Introduction
Leishmania donovani and Leishmania infantum protozoa are the etiological agents which cause visceral leishmaniasis (VL) in Old World and New World, respectively (Lukes et al., Reference Lukes, Mauricio, Schonian, Dujardin, Soteriadou, Dedet, Kuhls, Tintaya, Jirku, Chocholova, Haralambous, Pratlong, Obornik, Horak, Ayala and Miles2007). Considered as a neglected disease, VL is present worldwide with an estimative from 50 000 to 90 000 new cases per year (WHO, 2017). In the American continent, VL is described in 13 countries, and Brazil represents more than 95% of the cases reported in this continent (PAHO, 2017).
Brazil and other developing countries classify antimonial pentavalent as first-choice drugs for the treatment against VL. Other second-line compounds, such as pentamidine, amphotericin B and liposomal amphotericin B, paromomycin and miltefosine also have been added as therapeutic options (Sundar and Singh, Reference Sundar and Singh2018). However, the use of these drugs requires attention since hepatic and cardiac toxicity related to them have been registered. Furthermore, drug-resistant parasite strains also have been reported, which could demand an increase of the therapeutic dose or longer-term treatment of the patients, consequently, enhancing side-effects of these compounds (Freitas-Junior et al., Reference Freitas-Junior, Chatelain, Kim and Siqueira-Neto2012; Zulfiqar et al., Reference Zulfiqar, Shelper and Avery2017).
Miltefosine (hexadecylphosphocholine), initially developed as an anticancer drug, is a safe leishmanicidal agent with mild side-effects and the only one available to be administered orally (Croft et al., Reference Croft, Neal, Pendergast and Chan1987; Unger et al., Reference Unger, Damenz, Fleer, Kim, Breiser, Hilgard, Engel, Nagel and Eibl1989; Sundar et al., Reference Sundar, Jha, Thakur, Engel, Sindermann, Fischer, Junge, Bryceson and Berman2002; Palic et al., Reference Palic, Bhairosing, Beijnen and Dorlo2019). However, Miltefosine-resistant Leishmania parasites, isolated from patients who experienced therapeutic failure after miltefosine treatment, have been recorded in India and Brazil (Srivastava et al., Reference Srivastava, Mishra, Gupta, Singh, Shankar and Singh2017; Carnielli et al., Reference Carnielli, Monti-Rocha, Costa, Molina Sesana, Pansini, Segatto, Mottram, Costa, Carvalho and Dietze2019).
Recently, Roatt et al. employed a vaccine therapy in canine VL which promoted a significant improvement of clinical and immunological status leading to a reduction of the parasite burden in bone marrow and skin in naturally infected dogs (Roatt et al., Reference Roatt, Aguiar-Soares, Reis, Cardoso, Mathias, de Brito, da Silva, Gontijo, Ferreira, Valenzuela, Correa-Oliveira, Giunchetti and Reis2017), but these authors did not associate antileishmanial drugs with a vaccine therapy. In view of the current limited arsenal of drugs and increased resistance to them which represents an important concern, it is relevant to search other strategies that improve leishmanicidal activity and reduce the toxicity associated to conventional drugs. It is important to assess the immunological status against Leishmania-infection since it affects the drug's efficacy. Based on that, instead of relying only on chemotherapy and waiting for the host's immune response to expand in time to suppress parasitism, a promising alternative is to associate immunotherapy with chemotherapy to induce a quick effective immunological response concomitantly with a direct action of the drug against the infectious agent (Musa et al., Reference Musa, Noazin, Khalil and Modabber2010). Mayrink et al. (Reference Mayrink, Botelho, Magalhaes, Batista, Lima Ade, Genaro, Costa, Melo, Michalick, Williams, Dias, Caiaffa, Nascimento and Machado-Coelho2006) showed that patients with cutaneous leishmaniasis treated with an immunochemotherapy protocol consisting of killed L. amazonensis plus BCG adjuvant plus N-methyl meglumine antimoniate (Glucantime®) had almost 100% clinical cure with an 18% reduction in the volume of the drug and in the average treatment time (Mayrink et al., Reference Mayrink, Botelho, Magalhaes, Batista, Lima Ade, Genaro, Costa, Melo, Michalick, Williams, Dias, Caiaffa, Nascimento and Machado-Coelho2006). A preclinical study showed that L. donovani-infected mice and treated with dead L. donovani with sodium stibogluconate plus Monosphosphoryl lipid A (MPL-A) had a reduction in parasitic load as well as an increase in IFN-γ and a decrease in IL-10 and IL-4 (Joshi et al., Reference Joshi, Malla and Kaur2014). Besides, the association of immunostimulants in different formulations of adjuvants denominates Adjuvant Systems (AS) has been used to promote protective and efficient immunological responses following vaccination (Garcon and Van Mechelen, Reference Garcon and Van Mechelen2011). Noteworthy are AS02 (emulsion containing MPL-A and the purified fraction QS-21 of saponin), AS03 (emulsion containing α-tocopherol and squalene) and AS04 [Al (OH) 3 and MPL] used as prophylactic vaccines (Garcon and Di Pasquale, Reference Garcon and Di Pasquale2017).
Therefore, considering enhanced immune response acting synergistically with drugs to control the parasite load, the present work was designed to evaluate the therapeutic role never assessed before of a vaccine consisting of Leishmania braziliensis (LB) antigens combined with the AS containing saponin and MPL-A (LBSapMPL vaccine) (immunotherapy) plus miltefosine (immunochemotherapy) using hamsters Mesocricetus auratus as an experimental model for VL treatment.
Materials and methods
Animals, parasites and experimental infection
Male and female Syrian golden hamsters (M. auratus), 4–6 weeks old and weight range 60–80 g, were used for this present study. They were obtained from Centro de Ciência Animal, Universidade Federal de Ouro Preto (UFOP), Brazil, and they were maintained in appropriate cages with rodent food and water ad libitum throughout the experiment time.
For experimental infections, L. infantum parasites (strain MCAN/BR/2008/OP46) were used (Moreira et al., Reference Moreira, Vitoriano-Souza, Roatt, Vieira, Ker, de Oliveira Cardoso, Giunchetti, Carneiro, de Lana and Reis2012; Carvalho et al., Reference Carvalho, De Brito, Gusmao, de Oliveira Aguiar-Soares, Reis and Roatt2021). Hamsters were infected by the intraperitoneal route with 2 × 107 promastigotes of L. infantum in the stationary growth phase. They were treated 60 days after infection and euthanized 15 days after treatments. All the experimental procedures in this study were carried out in duplicate and a total of 16 animals per group were used. The number of animals was divided in half to assess the different parameters addressed in the work: half (n = 8) for blood count/biochemistry/parasite burden evaluation, half (n = 8) for humoral/cellular response.
Vaccine antigen and adjuvants
Promastigotes from Leishmania (Viannia) braziliensis (MHOM/BR/75/M2903) were grown in a blood-agar culture medium, ‘Nicolle-Novy-Neal’ (NNN), associated with ‘Liver Infusion Tryptose’ (LIT). Parasites were obtained by centrifugation (900 g, 15 min, 4° C) from 7-day-old cultures, washed 3 times in saline buffer. Then, promastigotes were disrupted by ultrasound (Sonifier Cell Disruptor® – Brason Sonic Power Co., Danbury, Connecticut, USA) (1 min, 40 W in an ice bath) according to Roatt et al. (2012). After this procedure, the vaccine antigen was aliquoted and frozen at −80°C. The protein concentration was measured by the method of Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall1951). The chosen adjuvants used in association with LB antigen were saponin (Sigma Chemical Co., St. Louis, USA) and MPL-A (Monophosphoryl lipid A, obtained from Salmonella enterica, serotype Minnesota RE 595) (Sigma Chemical Co.). Both were diluted in injection water at the time of inoculation, to avoid loss of stability. Therefore, the LBSapMPL vaccine used in this work for immunotherapy or immunochemotherapy protocols was composed of LB antigens associated with AS saponin plus MPL-A.
Treatment groups
Hamsters’ male and female were divided into different groups, with 16 animals per group. Sixty days after infection they were treated with the varied treatment protocols, described below and in Fig. 1:
-
Positive control: infected and untreated animals (IA group);
-
Immunotherapy: animals that received by subcutaneous route (SC): LB (60 μg dose−1) alone (LB group); or adjuvant system saponin (50 μg dose−1) + MPL-A (12.5 μg dose−1) (SapMPL group); or LB (60 μg dose−1) + saponin (50 μg dose−1) + MPL-A (12.5 μg dose−1) (LBSapMPL group); in 3 series of 5 days each, with a break of 5 days among the series (Fig. 1);
-
Chemotherapy: animals were treated by oral route (gavage) with miltefosine 2% (Milteforan™ – Virbac, Carros, França) (2 mg kg−1 dose−1) (Miltefosine group) for 28 uninterrupted days with an average volume of 100 μL of drug (Fig. 1);
-
Immunochemotherapy: animals were treated by oral route (gavage) with miltefosine 2% (2 mg kg−1 dose−1) for 14 uninterrupted days with an average volume of 100 μL of drug (half course of chemotherapy) and LB (60 μg dose−1) subcutaneously in 2 series of 5 days each, with a break of 5 days among the series (Milt-LB group); or miltefosine 2% (14 days; 2 mg kg−1 dose−1) and LB (60 μg dose−1) + saponin (50 μg dose−1) + MPL-A (12.5 μg dose−1) in 2 series of 5 days each, with a break of 5 days among the series (Milt-LBSapMPL group) (Fig. 1).
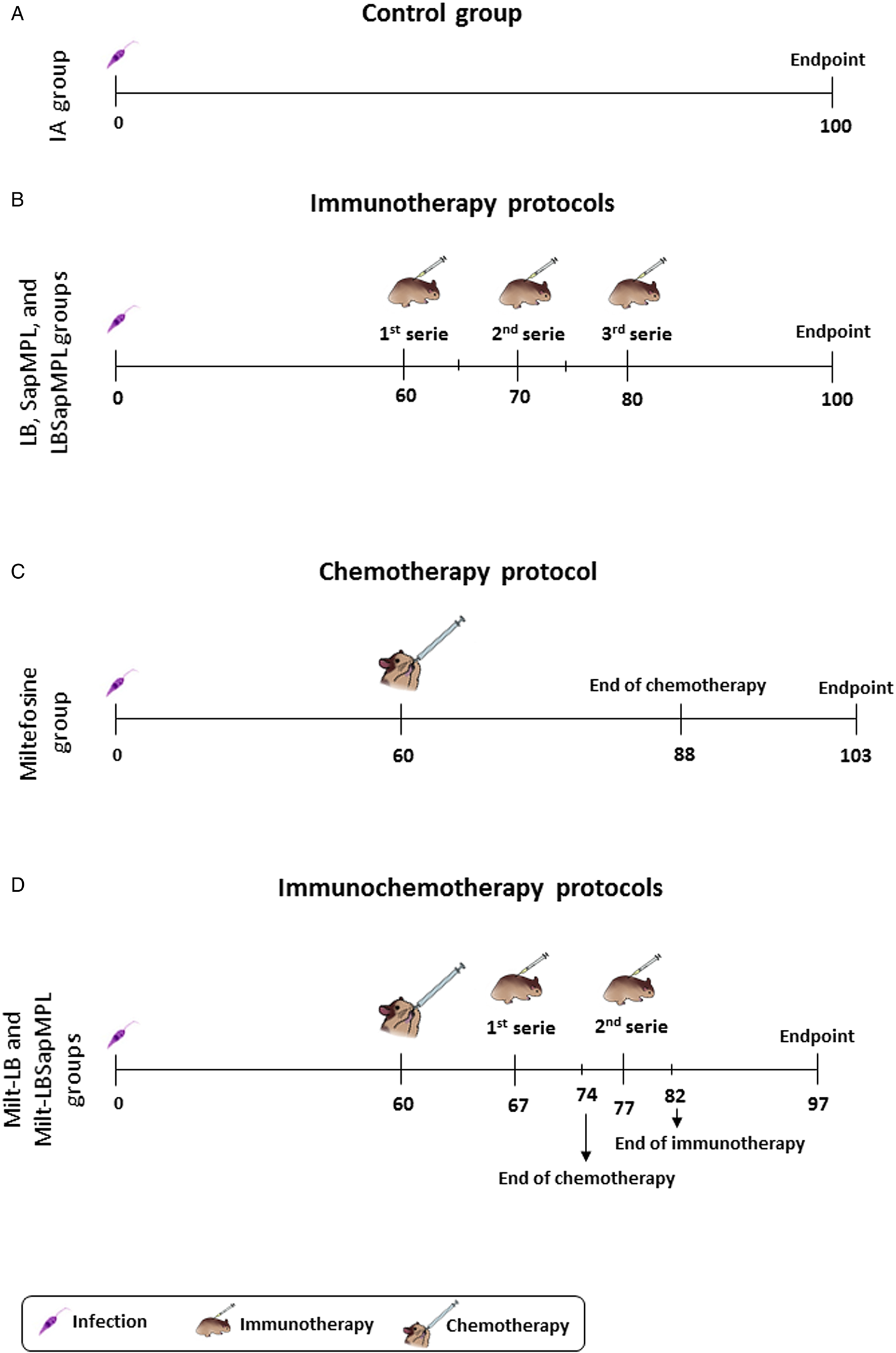
Fig. 1. Therapeutic protocols used to treat Leishmania infantum-infected hamsters. (A) Monitoring of infected and untreated animals (IA group); (B) treatment scheme using immunotherapy, administered by subcutaneous route, with Leishmania braziliensis (LB) antigen (LB group); SapMPL adjuvant (SapMPL group); or LBSapMPL vaccine (LBSapMPL group); (C) treatment scheme (chemotherapy) using miltefosine, per oral route (gavage) (Miltefosine group); (D) treatment scheme (immunochemotherapy) using miltefosine per oral route (gavage) associated with subcutaneous administration of LB antigen (Milt-LB group); or LBSapMPL vaccine (Milt-LBSapMPL) (n = 16/group). After 15 days of the end of different scheme treatments, the animals were euthanized, and different laboratory analyses were performed.
Cellular immune response and flow cytometry acquisition
The procedure was performed in accordance with Carvalho et al. (Reference Carvalho, De Brito, Gusmao, de Oliveira Aguiar-Soares, Reis and Roatt2021). Briefly, in a solution of RPMI (Sigma, St. Louis, MO, USA) plus heparin (Cristália, Itapira, SP, Brazil), splenocytes were obtained by maceration of spleens harvested from the animals under sterile conditions. After centrifugation at 450 g, 4°C for 10 min, the splenocytes were plated (5 × 105 cells well−1) in 96-well round-bottom (Costar, Cambridge, MA, USA) culture plates in a final volume of 200 μL and were incubated for 48 h at 37°C with 5% CO2 in RPMI, in the presence or absence of soluble Leishmania Antigen – SLiAg (50 μg mL−1) or with phorbol 12-myristate 13-acetate (PMA, 5 ng mL−1, Sigma) and ionomycin (ION, 0.2 μg mL−1, Sigma). Then, Brefeldin A (BFA, 10 μg mL−1, Sigma) and 2 mm ethylenediamine tetra acetic acid (EDTA) (Sigma) were added. Following centrifugation at 400 g at 4°C for 7 min, cells were labelled with Fixable Viability Stain 450 (FVS450, BD Biosciences, USA) (12.5 μg mL−1). Then, the splenocytes were stained with 6 μg mL−1 of the anti-mouse CD4 FITC (clone Gk1.5 – BD Biosciences Bioscience, USA) antibody and were fixed with FACS fixing solution. After washing and permeabilization steps, the cells were stained with anti-mouse TNF-α PerCP-Cy5.5 (clone MP6-XT22), anti-mouse IFN-γ AF-647 (clone XMG1.2) and anti-mouse IL-10 PE (clone JES5-2A5) (BD Biosciences Bioscience) in a concentration of 1.5 μg mL−1 of all the cytokines. It is important to notice that internal controls such as unstained cells and isotypic control of the monoclonal antibodies were used in all the assays.
The data analysis followed from singlet selection based on size (forward scatter area – FSC-A and forward scatter height – FSC-H) and dead cells exclusion by gating FVS450 cells (granularity vs FVS450 stain). Then, we gated FSC-A and granularity (side scatter area – SSC-A) to quantify lymphocytes and FSC-A vs AF647/PerCP-Cy5.5/PE-Fluorescence to quantify cytokines. Then, we analysed CD4+ lymphocytes by gating SSC-A vs FITC-Fluorescence and the cytokines-CD4+ cells by FITC-Fluorescence vs AF647/PerCP-Cy5.5/PE-Fluorescence. The cells were acquired (100 000 events per second) by LSR Fortessa Flow Cytometer (BD Biosciences) using FACSDiva software and for data analysis FlowJo software (BD Biosciences) was utilized (Supplementary Fig. 1).
Humoral immune response
The serum anti-Leishmania immunoglobulin G (IgG) isotype antibody was measured by conventional enzyme-linked immunosorbent assay (ELISA). Briefly, 1 μg well−1 of SliAg (Moreira et al., Reference Moreira, Vitoriano-Souza, Roatt, Vieira, Ker, de Oliveira Cardoso, Giunchetti, Carneiro, de Lana and Reis2012) was used to coat ELISA microplates (MaxiSorp, Nalge Nunc). After blocking free binding sites and washing step, the sera sample (diluted 80 times) was added. Following a washing step, the samples were incubated with goat anti-hamster IgG (1:1000, Goat Anti-Hamster – Caltag Laboratories). After a washing step, the plates were incubated with the substrate, and chromogen (O-phenylenediamine, Sigma–Aldrich) and sulfuric acid 2N was added to stop the reactions. Optical density values were read in ELISA reader (ELX800 Biotek Instruments VT, USA) at 490 nm. Positive and negative hamsters control sera, conjugate control, and reaction control – ‘blank’ were used. The cut-off point was established as mean absorbance value +2 s.d. from uninfected hamsters’ sera.
Quantification of spleen parasite burden
Splenic parasitism was determined by quantitative real-time PCR (qPCR) according to Moreira et al. (Reference Moreira, Vitoriano-Souza, Roatt, Vieira, Ker, de Oliveira Cardoso, Giunchetti, Carneiro, de Lana and Reis2012). Briefly, genomic DNA was isolated from spleens using the CTAB reagent. A conserved region of Leishmania kDNA (kinetoplast DNA) [forward primer: 5′-GGG(G/T) A GGG GCG TTC T(C/G) CGA A-3′; reverse primer: 5′-(C/G) (C/G) (C/G) (A/T) CTA T(A/T) TTA CAC CAA CCC C-3′] was evaluated to quantify L. infantum parasites using Bryt Fluorescent Signal as detection system (GoTaq® qPCR Master Mix – Promega Corporation, EUA). As a constitutive gene hamster, GAPDH primers (5′-TGG AGT CTA CTG GCG TCT TC-3′; reverse: 5′ GGA GAT GAT GAC CCT CTT G-3′) were used. The following steps were programmed: one step to activate Taq enzyme (95°C for 2 min) followed by 40 cycles of denaturation at 95°C for 15 s and annealing-extension at 60°C for 1 min. Each 96-well reaction plate contained a standard curve in triplicate (efficiency, 96.0%; r 2 – 0.99) in duplicate samples. The result was expressed as the number of amastigotes DNA copies per mg of the hamster's spleen.
Statistical analysis
GraphPad Prism 8.0 software (Prism Software, Irvine, CA, USA) was utilized to perform the statistical tests. Data normality was carried out using the Shapiro-Wilk test. For parametric tests, the analyses were performed using one-way ANOVA measures. To assess the correlation analyses, Pearson's r test was utilized. Differences with P values <0.05 (P < 0.05) were considered significant.
Results
Hamsters treated with vaccine therapy (LBSapMPL) and with chemovaccinetherapy (Milt-LBSapMPL) were better able to induce total T cells and T-CD4 splenocytes to produce high levels of IFN-γ and TNF-α and very low levels of IL-10
To evaluate the cellular immune response profile, we have assessed the intracytoplasmic cytokines IFN-γ, TNF-α and IL-10 produced by splenocytes after SLiAg antigen-specific stimulus (Fig. 2). It was found an increase (P < 0.05) in the percentage of total T splenocytes producing IFN-γ in LBSapMPL group in relation to IA group. Beyond that, in Miltefosine, Milt-LB and Milt-LBSapMPL groups, these cell percentages were also increased (P < 0.05) in comparison to IA and LB groups. There was also an increase (P < 0.05) of total T splenocytes secreting TNF-α in all treated groups in relation to IA group, being that in LBSapMPL and Milt-LBSapMPL groups also, there was an increase (P < 0.05) regarding LB, SapMPL and Milt-LB groups (Fig. 2). Besides that, the percentage of T CD4+IFN-γ + lymphocytes was enhanced (P < 0.05) in LBSapMPL, Miltefosine, Milt-LB and Milt-LBSapMPL groups in relation to IA and LB groups. While in Milt-LBSapMPL group, the production of this cytokine by T CD4+ cells was increased (P < 0.05) compared to SapMPL, Miltefosine and Milt-LB groups. As for T CD4+TNF-α + splenocytes, an enhance (P < 0.05) in LB, LBSapMPL, Miltefosine, Milt-LB and Milt-LBSapMPL groups compared to IA group was observed, while LBSapMPL and Milt-LBSapMPL groups also were increased when compared to LB and SapMPL groups (Fig. 2). Otherwise, when we have assessed the immunomodulatory cytokine, we have found a decrease (P < 0.05) in the number of total T splenocytes producing IL-10 in LB, LBSapMPL, Miltefosine, Milt-LB and Milt-LBSapMPL groups regarding IA group. In addition, the percentage of these cells was reduced (P < 0.05) in Milt-LBSapMPL group compared to LB, SapMPL, Miltefosine and Milt-LB groups. There was also a reduction (P < 0.05) in the percentage of T CD4+IL-10+ lymphocytes in LB, LBSapMPL and Milt-LBSapMPL groups compared to IA group. Likewise, in LBSapMPL and Milt-LBSapMPL groups, the percentage of these cells was decreased (P < 0.05) regarding LB, SapMPL, Miltefosine and Milt-LB groups (Fig. 2).

Fig. 2. Profile of intracytoplasmatic cytokines in splenocytes of hamsters infected by Leishmania infantum and submitted to different protocols of immunotherapy, chemotherapy and immunochemotherapy. Analysis of intracellular cytokines (IFN-γ, TNF-α and IL-10) in total and CD4+ T splenocytes in the spleen of hamsters intraperitoneal infected with 2 × 107 Leishmania infantum promastigotes after in vitro stimulation with soluble L. infantum antigens (SLiAg) in untreated (infected animals – IA) or treated with Leishmania braziliensis antigen (LB), adjuvant system (Saponin + MPL – SapMPL) and LBSapMPL therapeutic vaccine (LBSapMPL); chemotherapy with Miltefosine (Miltefosine); or immunochemotherapy Miltefosine with LB (Milt-LB) and Miltefosine with LBSapMPL therapeutic vaccine (Milt-LBSapMPL) groups (n = 8/group). The total and CD4+ T splenocytes cytokine indexes were calculated as the proportion of cytokine+ cells observed in SLiAg-stimulated cultures divided by the control culture (CC) (SLiAg/CC ratio). Significant differences (P < 0.05) are shown by the ‘*’ representing differences related to IA, ‘**’ representing differences related to IA and LB, and ‘***’ representing differences related to IA, LB and SapMPL. Connecting lines (—) represent differences between the LBSapMPL, Miltefosine, Milt-LB and Milt-LBSapMPL groups. The results are expressed as mean ± standard deviation.
As observed in radar charts (Supplementary Fig. 2) represented by the CD4 cytokine cell response, the IA and SapMPL groups showed an immunomodulatory profile (prominent IL-10 production) reflecting the cellular suppression caused by L. infantum infection. On the other hand, animals treated with LBSapMPL and Milt-LBSapMPL demonstrated a Th1 polarization (intense IFN-γ and TNF-α production) with a proinflammatory profile, indicating the activation of immune response associated with the use of immunotherapy (Supplementary Fig. 2).
Chemovaccinetherapy (Milt-LBSapMPL) was able to reduce the polyclonal activity of B cells leading IgG levels to a profile similar to that of hamsters treated with chemotherapy alone (Miltefosine)
The humoral immune response was assessed through the quantification of serum levels of anti-Leishmania total IgG (Fig. 3). There was a reduction (P < 0.05) in the amount of this immunoglobulin isotype antibody in Miltefosine, Milt-LB and Milt-LBSapMPL groups compared to IA group. Furthermore, in Miltefosine and Milt-LBSapMPL groups, circulating total IgG were decreased (P < 0.05) regarding LB, SapMPL, LBSapMPL and Milt-LB groups (Fig. 3).

Fig. 3. Humoral response of hamsters infected by Leishmania infantum and submitted to different protocols of immunotherapy, chemotherapy and immunochemotherapy. Analysis of IgG response in the sera of hamsters intraperitoneal infected with 2 × 107 Leishmania infantum promastigotes in untreated (infected animals – IA) or treated with Leishmania braziliensis antigen (LB), adjuvant system (Saponin + MPL – SapMPL) and LBSapMPL therapeutic vaccine (LBSapMPL); chemotherapy with Miltefosine (Miltefosine); or immunochemotherapy Miltefosine with LB (Milt-LB) and Miltefosine with LBSapMPL therapeutic vaccine (Milt-LBSapMPL) groups (n = 8/group). Significant differences (P < 0.05) are shown by the ‘*’ representing differences related to IA, ‘**’ representing differences related to IA and LB, and ‘***’ representing differences related to IA, LB and SapMPL. Connecting lines (—) represent differences between the LBSapMPL, Miltefosine, Milt-LB and Milt-LBSapMPL groups. The results are expressed as mean ± standard deviation.
Vaccine therapy (LBSapMPL) and chemovaccinetherapy (Milt-LBSapMPL) surpassed the therapeutic efficiency of chemotherapy (Miltefosine) alone, leading to a near parasitological cure
We have used the spleen as a target organ to assess therapeutic efficacy by quantifying L. infantum DNA using real-time PCR (qPCR) methodology (Fig. 4). It was shown a significant decrease (P < 0.05) of spleen parasite load of 94% in LBSapMPL, 91% in Miltefosine, 80% in Milt-LB and 96% in Milt-LBSapMPL groups in comparison to IA group. Also, a significant reduction (P < 0.05) of spleen parasitism in LBSapMPL, Miltefosine and Milt-LBSapMPL groups compared to LB and SapMPL groups was observed (Fig. 4). No significant difference was observed between the Miltefosine (28 days) and immunochemotherapy groups using the LBSapMPL vaccine and half time of treatment with Miltefosine (14 days).

Fig. 4. Parasite burden in the spleen of hamsters infected by Leishmania infantum and submitted to different protocols of immunotherapy, chemotherapy and immunochemotherapy. Analysis of parasite burden in the spleen of hamsters intraperitoneal infected with 2 × 107 L. infantum promastigotes in untreated (infected animals – IA) or treated with Leishmania braziliensis antigen (LB), adjuvant system (Saponin + MPL – SapMPL) and LBSapMPL therapeutic vaccine (LBSapMPL); chemotherapy with Miltefosine (Miltefosine); or immunochemotherapy Miltefosine with LB (Milt-LB) and Miltefosine with LBSapMPL therapeutic vaccine (Milt-LBSapMPL) groups (n = 8/group) and percentage of parasitism reduction compared to the IA group. Significant differences (P < 0.05) are shown by the ‘*’ and ‘***’ representing differences related to IA; and IA, LB and SapMPL groups, respectively.
Vaccine therapy (LBSapMPL) and chemovaccinetherapy (Milt-LBSapMPL) led to a strong positive correlation with increased IFN-γ and TNF-α production, low IL-10 production by total T splenocytes and T CD4+ accompanied by a drastic reduction in splenic parasite load
We also have performed correlation analyses between the spleen parasitism and IFN-γ, TNF-α and IL-10 cytokines secreted by T-total and T-CD4+ splenocytes, as well as among parasitic load and total IgG (Table 1). Regarding the spleen parasitism vs cytokines, our results demonstrated a negative correlation between parasitic burden and production of IFN-γ by CD4+ lymphocytes in IA (P = 0.015; Pearson's r = −0.578) and LBSapMPL (P = 0.039; Pearson's r = −0.701) groups. Also, a negative correlation between splenic parasitism and production of TNF-α by total lymphocytes was observed in LBSapMPL group (P = 0.031; Pearson's r = −0.799). On the other hand, a strong positive correlation among parasitic burden and total lymphocytes producing IFN-γ was noticed in Miltefosine group (P = 0.024; Pearson's r = 0.871). There was also a positive correlation between spleen parasitism and IL-10 produced by T-total splenocytes in IA (P = 0.038; Pearson's r = 0.556) and Milt-LBSapMPL (P = 0.024; Pearson's r = 0.871) groups. In addition, a strong positive correlation among parasitic load and T-CD4+IL-10+ splenocytes in IA (P = 0.005; Pearson's r = 0.706), LBSapMPL (P = 0.009; Pearson's r = 0.878) and Milt-LBSapMPL (P = 0.015; Pearson's r = 0.806) groups was showed (Table 1). In relation to spleen parasitism vs circulating anti-Leishmania IgG, it was observed a strong positive correlation in IA (P < 0.001; Pearson's r = 0.838) and Milt-LBSapMPL (P = 0.029; Pearson's r = 0.757) groups (Table 1).
Table 1. Correlation Indexes of hamsters infected by Leishmania infantum and submitted to different protocols of immunotherapy, chemotherapy and immunochemotherapy

Bold numbers represent positive or negative correlations presenting significant differences (P < 0.05).
Discussion
The control of VL is a global health challenge since there is no vaccine available for human and for mass immunization of dogs to date. Beyond that, current conventional treatment, based on chemotherapy, is hampered owing to several side-effects, difficult route of administration and many non-responders’ patients, suggesting the emergence of resistant parasite strains (Roatt et al., Reference Roatt, de Oliveira Cardoso, De Brito, Coura-Vital, de Oliveira Aguiar-Soares and Reis2020). Therefore, combined anti-Leishmania treatment using vaccines and drugs has become an attractive strategy in an attempt to reduce toxicity, increase efficacy in removal of parasites and stimulate a sustained immune response (Borja-Cabrera et al., Reference Borja-Cabrera, Santos, Santos, Trivellato, Kawasaki, Costa, Castro, Nogueira, Moreira, Luvizotto, Palatnik and Palatnik-de-Sousa2010; Dayakar et al., Reference Dayakar, Chandrasekaran, Kuchipudi and Kalangi2019). Furthermore, it is suggested that the system adjuvant compound of saponin and MPL-A used in this work is a potent activator of the innate and adaptative response with the induction of IFN-γ secretion by activated antigen-specific T cells (Garcon and Van Mechelen, Reference Garcon and Van Mechelen2011). Roatt et al. employed vaccinotherapy with a heterologous L. braziliensis vaccine plus MPL as an adjuvant to treat symptomatic dogs naturally infected with L infantum. These researchers highlighted the strong potential for the use of this heterologous vaccine therapy as an important strategy for VL treatment, since it was able to promote a significant improvement of clinical and immune status with reduction in parasite burden in treated animals (Roatt et al., Reference Roatt, Aguiar-Soares, Reis, Cardoso, Mathias, de Brito, da Silva, Gontijo, Ferreira, Valenzuela, Correa-Oliveira, Giunchetti and Reis2017). Herein, the use of these adjuvants had the objective of enhancing the immune response and thereby helping and improving the action of the immunological system and the drug against the parasite. Keeping this in mind, we assessed the in vivo antileishmanial efficacy of the immunochemotherapy protocol using Miltefosine along with LBSapMPL vaccine in L. infantum-infected hamsters, which resulted in a Th1 response immune activation with a decrease of the spleen parasitism. It has been known that the success of the antileishmanial treatment depends on the combined action of the drug and the immune response, since an impaired immune system can result in therapeutic failure (Aruleba et al., Reference Aruleba, Carter, Brombacher and Hurdayal2020). Besides, IFN-γ is one of the main anti-leishmanial cytokines, helping to induce the secretion of pro-inflammatory cytokines, such as TNF-α, IL-1 and IL-6, and playing an important role in the activation of macrophages which release oxygen radicals necessary for the removal of parasites (Hart et al., Reference Hart, Whitty, Piccoli and Hamilton1989; Nathan and Hibbs, Reference Nathan and Hibbs1991; Dayakar et al., Reference Dayakar, Chandrasekaran, Kuchipudi and Kalangi2019).
Therefore, investigating how the host's immune response reacts after treatment is crucial to assessing prognosis. In view of this, we evaluated by flow cytometry the cytokine profile produced by the T-total and T-CD4+ splenocytes after treatment protocols of infected hamsters. We found hamsters treated with vaccinotherapy (LBSap, LBSapMPL) and with chemovaccinetherapy (Milt-LBSapMPL) were better able to induce total T and T-CD4 splenocytes to produce high levels of IFN-γ and TNF-α and very low levels of IL-10 (Fig. 2). It does corroborate with a study in which a treatment consisting of an immuno-modulator (liposomal CpG-ODN-2006) and miltefosine in L. donovani-infected hamsters showed the dominance of Th1-type immune response with an increase of inducible nitric oxide synthase (Shivahare et al., Reference Shivahare, Vishwakarma, Parmar, Yadav, Haq, Srivastava, Gupta and Kar2014). Another study showed that hamsters immunotreated with recombinant proteins of L. donovani presented an increase of IFN-γ and TNF-α cytokines expression (Rawat et al., Reference Rawat, Yadav, Joshi, Ratnapriya, Sahasrabuddhe and Dube2018). Similar results, as higher levels of pro-inflammatory cytokines and decrease of interleukin-10 in spleen, also were demonstrated in hamsters treated with Miltefosine or its derivative (Gupta et al., Reference Gupta, Kushawaha, Samant, Jaiswal, Baharia and Dube2012; da Silva et al., Reference da Silva, Nunes, Gontijo, Malaquias, de Freitas, Alves, Colombo, Laurenti and Marques2020). In VL, Th1-Th2 dichotomy is intrinsically linked to disease progression and/or host resistance (Reis et al., Reference Reis, Martins-Filho, Teixeira-Carvalho, Giunchetti, Carneiro, Mayrink, Tafuri and Correa-Oliveira2009). Predominance of IFN-γ and TNF-α Th1-cytokines is related to protection against VL, since they work together activating the immune system to eliminate the pathogen (Dayakar et al., Reference Dayakar, Chandrasekaran, Kuchipudi and Kalangi2019). Otherwise, the interleukin-10 plays a key role in the susceptibility and progression of the disease, downregulating the innate and adaptive immune response (Mosmann, Reference Mosmann1991; Reis et al., Reference Reis, Giunchetti, Carrillo, Martins-Filho and Moreno2010). Our data corroborate with these concepts, whereas we observed a high spleen parasitism accompanied by lower percentage of IFN-γ and increased levels of IL-10 in infected and untreated hamsters (IA group) (Figs 2, 4 and Table 1). Similarly, other studies also showed the growth of IL-10 expression in hamsters infected (Medina-Colorado et al., Reference Medina-Colorado, Osorio, Saldarriaga, Travi, Kong, Spratt, Soong and Melby2017; Moulik et al., Reference Moulik, Karmakar, Joshi, Dube, Mandal and Chatterjee2021). Beyond that, a strong positive correlation was found between the reduction of the splenic parasite burden and T-CD4+IL-10+ lymphocytes levels reduced in LBSapMPL and Milt-LBSapMPL groups (Table 1). That is, the vaccinetherapy and chemovaccinetherapy proposed here were able to control the spleen parasitism that is dependent on reduced IL-10 secretion by CD4+ T cells.
As we know, with the evolution of VL to severe conditions, there is a loss of cellular response against the parasite's antigens (cellular energy) with the establishment of a strong type 2 response and an elevated polyclonal activity of B-cells that start to secrete exorbitant amounts of different class and subclasses of immunoglobulins (hypergammaglobulinemia). These immunoglobulins, in turn, cannot act in amastigote forms that do not stop multiplying inside macrophages, on the contrary, the Ig(s) will contribute to worsen the clinical picture, leading to a series of aggravations, culminating in renal failure and multiple organ failure (Giunchetti et al., Reference Giunchetti, Silveira, Resende, Leite, Melo-Junior, Rodrigues-Alves, Costa, Lair, Chaves, Soares, de Mendonca, Lanna, Ribeiro, Maia-Goncalves, Santos, Roatt, Aguiar-Soares, Vitoriano-Souza, das Dores Moreira, Mathias, Cardoso, Coura-Vital, Galdino, Viana, Martins-Filho, Silveira-Lemos, Dutra and Reis2019). We also analysed the humoral response quantifying anti-Leishmania total immunoglobulin G in sera of the hamsters and our results showed an increased amount of IgG in animals of the IA group as expected, on the other hand, we observed a reduction of total IgG in hamsters treated with chemovaccinetherapy (Milt-LBSapMPL) was able to reduce the polyclonal activity of B cells leading IgG levels to a profile similar to that of hamsters treated with chemotherapy alone (Miltefosine) (Fig. 3). High levels of parasite-specific IgG are found in human, dogs and hamsters infected by Leishmania viscerotropic species, being considered a biomarker of active VL (Neogy et al., Reference Neogy, Nandy, Ghosh Dastidar and Chowdhury1987; Reis et al., Reference Reis, Teixeira-Carvalho, Vale, Marques, Giunchetti, Mayrink, Guerra, Andrade, Correa-Oliveira and Martins-Filho2006; Moreira et al., Reference Moreira, Vitoriano-Souza, Roatt, Vieira, Ker, de Oliveira Cardoso, Giunchetti, Carneiro, de Lana and Reis2012). Indeed, the present work showed that the maintenance of splenic parasitism led to an increase of anti-Leishmania IgG in the serum of the hamsters infected and untreated (Table 1). Similar results are found by Carvalho et al. (Reference Carvalho, De Brito, Gusmao, de Oliveira Aguiar-Soares, Reis and Roatt2021) that showed a strong positive correlation between spleen parasitic burden and levels of sera IgG in L. infantum-infected hamsters (Carvalho et al., Reference Carvalho, De Brito, Gusmao, de Oliveira Aguiar-Soares, Reis and Roatt2021). In addition, it was ascertained that those hamsters infected present high avidity antibodies without evolutionary maturation relating to the progression of the disease (de Carvalho et al., Reference de Carvalho, Ferrao, Cavalcante, de Freitas, Meireles and de Andrade Junior2020).
To assess the efficacy of the treatments against experimental VL, the splenic parasite load was analysed by qPCR. We observed an intensive decrease of parasitism in animals treated with LBSapMPL vaccine, Miltefosine or the combinatorial protocol Miltefosine plus LBSapMPL (Fig. 4). Eberhardt et al. (Reference Eberhardt, Mondelaers, Hendrickx, Van den Kerkhof, Maes and Caljon2016) showed that there is a homogeneity of infection by L. infantum in the spleen and liver of hamsters and the Miltefosine efficacy of parasite clearance seems to be organ-specific, being more prominent in the spleen (Eberhardt et al., Reference Eberhardt, Mondelaers, Hendrickx, Van den Kerkhof, Maes and Caljon2016). In this context or data, it was shown that vaccine therapy (LBSapMPL) and chemovaccinetherapy (Milt-LBSapMPL) surpassed the therapeutic efficiency of chemotherapy (Miltefosine) alone, leading to a near parasitological cure.
Altogether, our data reinforce that the presence of the L. infantum parasites is related with high levels of IgG antibodies and interleukin-10 as well as a lower amount of Th1 cytokines such as IFN-γ and TNF-α. Therefore, the establishment of a therapeutic scheme that can change these parameters is fundamental for treatment success. Accordingly, in the present work, we demonstrated that the immunotherapy with LBSapMPL vaccine and the immunochemotherapy using miltefosine plus LBSapMPL vaccine promoted increased levels of IFN-γ and TNF-α; beyond that, the immunochemotherapy was able to reduce significantly the amount of sera anti-Leishmania IgG antibodies, accompanied by strong reduction of splenic parasite burden. Thereupon, the treatment with Miltefosine plus LBSapMPL vaccine raised here, although there is a need for additional studies, is a promising proposal since the complementary action between the drug and the vaccine triggers a potent induction of Th1 subtype response immune enough to control the spleen parasitism even though with reduced treatment time. With that, we hope that this treatment protocol has a better chronicity profile of the disease in the long term, with the parasite load taking longer to rise/return, and thus have shorter treatment intervals or even parasitological cure the animal.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001906
Author contributions
L.M. Carvalho wrote the paper, performed research, methodology and analysed data. M.R. Gusmão, A.F.P. Costa and J.M. de O. Cardoso performed methodology, validation and review. R.D. de O. Aguiar-Soares and R.C.F. de Brito performed visualization, contributed new reagents or analytic tools and review. A.B. Reis and C.M. Carneiro performed review, editing, visualization and funding acquisition. B.M. Roatt performed review, editing, visualization, funding acquisition, supervision and project administration.
Financial support
The authors acknowledge the Brazilian agencies CNPq (grant number 435224/2018-2), FAPEMIG (grant number APQ-03505-13 – PROGRAMA DE PESQUISA PARA O SUS–PPSUS, APQ-01373-14 – PRONEX, APQ 02577-18 and APQ-02556-18), CAPES [this study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES) – Finance Code 001] and Universidade Federal de Ouro Preto – UFOP for funding. A.B.R., C.M.C. and B.M.R. are grateful to CNPq for fellowships.
Conflict of interest
None.
Ethical standards
The procedures were approved by Ethical Committee for the use of Experimental Animals from UFOP (protocol CEUA ID number – 2016/57) and they were performed according to the guidelines of Brazilian regulations for experimental animals. The L. infantum OP46 strain used in this study has approval from the National System for the Management of Genetic Heritage and Associated Traditional Knowledge – SISGen (A55DE5A).