Introduction
Toxoplasma gondii is the only obligatory intracellular protozoan infecting almost all warm-blooded animals. Approximately one-third of the world's population is estimated to be infected with this parasite (Guo et al., Reference Guo, Dubey, Hill, Buchanan, Gamble, Jones and Pradhan2015). Toxoplasma gondii infection in the general population can remain asymptomatic, but could be fatal in the immunocompromised patients. Pregnant women are a very important group, because this parasite may lead to miscarriage or neurological disorders in the fetus (Dong et al., Reference Dong, Su, Lu, Wang, Liu, Jian and Yang2018b). The route of transmission of T. gondii to humans could be vertical or horizontal via the consumption of resistant oocysts from the environment or uncooked meat of infected animals (Belluco et al., Reference Belluco, Patuzzi and Ricci2018). Approximately, 60% of toxoplasmosis occurs horizontally, which varies from country to country. Intermediate hosts, such as livestock animals play an important role as reservoirs of infection for humans. Studies in six major European cities, estimated that, 30–63% of the infections in pregnant women are meat-borne (Cook et al., Reference Cook, Holliman, Gilbert, Buffolano, Zufferey, Petersen, Jenum, Foulon, Semprini and Dunn2000). Direct microscopic observations, polymerase chain reaction (PCR), bioassay and antibody detection by serological techniques are common methods of diagnosing T. gondii. But, in epidemiological studies, serological methods are preferred for the diagnosis of T. gondii infection in animals. It is because other methods are not suitable for the analysis of a large sample size (Montoya, Reference Montoya2002; Ferra et al., Reference Ferra, Holec-Gąsior and Kur2015).
However, bovines do not appear to be a suitable host for T. gondii, but cattle are considered as one of the sources of T. gondii infection for humans (Hoffmann et al., Reference Hoffmann, Devleesschauwer, Aspinall, Cooke, Corrigan, Havelaar, Angulo, Gibb, Kirk and Lake2017). In fact, the meat and milk contaminated with T. gondii are the risk factor for T. gondii infection (Dubey and Thulliez, Reference Dubey and Thulliez1993; Hill and Dubey, Reference Hill and Dubey2013; Kalita and Sarmah, Reference Kalita and Sarmah2015). Further research is needed to determine the prevalence of T. gondii infection in bovines worldwide and the associated factors to help control it in these animals. Since T. gondii is a common important pathogen in humans and livestock, a more comprehensive understanding of the occurrence of T. gondii in the animal is essential. Although numerous valuable studies have been performed on the seroprevalence of T. gondii worldwide, to our knowledge, no global meta-analyses have been performed based on the systematic review of the literature. Therefore, the present systematic review and meta-analysis has been accomplished to draw a global perspective on the seroprevalence of T. gondii in bovines to better understand the global seroprevalence and importance of the bovine T. gondii infection.
Methods
Design and protocol registration
The present systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA) as described previously (Shamseer et al., Reference Shamseer, Moher, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015). The details of the study protocol are available on the website of the International Prospective Register of Systematic Reviews with the identifier Central Registration Depository of CRD42019107442 (Daryani et al., Reference Daryani, Shariatzadeh, Pagheh, Sharif and Sarvi2018).
Search strategy
Seroprevalence of T. gondii in bovines including cattle (Bos taurus), buffalo (Bubalus bubalis), yak (Bos grunniens) and bison (Bison bonasus) were of interest in this global review. To begin, we searched scientific databases for all the articles on the prevalence of bovine T. gondii infection published up to 30 May 2019. These keywords used alone or in combination were as follows: ‘Toxoplasma gondii’, ‘T. gondii’, ‘toxoplasmosis’, ‘seroprevalence’, ‘prevalence’, ‘serological test’, ‘bovine’, ‘cattle’, ‘heifer’, ‘calf’, ‘buffalo’, ‘yak’ and ‘bison’. The searching process of articles was carried out using English language databases including ‘Web of Science’, ‘Scopus’, ‘PubMed’, ‘Science Direct’, ‘ProQuest’ and ‘Google Scholar’. The systematic search of articles was conducted from 20 April to 30 May 2019 by two researchers independently (SAS and ASP).
Inclusion and exclusion criteria
Abstracts and full texts were assessed independently by the two researchers using a piloted form. The final decisions about the inclusion or exclusion of studies were made separately. Disagreements were resolved through arbitration by another author. Following the removal of duplicate entries, articles were evaluated according to the following criteria: (1) cross-sectional studies about the prevalence of T. gondii infection in bovines, (2) studies conducted only on cattle, buffalo, yak and bison and (3) studies where T. gondii infection in bovines was diagnosed by detecting immunoglobulin G (IgG) and/or IgM antibodies against T. gondii. The exclusion criteria comprised of: (1) case-control studies, review articles, dissertations, letters and animal models, (2) studies provided unclear data, (3) articles that were not available in the English language, (4) studies conducted on human and other animals and (5) conference abstracts.
Data extraction and the study quality assessment
All necessary information on the studies was recorded using Microsoft Excel software. The following data were collected for each study: the first author's name, publication year, country, continent, location, sample size, number of positives and negatives cases, bovine species, gender and age of each animal and type of serological method.
The Joanna Briggs Critical Evaluation Checklist was implemented to assess the quality of included records (Munn et al., Reference Munn, Moola, Lisy and Riitano2014). This checklist contains ten questions with four options designated as yes, no, unclear and not-applicable, regarding study quality. The papers with total scores of <5, 6 and 7–10 were considered to be of low, moderate and high quality, respectively. Our decisions to include or exclude the articles were influenced by the score of quality assessment and the articles with a quality score <5 were deleted.
Data synthesis and statistical analysis
In this study, the pooled rate seroprevalence of T. gondii with a 95% confidence interval (CI) was calculated using the random-effects model using StatsDirect software, version 2.8.0 (http://statsdirect.com). Heterogeneity among included studies was examined by Cochran's Q and I 2 statistics test. I 2 values of 50% or more were considered heterogeneous. A forest plot in the random effects model was used to calculate the pooled seroprevalence of T. gondii in bovines. To determine the source of heterogeneity, subgroup analyses were carried out. In a subgroup analysis, the seroprevalence of T. gondii was estimated based on year, gender, the continent of origin, type of animal, type of diagnostic method and sample size. Publication bias was evaluated graphically and statistically by applying Egger's and Begg's tests. In all statistical analyses, P value <0.05 was considered significant.
Results
A graphical summary of this study on global seroprevalence of bovine Toxoplasma infection is shown in Fig. 1. Our preliminary search of seven databases yielded 7691 articles but 710 were excluded from the study due to duplication. After a primary screening of the titles of the articles based on keywords, 5356 studies were extracted. In the next step, by screening the abstracts of the articles and based on the inclusion/exclusion criteria, 1407 of them were excluded. After reading the full text of the articles, 40 other papers were excluded. Finally, 178 (overall, 108 688 bovines, including 90 732 cattle, 11 791 buffaloes, 5816 yaks and 349 bison) of these articles (total data: 193) were entered into the meta-analysis with respect to the inclusion/exclusion criteria (Fig 2). The publication date of the studied articles was from 1967 to 2019.
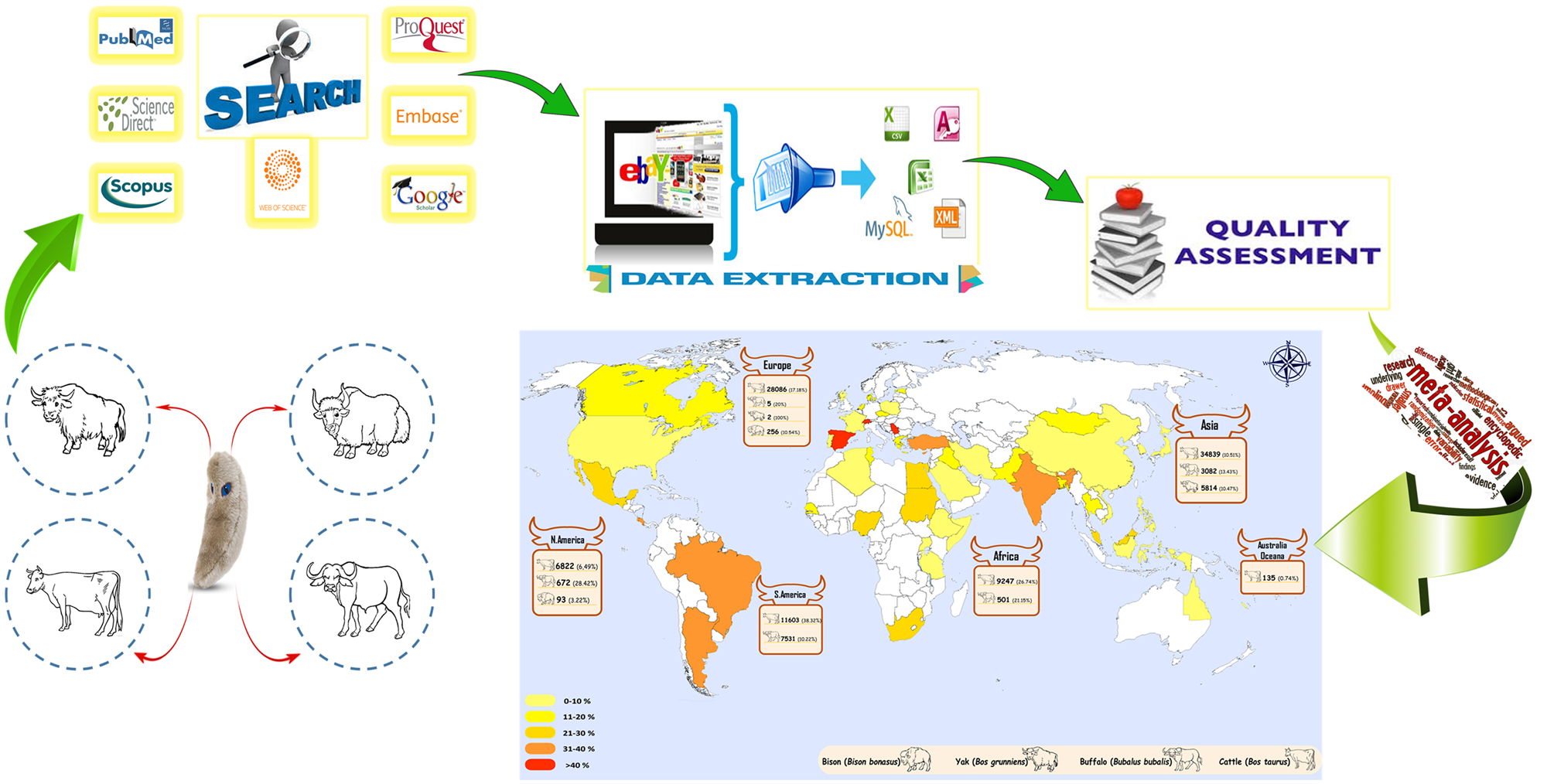
Fig. 1. Graphical summary of this study on global seroprevalence of bovine Toxoplasma infection.

Fig. 2. Flow chart of the study selection process showing inclusion and exclusion of studies identified.
Overall, there were 25 studies (28 349 bovines) in Europe, 84 studies (43 735 bovines) in Asia, 31 studies (9748 bovines) in Africa, 36 studies (26 721 bovines) in America and 2 studies (135 bovines) in Australia/Oceania. Among the included articles in this systematic review, enzyme-linked immunosorbent assay (ELISA) was the most common diagnostic method (53 studies), followed by modified agglutination test (MAT) in 24 studies, indirect immunofluorescence antibody test (IFAT) in 41 studies, direct agglutination test (DAT) in seven studies, latex agglutination test (LAT) in 30 studies, Sabin–Feldman dye test (SFDT) in 8 studies, indirect haemagglutination (IHA) in 25 studies, 2-mercaptoethanol (2ME) in one study, complement fixation test (CFT) in one study, Lateral flow chromatographic immunoassay (LFCIA) in one study, Indirect agglutination test (IAT) in one study and Micro precipitation in agar gel (MPA) in one study.
The main characteristics of the included studies are given in Table 1. In the studies, seroprevalence of T. gondii among bovines varied (from 0 to 100%) and a heterogeneity in the papers was observed (Cochran Q = 28 162.82; I 2 = 99.3%; df = 192; P < 0.001). Moreover, the results of the Begg–Mazumdar and Egger's regression test conducted to determine publication bias indicated no considerable effect on the total prevalence estimate (Begg–Mazumdar: 7.34 × 10−3, Egger's bias = 10.56, P = 0.88 and P < 0.001) (Fig 3).

Fig. 3. Bias assessment plot from the Egger's test based on standard error.
Table 1. Baseline characteristics of the included studies in this systematic review and meta-analysis based on years

ELISA, enzyme-linked immunosorbent assay; MAT, modified agglutination test; IFAT, indirect immunofluorescence antibody test; DAT, direct agglutination test; LAT, latex agglutination test; SFDT, Sabin–Feldman dye test; CFT, complement fixation test; LFCIA, lateral flow chromatographic immunoassay; IAT, indirect agglutination test; MPA, micro precipitation in agar gel; IHA, indirect haemagglutination.
The global pooled and weighted seroprevalence of T. gondii among bovines was 17.91% (95% CI: 15.32–20.6). The forest plot diagram of the present systematic review is illustrated in Fig 4. Moreover, the weighted prevalence of this parasite in bovines based on the host was as follows: cattle 16.94% (95% CI: 14.25–19.81), buffalo 22.26% (95% CI: 16.8–29), yak 23% (95% CI: 14–33) and bison 8.1% (95% CI: 3.9–13.7). Significant geographic differences in pooled T. gondii seropositivity rates among bovine were estimated. The seroprevalence was higher in America and Europe, where the T. gondii seropositivity rate was 22.2% (95% CI: 15.2–30.1) and 21.93% (95% CI: 15.98–28.53), respectively. The lowest seroprevalence was estimated in Australia/Oceania with the rate of 1.36% (95% CI: 0.02–7.65). A geographical map summarizing the seroprevalence of T. gondii among bovine in the world is shown in Fig 5.

Fig. 4. Forest plot displaying the global seroprevalence of Toxoplasma gondii in bovines.

Fig. 5. Global distribution map of seroprevalence of T. gondii in bovines worldwide.
The pooled seroprevalences of T. gondii in male and female bovine were 18.9% (95% CI: 13.8–24.7) and 17.78% (95% CI: 13.2–22.86), respectively. In a subgroup analysis based on diagnostic methods, the highest T. gondii seroprevalence detected by SFDT was 30% (95% CI: 17–45), followed by DAT 27% (95% CI: 13–43), IFAT 20.3% (95% CI: 13.9–27.6), MAT 19.7% (12.3–28.5), ELISA 18.52% (95% CI: 13.74–23.84), LAT 14.7% (95% CI: 8.9–21.8) and IHA 12.81% (95% CI: 7.82–18.81). The seroprevalence was higher in 2001–2005 and 2005–2010, whereas the T. gondii seropositivity rates were approximately 23.5% (95% CI: 6.8–46.3) and 23.5% (95% CI: 14.4–34.1), respectively. The lowest seroprevalence was estimated in ⩽2000 with the rate of 12.36%% (95% CI: 8.19–17.24). In a subgroup analysis based on sample size, the highest T. gondii seroprevalence was detected in sample size of 601–900 with the prevalence of 23% (95% CI: 13–35) followed by ⩽300 with the prevalence of 19.23% (95% CI: 15.17–23.63), 301–600 with the prevalence of 16.4% (95% CI: 11.7–21.8) and ⩾901 with the prevalence of 13.6% (95% CI: 8.3–19.9).
Table 2 summarizes the results of subgroup analysis and its details. Based on subgroup analysis, a statistically significant difference was observed in the overall prevalence of T. gondii in bovine based on year (χ 2 = 1346.94, P < 0.001), continent (χ 2 = 4145.1, P < 0.001), bovine species (χ 2 = 2632.73, P < 0.001), gender (χ 2 = 4.62, P = 0.032), method (χ 2 = 2833.23, P < 0.001) and sample size (χ 2 = 363, P < 0.001).
Table 2. Subgroup meta-analysis (variables such as year, continent, bovine species, gender, method and sample sizes) of global seroprevalence of Toxoplasma gondii in bovine

ELISA, enzyme-linked immunosorbent assay; MAT, modified agglutination test; IFAT, indirect immunofluorescence antibody test; DAT, direct agglutination test; LAT, latex agglutination test; SFDT, Sabin–Feldman dye test.
Discussion
Toxoplasmosis is one of the most common parasitic diseases in warm-blooded animals and in livestock poses a risk to the general public health, as the consumption of raw or uncooked meat can facilitate T. gondii transmission to humans (Tilahun et al., Reference Tilahun, Tolossa, Tilahun, Ashenafi and Shimelis2018). Therefore, it is difficult to implement the prevention and control programmes without sufficient information on the prevalence of T. gondii infection in animals, because they are the main source of zoonosis. Bovines are one of the most important sources of meat for humans in the world. However, the clinical symptoms of toxoplasmosis in bovines are much milder or more unknown than in the sheep, goats and pigs. However, it seems that accurate estimation on the prevalence of the infection in bovines for large-scale screening is essential in order to elucidate the role of bovines as carriers of T. gondii tissue cysts and to clarify the role of beef and milk in transmitting T. gondii to humans for health. The purpose of the analysis and interpretation presented in this review is to better understand the global prevalence of T. gondii among bovines. Overall, 108 688 bovines including 90 732 cattle, 11 791 buffaloes, 5816 yaks and 349 bisons were investigated by various serological methods. The data demonstrate that the weighted global seroprevalence in bovines was 17.91% and the prevalence range was between 0 and 100. There are numerous differences in the seroprevalence of bovine T. gondii infection in the world that could be categorized as follows: (i) biological aspects of parasites including geographical location, climate change, humidity and temperature, which can be effective in the parasitic life cycle and facilitate transmission, (ii) behavioural aspects including animal husbandry industry, the diet in different nations, especially the type of cooking, and public health management, which are considered as important variables in the prevalence of the infection in different communities and (iii) investigative aspects including study population, sample size, type of sampling and diagnostic method, which influences different findings in each study.
To investigate the causes of heterogeneity in the findings of studies included in this systematic review, the subgroup analysis was performed on various variables such as year, continent, and bovine species, gender, diagnostic methods and sample size. Our findings indicated a significant difference between the seroprevalence of T. gondii and the bovine species. The pooled seroprevalence of T. gondii in yak, buffalo, cattle and bison were 23, 22.26, 16.9, and 8.1%, respectively. The most prevalent rate was noted in yaks because the animals are free-grazing bovines that live with other wild and domestic animals. Yak (B. grunniens) is a long-haired male bovine species that lives in Afghanistan, the Himalayas, the Tibetan Plateau and Mongolia (Liu et al., Reference Liu, Cai, Zhang, Liu, Chen, Han and Liu2008). Yaks graze in pastures that are exposed to more T. gondii oocysts followed by the consequences of increasing the risk of infection (Qin et al., Reference Qin, Zhou, Cong, Zhang, Lou, Yin, Tan and Zhu2015). Also, the investigated population in various studies on yaks (6 data) is less than cattle (155 data) and buffalo (31 data). In the case of buffaloes, although they are similar to yaks in grazing, perhaps this less common difference than in the yaks is related to the more studied population of buffaloes (Alvarado-Esquivel et al., Reference Alvarado-Esquivel, Romero-Salas, Garcia-Vazquez, Cruz-Romero, Peniche-Cardena, Ibarra-Priego, Aguilar-Dominguez, Perez-de-Leon and Dubey2014). The prevalence of toxoplasmosis among buffaloes and yaks were almost similar, which is related to their way of life and breeding. These types of livestock are not bred centrally at all and are fed on open grazing in pastures, but cattle are usually kept centrally and are raised industrially. Obviously, in this type of breeding, the animals have much less access to free water or oocyst-contaminated forage. Although in traditional breeding, livestock has high and easy access to pastures, forage and the oocyst-contaminated water. The low prevalence of T. gondii in bison (8.1%) could be attributed to the fact that they are less exposed to cats and in contact with less occurrence of parasite life cycle, as a result, less risk of contamination. Of course, the number of studies on bison is much less compared to other bovines. Perhaps, if the population of this animal had been examined in greater numbers, the prevalence could have been changed as well (Wang et al., Reference Wang, Wang, Ye, Meng, Yin and Zhang2012; Alvarado-Esquivel et al., Reference Alvarado-Esquivel, Romero-Salas, Garcia-Vazquez, Cruz-Romero, Peniche-Cardena, Ibarra-Priego, Aguilar-Dominguez, Perez-de-Leon and Dubey2014; Brasil et al., Reference Brasil, Parentoni, Feitosa, Bezerra Cde, Vilela, Pena and de Azevedo2015).
Among the studies, there was a slight difference in the rate of infection by sex; therefore, the rate of infection in males (18.9%) was higher than that in the females (17.78%). Although in most studies the seroprevalence is higher in females. It could be attributed to the low immune system in females for the reasons, such as pregnancy and lactation. In most studies, the population of industrial dairy cattle was investigated (Bao et al., Reference Bao, Tian, Zhou, Xu, Wu, Wang, Tang, Qu and Song2012; FK and Shah, Reference FK and Shah2016). When the study population is higher in the industrial farms where cattle are usually not in contact with pastures and are less exposed to the parasites present in pastures. Under this condition, the lower percentage of infection in the male is more reasonable. Differences in the prevalence of T. gondii infection in females compared to males may be attributed to differences in sex hormones. Some researchers believe that female bovines are more resistant to infection compared to males, because the female hormones such as oestrogen strengthen and boost the immune system compared to androgen in males. Androgens make males more susceptible to infection due to a weakening immune system (Diakou et al., Reference Diakou, Papadopoulos, Haralabidis, Papachristou, Karatzias and Panousis2005; Albuquerque et al., Reference Albuquerque, Munhoz, Teixeira, Flausino, de Medeiros and Lopes2011; FK and Shah, Reference FK and Shah2016).
Based on our analysis, the most prevalence rate of T. gondii infection in cattle was observed in studies that used the Sabin–Feldman, DAT, IFAT, MAT, ELISA, LAT and IHA, respectively. MAT is more accurate and suitable compared to other agglutination methods but is unsuitable for investigation in the field, because it requires a large number of tachyzoites of T. gondii (Jones and Dubey, Reference Jones and Dubey2012). ELISA is a simple method for T. gondii diagnosis, but it requires species-specific combinations and an ELISA reader device. The sensitivity and specificity of serological assays are varied. Seroprevalence of T. gondii in cattle can be up to 90%, while isolation of live T. gondii from cattle has generally been unsuccessful (Dubey and Beattie, Reference Dubey and Beattie1988). Serological tests, especially MAT, IFAT and ELISA are the most common methods for diagnosing T. gondii infection in animal products and meat. Serological methods are often used as the first screening method to identify infected animals. The selection of a reliable and precise method can effectively interpret the results of T. gondii serological studies (Sharma et al., Reference Sharma, Sandhu, Bal, Kumar, Verma and Dubey2008; Sroka et al., Reference Sroka, Karamon, Cencek and Dutkiewicz2011; Fereig et al., Reference Fereig, Mahmoud, Mohamed, AbouLaila, Abdel-Wahab, Osman, Zidan, El-Khodary, Mohamed and Nishikawa2016).
The prevalence of T. gondii in bovines varied in different years. It seems that since the beginning of 2000, with the introduction of different methods, the prevalence has increased slightly compared to the reports prior to 2000. However, this prevalence rate of infection has decreased in the last 5 years, despite numerous studies with higher sample sizes, which may be related to the improvement of health index in animal husbandry, especially industrial breeding of livestock, more attention to animal health, the development of animal and veterinary sciences in the case of chronic diseases in large livestock (De Oliveira et al., Reference De Oliveira, Casseb, Ramos, Filho, Nogueira, Pinho and Pereira2018). The trend of global climate change, the reduction of rainfall, declining groundwater levels and numerous droughts in different parts of the world had an effective role in this declining trend since the beginning of the 20th century. Environmental and geographical factors, such as temperature, rainfall and geographical coordination can change the prevalence of infection in different populations (de Souza et al., Reference de Souza, Soares, Maia, Pereira, Ferraudo, Cruz, Pires Teixeira, Felippelli, Maciel, Goncalves, da Costa and Zanetti Lopes2016; De Oliveira et al., Reference De Oliveira, Casseb, Ramos, Filho, Nogueira, Pinho and Pereira2018).
Based on subgroup meta-analysis, there was a significant relationship between sample size and the prevalence of T. gondii infection, therefore increase of sample size, is followed by increase of the infection prevalence (Table 2). In the studies with a sample size above 900, the prevalence of T. gondii was lower than the other groups under study (Table 2). Most studies that had a sample size of more than 900 were conducted in a wider geographical area (e.g. a country or a vast province or the Tibetan Plateau, etc.) than the other three groups. However, when more livestock are studied in a large area (province or country, etc.), that study naturally notices more geographical and climatic diversity, and as a result, the chances of finding positive cases may be lower (Dubey et al., Reference Dubey, Hill, Jones, Hightower, Kirkland, Roberts, Marcet, Lehmann, Vianna, Miska, Sreekumar, Kwok, Shen and Gamble2005; Santos et al., Reference Santos, Costa, Toniollo, Luvizotto, Benetti, Santos, Matta, Lopes, Oliveira and Oliveira2009; Gharekhani, Reference Gharekhani2014b)
The strengths of this systematic review include the quality assessment by a checklist for incidence and prevalence study (the Joanna Briggs Critical Appraisal Checklist for Studies Reporting Prevalence Data), the high sample size in the global area, diversity of studies and analysis of subgroups according to geographical location, sex, sample size, year and type of serological method. In this study, we tried to pay attention to the maximum datasets in the article, despite the existence of 178 studies, the number of datasets reached 194, which indicates the accuracy of data extraction.
The main limitation expressed in the included articles was related to the selection of only English-language studies. For example, in Brazil which is one of the largest producers and exporters of beef in the world due to our limited access to local Brazilian journals, the number of included studies in this systematic review was lower than the number of studies conducted in the country.
On the contrary, the prevalence of T. gondii infection in bovines has been evaluated with different serological methods, while the gold standard method for diagnosing T. gondii infection in bovines is the bioassay method (Dehkordi et al., Reference Dehkordi, Haghighi Borujeni, Rahimi and Abdizadeh2013). The use of different laboratory diagnostic methods with varying sensitivity and specificity can affect the test results. It is better for researchers to use a reliable and precise serological test to measure the prevalence of T. gondii in bovines (Khames et al., Reference Khames, Yekkour, Fernández-Rubio, Aubert, Nguewa and Villena2018). It seems that in order to better understand the prevalence of T. gondii in bovines, it is necessary to use more sensitive and accurate methods such as PCR along with demographic screening methods, such as serological methods (Majeed and Abbas, Reference Majeed and Abbas2018). Although the serological methods have many advantages, but the variation in the cut-off value of each method cannot be a definitive indicator to estimate the infection in the animal population (Sharma et al., Reference Sharma, Parker, Al-Adhami, Bachand and Jenkins2019). The data presented, showed significant heterogeneity among the included studies based on livestock habitat. Some of these studies investigated the prevalence rate of T. gondii in industrial livestock that had fencing and protection and was less access to cats as a final host of this parasite, but other studies used domestic livestock with open grazing and with traditional breeding methods, which were more exposed to feces of cats. Some studies carried out on free-ranging wild bovines, such as bison. These animals not only live next to domestic cats but are also exposed to the wild cat's life cycle.
The type of sampling in most studies is not carried out uniformly or randomly or clustered. In fact, in most studies, it is not clear whether the study population represents the entire community in the region or not. In some studies on industrial livestock sampling, the sampling was performed only from livestock in a farm, the correct sampling or an acceptable estimate of the sample size was not mentioned.
Finally, due to the incomplete data in the included studies, our meta-analysis could not evaluate risk factors, such as breed, storage location, feeding age, type of breeding, etc. Evaluating these risk factors could provide a more comprehensive analysis with better dimensions of the problem.
Conclusion
To the best of our knowledge, this is the first systematic review and meta-analysis that provides a comprehensive view of the global seroepidemiology of bovine toxoplasmosis. The obtained result of this review showed that the global seroprevalence of bovine T. gondii infection is relatively high and it emphasizes the need for implementing screening and management of large animal meat products. This type of review analysis gives us an estimation on the prevalence in the world and completes the puzzle of the challenges facing one of the most important slogans of the World Health Organization, entitled ‘Food safety from farm to fork’. Finally, investigation on the role of bovine products, such as milk, meat and oral viscera, known as the source of transmission of infection be examined in detail on a global scale to clear the data on bovine toxoplasmosis and its role in humans.
Acknowledgements
The authors thank vice chancellors for research of Mazandaran University of Medical Sciences.
Financial support
This research received no grants.
Conflict of interest
The authors declare no conflicts of interest.