was similar between treatments (table 2): their percentage in generation P35 was similar to that in P40 (table 2, χ2 = 5.79, P = 0.7603, df = 19) and P45 (table 2, χ2 = 2.24 P = 0.9871, df =19) as well as in P0 (table 2, χ2 = 8.16, P = 0.5180, df = 19). Likewise, the percentage of ‘deformed’ individuals that emerged in generation P40 was similar to P45 (table 2, χ2 = 4.90, P = 0.8431, df = 19) and to P0 (table 2, χ2 = 5.94, P = 0.7456, df = 19). Similarly, the percentage of ‘deformed’ individuals that emerged in P45 was similar to P0 (table 2, χ2 = 7.46, P = 0.5889, df = 19).
Parasitism capacity on S. frugiperda eggs of T. remus reared on C. cephalonica eggs for consecutive generations
Parental T. remus female longevity (P = 0.5141; F = 0.70), lifetime number of parasitized eggs/parasitoid females (P = 0.0767; F = 5.22), viability of parasitism (% of parasitoids emerged from parasitized eggs) (P = 0.2005; F = 1.84), and progeny sex ratio (P = 0.6395; F = 0.46) did not differ between parasitoid generations (table 3). In contrast, egg-to-adult duration (days) was around 2 days shorter for P45 compared with P40 (P < 0.0001; F = 6768.89) (table 3).
Table 3. Biological characteristics of Telenomus remus reared on Corcyra cephalonica eggs for successive generations parasitizing Spodoptera frugiperda eggs (bioassay 3) under controlled environmental conditions (25 ± 2°C, 80 ± 10% RH, and photoperiod of 14/10 h L/D) (N = 15).

1 Means ± SE followed by the same letter in the column are not significantly different from each other (Tukey's test, P > 0.05); – Data not evaluated; nsANOVA non-significant.
More than 80% of the lifetime parasitism on S. frugiperda eggs of T. remus reared on eggs of C. cephalonica from generations P35 (fig. 2a), P40 (fig. 2b), and P45 (fig. 2c) was reached on the 2nd, 1st, and 1st day of parasitism, respectively. The number of parasitized eggs per day varied with parasitoid generations, but was higher in the first 24 h for all generations. In all treatments, the number of eggs parasitized per day decreased with time (fig. 2a–c).
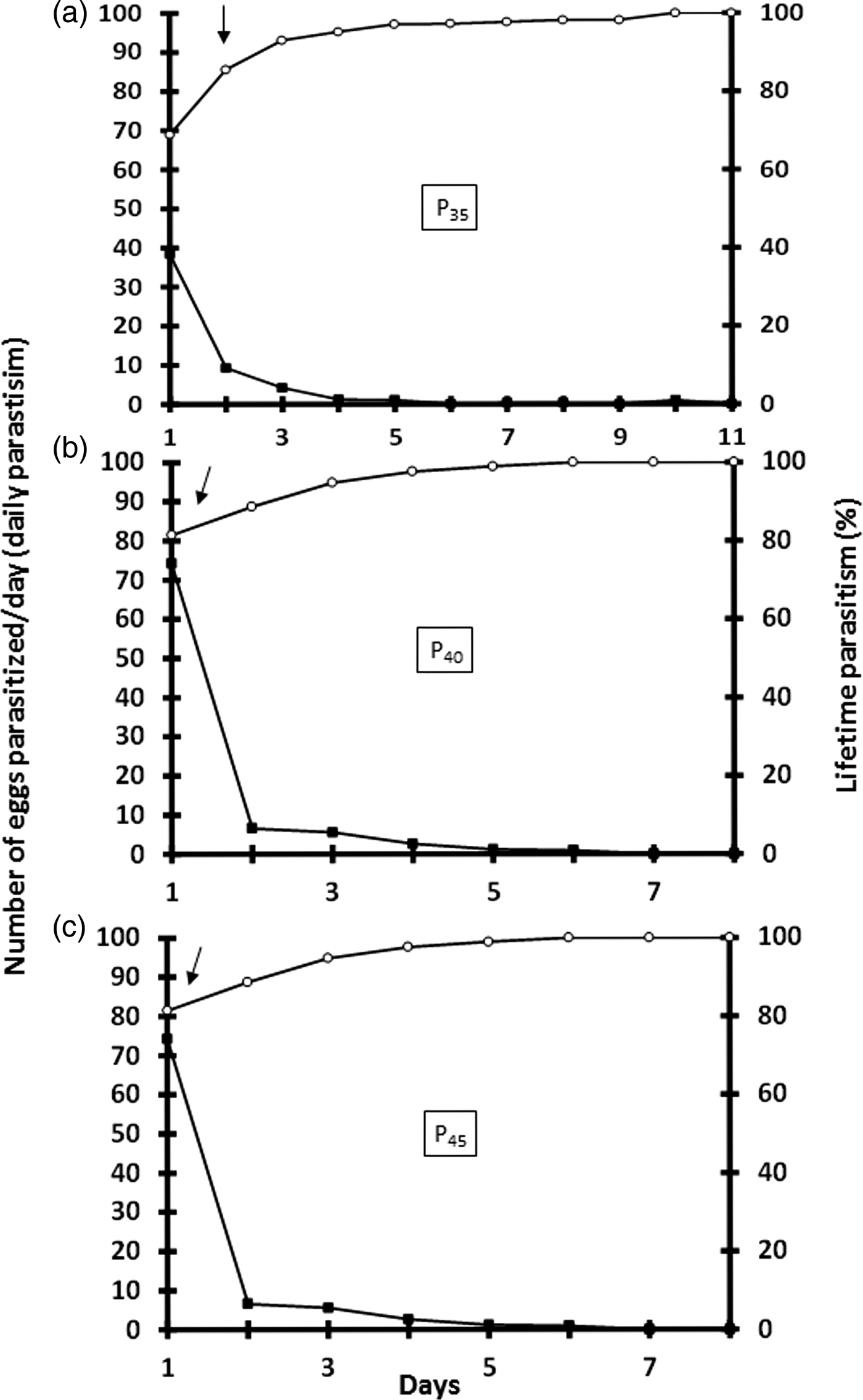
Fig. 2. Number of Spodoptera frugiperda eggs parasitized per day, and lifetime parasitism (%) of Telenomus remus reared on Corcyra cephalonica eggs for a variety of generations under controlled environmental conditions (25 ± 2°C, 80 ± 10% RH, and a photoperiod of 14/10 h L/D) (N = 15). (a) 35, (b) 40, and (c) 45 generations.
Discussion
Our research aimed to evaluate the quality of T. remus after being reared on eggs of the factitious host C. cephalonica for successive generations (P35, P40, and P45) by measuring parasitoid size and flight activity. This quality control was performed to test the fitness of these laboratory-produced parasitoids in field releases to control Spodoptera spp. The recorded results indicate that the quality of T. remus did not decrease significantly after rearing on C. cephalonica eggs for 45 generations. Therefore, we conclude that rearing of T. remus on the factitious host C. cephalonica promises to be successful.
The evaluated morphological characters (wing length, wing width, body length, and right hind length) have been suggested as adequate indicators of parasitoid fitness in insect-rearing facilities (Sequeira & Mackauer, Reference Sequeira and Mackauer1992a). Because parasitoid size can be directly related to its dispersal capacity in the field (Gardner & Lenteren, Reference Gardner and Van Lenteren1986), these parameters must be considered when evaluating the quality of T. remus reared on C. cephalonica eggs for its successful use in applied biological control programs (Vaz et al., Reference Vaz, Tavares and Lomônaco2004).
Although wing length, wing width, body length, and right hind length of T. remus were previously examined for parasitoids reared successively on C. cephalonica eggs, this was only done for 19 generations (Pomari-Fernandes et al., Reference Pomari-Fernandes, Bueno and Bortoli2016). The authors reported smaller values for morphological characters of parasitoids reared on C. cephalonica eggs compared with parasitoids reared on S. frugiperda eggs. Although smaller, they showed the same flight activity (percentage of ‘flyers’, ‘walkers’, and ‘deformed’) as bigger T. remus individuals reared on S. frugiperda eggs, indicating that a reduction of parasitoid size on the factitious host does not reduce its dispersal capacity, and is therefore adequate for biological control purposes. However, Pomari-Fernandes et al. (Reference Pomari-Fernandes, Bueno and Bortoli2016) studied neither those morphological characters nor the flight activity of more advanced generations of T. remus reared in the factitious host. It is crucial to understand whether successive T. remus rearing on C. cephalonica eggs for longer periods impairs parasitoid fitness over time.
The morphological characters of T. remus evaluated in our study (wing length, wing width, body length, and right hind length) were not reduced from generation P35 to generation P45. Moreover, our results (from generation P35 to P45) are similar to previous results reported by Pomari-Fernandes et al. (Reference Pomari-Fernandes, Bueno and Bortoli2016) for generations P1 to P19. More importantly, T. remus reared on C. cephalonica for 45 generations (P45) had the same percentage of ‘flyers’ than the parasitoid reared on the natural host, S. frugiperda. Our results suggest that the dispersal capacity of smaller T. remus reared on C. cephalonica eggs was not impaired, similar to that of individuals from earlier generations (Pomari-Fernandes et al., Reference Pomari-Fernandes, Bueno and Bortoli2016).
The similarity in percentages of flying individuals (‘flyers’) from the factitious host C. cephalonica (generation P45) and from the natural host S. frugiperda indicates that the size reduction of the recorded morphological characters was not sufficient to compromise flight ability. Even though the number of ‘flyers’ in generations P35 and P40 was smaller, their percentage was still close to 80% and that of individuals with deformities was only around 3%. The average percentage (≈80% or higher) of ‘flyers’ was similar to that found by other authors using the same protocol for other parasitoid species. Rodrigues et al. (Reference Rodrigues, Sampaio and Miranda2009) found averages from 85.9 to 97.7% of flying individuals and Prezotti et al. (Reference Prezotti, Parra, Vencovsky, Dias, Cruz and Chagas2002) reported mean percentages between 74.7 and 90.6% for Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). The similarity between those and our results suggests that T. remus rearing on C. cephalonica eggs provides an acceptable number of ‘flyers’ even when reared for successive generations (until generation P45). Despite these findings, it is important to continuously examine flight ability in quality control procedures during parasitoid rearing.
In addition to flying, walking is another significant indicator of the performance of natural enemies in field conditions, as both relate to foraging and dispersal. Therefore, the number of ‘walkers’ is also important for the evaluation of parasitoid quality. The average percentage of ‘walkers’ (from 7.58 to 26.01%) was higher than that obtained in studies using T. pretiosum (Prezotti et al., Reference Prezotti, Parra, Vencovsky, Dias, Cruz and Chagas2002; Rodrigues et al., Reference Rodrigues, Sampaio and Miranda2009), which might be related to interspecific behavioral differences. The percentage of individuals with deformities such as stunted or folded wings was less than 4%.
Host quality is related to its size, reflecting the biomass available for consumption by the parasitoid (Chau & Mackauer, Reference Chau and Mackauer2001; Jones et al., Reference Jones, Bilton, Mak and Sait2015). For example, the parasitoid Monoctonus paulensis (Ashmead) (Hymenoptera: Braconidae) shows preference for larger aphids in order to increase its fecundity (number, size, and egg quality) (Chau & Mackauer, Reference Chau and Mackauer2001). Another important factor is the duration of parasitoid development (egg–adult), which the species can extend as a compensatory action to recover from low-quality food and in order to reach a larger adult size (Sequeira & Mackauer, Reference Sequeira and Mackauer1992b). Overall, T. remus developed more slowly on C. cephalonica eggs than on S. frugiperda eggs (Bueno et al., Reference Bueno, Bueno, Xavier and Carvalho2014). Some differences in parasitoid development observed between C. cephalonica and S. frugiperda as hosts might be associated with the different quality of their eggs (Smith, Reference Smith1996). Differences between eggs of different host species were previously identified as an important factor for survival and development of parasitoid species, such as Trichogramma spp. (Cônsoli et al., Reference Cônsoli, Kitajima and Parra1999) and T. remus (Bueno et al., Reference Bueno, Bueno, Xavier and Carvalho2014). Egg surface, egg size, chorion structures and other egg properties differ between host species, such as color during embryonic development, and volume. In addition, S. frugiperda lays its eggs in superposed masses while C. cephalonica lays individual eggs. All of these differences can affect not only parasitoid handling time and exploitation, but also the host's suitability for parasitoid development, which influences egg-to-adult development time (Cônsoli et al., Reference Cônsoli, Kitajima and Parra1999).
The lifetime number of parasitized eggs may be of even greater importance than egg-to-adult duration because it determines the efficiency of biological control in the field. Lifetime numbers of parasitized eggs in our trials were smaller than those reported for parasitoids reared on eggs of S. frugiperda (Pomari et al., Reference Pomari, Bueno, Bueno and Menezes Junior2012). However, even though it might be necessary to release a higher number of T. remus when the parasitoid is reared on a factitious host, laboratory-produced parasitoids of our study reared on C. cephalonica eggs did not lose their capacity to control S. frugiperda eggs. Similarly, T. remus reared for 75 generations on C. cephalonica eggs was able to parasitize Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) over all tested generations with a viability higher than 98% and a sex ratio above 0.50 (Kumar et al., Reference Kumar, Pawar and Divakar1986).
The parasitization period (the period during which females are active) may vary due to differences in hosts (Reznik et al., Reference Reznik, Umarova and Voinovich2001) or parasitoid species/strain (Pratissoli & Parra, Reference Pratissoli and Parra2000; Pizzol et al., Reference Pizzol, Pintureau, Khoualdia and Desneux2010), and can influence the success of biological control programs using egg parasitoids (Wajnberg & Hassan, Reference Wajnberg and Hassan1994; Smith, Reference Smith1996), or at least define the strategies for parasitoid release. Thus, whether parasitism is more intense in the first days of life or evenly distributed throughout adulthood is an important characteristic to be considered when choosing the best parasitoid release strategy (Bueno et al., Reference Bueno, Carneiro, Bueno, Pratissoli, Fernandes and Vieira2010). Parental T. remus lived for 5.6–6.1 days, but always reached 80% of its parasitism on the first or second day of parasitism. This might be a consequence of a pro-ovigenic development of T. remus. Some parasitoid species have the capacity to store a full complement of mature eggs in the ovaries or oviducts and complete oogenesis either before or shortly after adult emergence (pro-ovigenic parasitoids) (Mills & Kuhlmann, Reference Mills and Kuhlmann2000). Therefore, adults emerge ready to lay eggs, as it seemed to be the case for T. remus in our trials. The sooner the parasitoid reaches 80% of its lifetime parasitism, the better, because while exposed to field conditions parasitoids might be susceptible to biotic and abiotic factors that can impair their action (Bueno et al., Reference Bueno, Parra and Bueno2012). Examples of such factors are the spraying of fungicides or herbicides used in crop management or an abrupt change in temperature that could kill the parasitoids (Carmo et al., Reference Carmo, Bueno and Bueno2010; Denis et al., Reference Denis, Pierre, Van Baaren and Van Alphen2011) but not the pests.
In conclusion, our data indicate that the quality of T. remus was not greatly impacted by successive rearing on C. cephalonica eggs. Most importantly, this laboratory-reared parasitoid did not lose its ability to parasitize S. frugiperda eggs. Therefore, rearing of T. remus on C. cephalonica eggs may be a successful strategy. The results presented here are from laboratory studies, requiring additional studies under field conditions to test the postulated hypotheses and to fully develop a successful biological control program using this egg parasitoid.
Acknowledgements
The authors would like to thank Embrapa Soja, the ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)’, and the ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)’, for funds that supported this research. Thanks are also extended to Maria Cristina Neves de Oliveira for the help with the statistics, Adair Vicente Carneiro for help with fig. 1 and Dagmar Frisch for the help with the English editing.







