Introduction
The Angoumois grain moth, Sitotroga cerealella Olivier (Lepidoptera: Gelechiidae), is an important pest of wheat (Triticum aestivum L.) and other grains throughout the world (Weston & Rattlingourd, Reference Weston and Rattlingourd1999; Shukle & Wu, Reference Shukle and Wu2003). The larvae of S. cerealella cause serious damage by feeding on kernels and producing feces (Khattak et al., Reference Khattak, Munaf, Khalil, Hussain and Hussain1996; Ahmed et al., Reference Ahmed, Ul-Hasan and Hussain2002; Throne & Weaver, Reference Throne and Weaver2013). However, physical and chemical characteristics of host grains can influence the feeding potential of S. cerealella (Ahmed & Raza, Reference Ahmed and Raza2010).
Pesticides have been the primary tools for controlling grain pests (Hosseininaveh et al., Reference Hosseininaveh, Bandani, Azmayeshfard, Hosseinkhani and Kazzazi2007); however, the use of these pesticides is limited because of their adverse effects on humans and the environment. It is now necessary to develop more environmentally benign alternatives for controlling stored-products pests. Among available options, host-plant resistance offers one of the most successful preservation methods for many agricultural and food crops (Hagstrum & Subramanyam, Reference Hagstrum and Subramanyam1996; Jayas & Jeyamkondan, Reference Jayas and Jeyamkondan2002; Hashem, et al., Reference Hashem, Risha, El-Sherif and Ahmed2012).
The life history of insects has been reported to be affected by different factors. Various macronutrients especially protein and carbohydrate content, have a significant effect on the life history of insect pests (Ohmart, Reference Ohmart, Raske and Wickman1991, Hanks et al., Reference Hanks, McElfresh, Millar and Paine1993; El Atta, Reference El Atta2000). There are comprehensive studies on the life history of S. cerealella fed on different grain cultivars (Khan et al., Reference Khan, Afsheen, Din, Khattak, Khalil, Hayat and Lou2010; Saikia et al., Reference Saikia, Goswami and Bhattacharyya2014). For example, Consoli & Filho (Reference Consoli and Filho1995) studied the effects of different corn genotypes on the development and reproduction of S. cerealella and reported that the longest larval period and the lowest fecundity was on genotype Shrunhen. Shafique et al. (Reference Shafique, Ahmad and Chaudry2006) examined the effect of different wheat cultivars on the egg hatch of S. cerealella and showed that Punjab-96 and Chenab-99 were the least suitable wheat cultivars for this pest.
Invertebrate herbivores are faced with nutritional challenges, such as harmful secondary metabolites, proteinaceous inhibitors and imbalanced nutrition, and these affect the optimal growth, development, reproductive potential and population dynamic of insects (Awmack & Leather, Reference Awmack and Leather2002; Babic et al., Reference Babic, Poisson, Darwish, Lacasse, Merkx-Jacques, Despland and Bede2008). These herbivores regulate nutrients to increase growth performance (Simpson et al., Reference Simpson, Sibly, Lee, Behmer and Raubenheimer2004; Behmer, Reference Behmer2009). Post-ingestive regulation is one of the strategies for dealing with nutritional imbalances and proteinaceous inhibitors in the restricted range of available foods (Kotkar et al., Reference Kotkar, Sarate, Tamhane, Gupta and Giri2009).
Alpha-amylases are widespread in nature, being found in animals, microorganisms and plants (Terashima & Katoh, Reference Terashima and Katoh1996; Franco et al., Reference Franco, Rigden, Melo, Bloch, Silva and Grossi de Sa2000). S. cerealella lives on a starch-rich diet and relies on the effectiveness of its α-amylases for development. The α-Amylase activity has been described in several stored-product pests: Prostephanus truncatus Horn (Coleoptera: Bostrichidae) (Mendiola-Olaya et al., Reference Mendiola-Olaya, Valencia-Jimenez, Valdes-Rodriguez, Delano-Frier and Blanco-Labra2000); Rhyzopertha dominica Fabricius (Coleoptera: Bostrichidae) (Cinco-Moroyoqui et al., Reference Cinco-Moroyoqui, Rosas-Burgos, Barboa-Flores and Cortez-Rocha2006); Plodia interpunctella Hubner (Lepidoptera: Pyralidae) (Bouayad et al., Reference Bouayad, Rharrabe, Ghailani and Sayah2008); Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) (Pytelkova et al., Reference Pytelkova, Hubert, Lepsik, Sobotnik, Sindelka, Krizkova, Horn and Mares2009); Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae) (Dastranj et al., Reference Dastranj, Bandani and Mehrabadi2013); Trogoderma granarium Everts (Coleoptera: Dermestidae) (Hosseininaveh et al., Reference Hosseininaveh, Bandani, Azmayeshfard, Hosseinkhani and Kazzazi2007; Borzoui et al., Reference Borzoui, Naseri and Namin2015). Despite the economic importance of S. cerealella on wheat grains, there is no published information regarding the digestive amylolytic activity of this pest. Therefore, this study is the first attempt to investigate the digestive α-amylase activity of S. cerealella on different wheat cultivars.
The goal of this research was to study the biological characteristics and midgut α-amylase activity of S. cerealella larvae when fed on different wheat cultivars. An understanding of how digestive α-amylase function and the nutritional physiology of S. cerealella on wheat cultivars, and determining the factors affecting its life history is required when developing methods of pest management, such as the application of resistant cultivars to control the pest.
Materials and methods
Wheat sources
Seed of six wheat cultivars including Arg, Bam, Nai 60, Pishtaz, Sepahan and Shanghai were obtained from the Plant and Seed Improvement Research Institute (Karaj, Iran).
Insect rearing
The adults of S. cerealella were collected from stored maize seeds in Ardabil, Iran. Their larvae were fed on maize seeds and maintained at 25 ± 1°C, relative humidity of 65 ± 5%, and a photoperiod of 16:8 (L:D) h, as described by Throne & Weaver (Reference Throne and Weaver2013). After colonization of S. cerealella, the insects tested on different wheat cultivars were reared for three generations on the same cultivars. Moisture content of wheat cultivars was measured using the hot air oven method (AOAC, 1984) and varied from 10.2 to 11.8%.
Developmental time and survival rate of S. cerealella
A quantity of 500 g of each wheat cultivar was held in the experimental room for 48 h before the experiments. One hundred newly deposited eggs (within 24 h) of S. cerealella were individually transferred into Petri dishes (diameter 6 cm, depth 1 cm), containing one seed of each wheat cultivar. Petri dishes were visited daily, and the duration of immature stages (from egg to emergence of adult), immature survival and adult longevity were recorded.
Realized fecundity and egg fertility
Newly emerged adults (one male and one female) were transferred to plastic vials (diameter 5 cm, depth 10 cm) to evaluate realized fecundity. Black paper sheets were used as the substrate of deposited eggs (Ellington, Reference Ellington1930). The paper sheets were removed daily and the number of deposited eggs was recorded until the female's death. The eggs were maintained for 12 days to estimate the percentage of hatched eggs (fertility).
Weight of adults
The newly deposited eggs (within 24 h) of S. cerealella were individually transferred into glass dishes (diameter 15 cm, depth 20 cm), containing 200 g of each wheat cultivar. These glass dishes were held in the experimental room until adults emerged. The male (25–41 replicates) and female (25–49 replicates) adults emerged from larvae reared on each wheat cultivar were weighed separately.
Extraction of the proteinaceous inhibitors
The proteinaceous inhibitor content of the tested wheat cultivars was extracted based on the methods of Baker (Reference Baker1987) and Melo et al. (Reference Melo, Sales, Silva, Franco, Bloch and Ary1999). Separately, each wheat cultivar was powdered thoroughly, and then 30 g of powdered wheat was mixed with a solution of 0.1 m NaCl and stirred for 1.5 h, followed by centrifugation at 8000 g for 30 min. The supernatant was taken and heated at 70°C for 30 min to inactivate endogenous enzymes. Proteins were concentrated using a saturation of 70% ammonium sulfate followed by centrifugation at 8000 g for 30 min at 4°C. Fractionation of supernatant was done using 20, 40, 60 and 80% ammonium sulfate saturations. Pellets obtained from 80% saturation of ammonium sulfate were dissolved in the phosphate buffer (0.02 m, pH 7) and dialyzed against the same buffer overnight. This dialyzed solution was used as a source of inhibitor in enzymatic assays.
Protein concentration of inhibitors of the tested wheat cultivars was measured using bovine serum albumin (Roche Co., Germany) (Bradford, Reference Bradford1976).
Enzymes preparation
The fourth instar larvae of S. cerealella fed on each cultivar were carefully dissected in pre-cooled distilled water under stereomicroscope (Stemi SV6 ZEISS, Germany) (Borzoui & Bandani, Reference Borzoui and Bandani2013). Midguts were separated and homogenized on ice using a pre-cooled homogenizer (Teflon pestle). The homogenates were centrifuged at 15,000 g for 15 min at 4°C. The supernatants were pooled and stored in aliquots at −20°C as an enzyme source for subsequent use. For inhibition experiment, the enzyme extract prepared from S. cerealella larvae fed on maize was used.
Effect of pH on digestive amylolytic activity
The effect of pH on α-amylase activity was examined using α-amylase extracted from fourth instar larvae fed on maize. Optimal pH for α-amylase activity was determined using universal buffer (0.02 m) (Hosseinkhani & Nemat-Gorgani, Reference Hosseinkhani and Nemat-Gorgani2003; Borzoui & Bandani, Reference Borzoui and Bandani2013) with pH set at 5–12.
Amylolytic activity assay
The dinitrosalicylic acid (DNS) method (Bernfeld, Reference Bernfeld1955), with 1% soluble starch (Sigma Chemical Co., St Louis, USA) as substrate at the optimal pH was used to assay the digestive amylolytic activity of S. cerealella larvae fed on different wheat cultivars. Twenty microliters of the enzyme extracted from 30 midguts were incubated with 500 µl of glycine–NaOH buffer (pH 11.5) (Borzoui & Bandani, Reference Borzoui and Bandani2013) and 40 µl of soluble starch for 30 min at 37°C. The reaction was stopped by adding 100 µl of DNS (Sigma Chemical Co., St Louis, USA) and heating in boiling water for 10 min. The absorbance of the mixture was read at 540 nm after being cooled on ice. One unit of α-amylase activity was defined as the quantity of enzyme required to produce 1 mg maltose at 37°C min−1. A standard curve of absorbance against the amount of maltose (Sigma Chemical Co., St Louis, USA) released was constructed to enable its calculation during α-amylase assays. A control containing no α-amylase extract with substrate was run simultaneously with the reaction mixture.
In-gel amylolytic activity assay
The amylolytic activity of S. cerealella in the gel was detected using the method of Kazzazi et al. (Reference Kazzazi, Bandani and Hosseibkhani2005). Briefly, polyacrylamide gel electrophoresis (PAGE) was performed in a 10% (w/v) separating gel and a 4% stacking gel with 0.05% SDS. Twenty microliters of the enzyme extracts (from 30 larval midguts) were mixed with an equal volume of zymogram sample buffer. Thirty five microliters of each sample were loaded on each well of zymogram gel. Electrophoresis was run until the blue dye reached the bottom of the gel. The gel was then rinsed with distilled water and left in a solution of 1% (v/v) Triton X-100 for 15 min. Thereafter, the gel was taken and put in a solution of glycine–NaOH (pH 11.5) containing 1% starch solution, 2 mm CaCl2 and 10 mm NaCl for 1.5 h at 37°C. To stop the reaction and stain the un-reacted starch background, the gel was treated with a solution of 1.3% I2 and 3% KI. Finally, light bands of α-amylase activities appeared against a dark background.
Inhibition of S. cerealella α-amylases by wheat cultivars
In this experiment, 1.5 mg proteinaceous extract of each cultivar was incubated (30 min at 37°C) with amylase extracted from fourth instar larvae fed on maize (Baker, Reference Baker1987; Melo et al., Reference Melo, Sales, Silva, Franco, Bloch and Ary1999). For the control group, we used the same enzyme extract of fourth instar larvae without inhibitor. The procedure for the α-amylase assay was conducted as noted in the ‘Amylolytic activity assay’ section. The percentage of α-amylase inhibition was calculated according to Borzoui & Bandani (Reference Borzoui and Bandani2013).
Weight of tested wheat cultivars
The weight of the grains of tested wheat cultivars was measured as the mean hundred-wheat weight (Fouad et al., Reference Fouad, Faroni, de Lima and Vilela2013). Five hundred seeds of each cultivar (in five replicates, each including 100 seeds) were randomly collected and weighed using an electronic balance.
Starch determination of wheat cultivars
The starch content of tested cultivars was quantified by the method of Bernfeld (Reference Bernfeld1955) using starch as a standard. Briefly, each wheat cultivar was powdered thoroughly, and then 200 mg of each cultivar were homogenized in 35 ml of distilled water and heated to boiling point. One hundred microliters of each sample was added to 2.5 ml of iodine reagent (0.02% I 2 and 0.2% KI), and the absorbance was read at 580 nm.
Statistical analysis
Before analysis, all the data were examined for normality using Kolmogorove–Smirnov test (PROC GLM; SAS Institute, 2003). Since the data were normally distributed, no data transformation was conducted. Life history and digestive α-amylase activity of S. cerealella were analyzed, based on a completely randomized design, by one-way analysis of variance (ANOVA) using SAS ver.9.2 program (PROC GLM; SAS Institute, 2003). Finally, the statistical differences among the means were appraised using the least significant difference (LSD) test at α = 0.05. Correlation analysis of the examined biological and physiological characteristics of S. cerealella fed on different wheat cultivars with inhibition of α-amylase, soluble starch content and hundred-wheat weight was performed using SPSS 16.0.
Results
Developmental time and survival rate
The results of the effect of various wheat cultivars on developmental time of S. cerealella are given in table 1. The shortest developmental time of immature stages (from egg to emergence of adult) (F = 85.69; df = 5, 407; P < 0.0001) was on cultivar Bam (23.65 ± 0.15 days), and the longest time was on cultivar Sepahan (32.63 ± 0.66 days). Different cultivars showed a significant effect on the longevity of male (F = 23.43; df = 5, 195; P < 0.0001) and female (F = 33.48; df = 5, 206; P < 0.0001) of S. cerealella, which was shortest on cultivar Sepahan (5.96 ± 0.20 and 6.80 ± 0.20 days, respectively), and longest on cultivar Bam (8.14 ± 0.10 and 9.38 ± 0.11 days, respectively) (table 1).
Table 1. Mean (±SE) duration (days) of immature stages and longevity of S, cerealella fed on different wheat cultivars.

The means followed by different letters in the same column are significantly different (LSD, P < 0.05).
1 The n value shows the sample size for each parameter.
The survival rates of S. cerealella immature stages on six wheat cultivars are shown in fig. 1. The lowest survival rate (F = 28.09; df = 5, 24; P < 0.0001) of immature stages was observed on cultivars Nai 60 (54.66 ± 2.49%) and Sepahan (49.33 ± 4.52%), and the highest survival rate was seen on cultivar Bam (93.33 ± 2.10%) (fig. 1).

Fig. 1. Mean (±SE) percentage survival of S. cerealella immature stages (n = 49–91) on different wheat cultivars. The means followed by different letters are significantly different (LSD, P < 0.05).
Realized fecundity and egg fertility
The results of realized fecundity and egg fertility of S. cerealella are shown in table 2. The highest realized fecundity (F = 136.33; df = 5, 206; P < 0.0001) and egg fertility (F = 53.79; df = 5, 206; P < 0.0001) of S. cerealella were recorded for females developed from larvae reared on cultivar Bam (93.30 ± 2.10 eggs/female and 91.90 ± 3.10%, respectively), and the lowest values of these parameters were recorded on cultivar Sepahan (49.30 ± 4.50 eggs/female and 67.4 ± 11.1%, respectively) (table 2).
Table 2. Mean (±SE) realized fecundity (eggs laid per female) and egg fertility (percentage of hatched eggs per female) of S. cerealella fed on different wheat cultivars.

The means followed by different letters are significantly different (LSD, P < 0.05).
1 The n value shows the sample size for each parameter.
Weight of adults
The results of adults' weight of S. cerealella on different wheat cultivars are shown in fig. 2. The male adults of S. cerealella which came from larvae fed on cultivar Bam were heavier (2.97 ± 0.02 mg) than those reared on any other cultivar (F = 176.40; df = 5, 196; P < 0.0001). In addition, females (F = 297.56; df = 5, 206; P < 0.0001) which came from larvae reared on cultivar Bam showed the highest weight (4.80 ± 0.01 mg) as compared with other cultivars tested (fig. 2). By contrast, the lowest weight of male adults of S. cerealella was seen in the insects that came from larvae reared on cultivars Nai 60 (2.17 ± 0.03 mg) and Sepahan (2.15 ± 0.03 mg). Moreover, the lowest weight of female adults was seen in the insects that came from larvae fed on cultivar Sepahan (3.65 ± 0.03 mg).
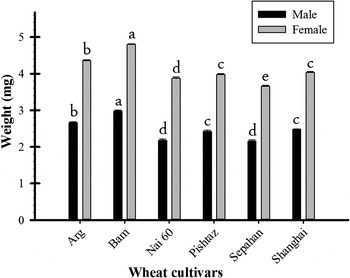
Fig. 2. Mean (±SE) weight of male (n = 25–41) and female (n = 25–49) adults of S. cerealella fed on different wheat cultivars. The means followed by different letters are significantly different (LSD, P < 0.05).
Optimal pH of α-amylase
The effect of pH on amylolytic activity of the midgut extract from the fourth instar larvae of S. cerealella is presented in fig. 3 (F = 201.22; df = 14, 30; P < 0.0001). Amylolytic activity with starch as substrate occurred over an alkaline pH range (pH 9–11), with maximum activity at pH 11.5. In acidic conditions (pH 5–7), little activity (under 20% of maximum activity) was observed. In highly alkaline conditions (pH 12), approximately 70% of maximum activity was recorded.
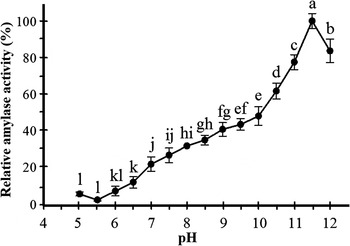
Fig. 3. Effect of pH on the amylolytic activity (n = 3) of S. cerealella. Activity was determined using universal buffer. The means followed by different letters are significantly different (LSD, P < 0.05).
Enzyme assay
Amylolytic activity for the fourth instar larvae of S. cerealella feeding on different wheat cultivars is shown in fig. 4 (F = 19.60; df = 5, 12; P < 0.0001). The fourth instar larvae reared on cultivar Bam had the highest level of amylolytic activity (0.89 ± 0.04 mg maltose min−1). By contrast, the lowest levels of amylolytic activity were seen on cultivars Nai 60 and Sepahan (0.42 ± 0.03 and 0.39 ± 0.04 mg maltose min−1, respectively).

Fig. 4. Mean (±SE) α-amylase activity of midgut extracts (n = 3) from S. cerealella larvae fed on different wheat cultivars. The means followed by different letters are significantly different (LSD, P < 0.05).
In-gel enzyme analysis
Amylolytic activity pattern of the fourth instar larvae of S. cerealella feeding on different wheat cultivars is shown in fig. 5. One prominent α-amylase isozyme was detected in the midgut extracts of larvae fed on the tested wheat cultivars, but, the intensity of individual α-amylase isozymes varied by cultivars. According to the results of this study, larvae reared on cultivar Sepahan showed a weak intensity of amylolytic band as compared with those reared on cultivars Bam, Arg and Shanghai. Furthermore, larvae fed on cultivar Bam had a stronger intensity than those fed on the other cultivars (fig. 5).

Fig. 5. In-gel visualization of α-amylase activity isozyme in the midgut of S. cerealella larvae fed on six wheat cultivars. One band of the α-amylase activity was observed in each sample, and this band was designated as a major isoenzyme.
Inhibition of α-amylases by wheat cultivars
The effect of inhibitor extracted from wheat cultivars on digestive α-amylase activity of S. cerealella is shown in fig. 6 (F = 12.93; df = 5, 12; P < 0.0001). Maximum inhibition of α-amylase activity was estimated by incubating midgut extracts from the fourth instar larvae with proteinaceous extract of cultivars Sepahan and Nai 60 (85.32 ± 5.90 and 75.91 ± 1.83%, respectively).

Fig. 6. Mean (±SE) percentage inhibition of amylolytic activity of S. cerealella larval midgut (n = 3) by crude inhibitor extracted from different wheat cultivars. Enzyme and inhibitor were incubated for 30 min prior to the addition of starch to measure enzymatic activity. The means followed by different letters are significantly different (LSD, P < 0.05).
Dietary starch content
Dietary starch content of different wheat cultivars used for feeding assays of S. cerealella is shown in fig. 7. Cultivar Bam had the highest starch content (413.66 ± 5.81 mg g−1 fresh weight) and cultivar Sepahan had the lowest (363.33 ± 12.77 mg g−1 fresh weight) (F = 4.75; df = 5, 12; P < 0.0001). However, there were no significant differences in starch content among cultivars Bam, Nai 60, Arg and Pishtaz.

Fig. 7. Soluble starch content of different wheat cultivars used for feeding of S. cerealella larvae. Each point is average of three replications. The means followed by different letters are significantly different (LSD, P < 0.05).
Weight of tested wheat cultivars
Fig. 8 shows the mean hundred-wheat weight of tested cultivars (F = 12.93; df = 5, 12; P < 0.0001). The mean hundred-wheat weight was significantly heavier (5.92 ± 0.19 g) in cultivar Bam than in the other cultivars. By contrast, the weight of cultivar Sepahan was the lightest (4.02 ± 0.19 g) compared with the other cultivars.

Fig. 8. The mean hundred-wheat weight of different tested wheat cultivars. The means followed by different letters are significantly different (LSD, P < 0.05).
Correlation analysis
The analysis of correlation coefficients of the examined biological and physiological characteristics of S. cerealella fed on different wheat cultivars with inhibition of α-amylase, soluble starch content and hundred-wheat weight is shown in table 3. The results of this study showed that very high correlations existed between the immature period, fecundity and fertility on one side and inhibition of α-amylase, soluble starch content and hundred-wheat weight on the other. Very high positive correlations were also found between fecundity (r = 0.876 and 0.989, respectively) and fertility (r = 0.864 and 0.990, respectively) and soluble starch content and hundred-wheat weight. Moreover, a negative correlation was found between fecundity and fertility (r = −0.896 and −0.896, respectively) and inhibition of α-amylase. The immature period exhibited a negative correlation with soluble starch content and hundred-wheat weight (r = −0.839 and −0.983, respectively) and a positive correlation with inhibition of α-amylase (r = 0.946). Correlation coefficients showed that amylolytic activity exhibited a positive correlation with inhibition of α-amylase (r = 0.842) and a very high positive correlation with hundred-wheat weight (r = 0.967) (table 3).
Table 3. Correlation coefficients (r) of some biological and physiological characteristics of S. cerealella fed on different wheat cultivars with inhibition of α-amylase, soluble starch content and hundred-wheat weight.

Discussion
In this study, the biological and physiological properties of S. cerealella were influenced when exposed to different wheat cultivars. S. cerealella required a longer time to complete its immature stages when reared on cultivar Sepahan than when reared on the other cultivars. The quality and quantity of food have a direct effect on basic aspects of the biology of insects (Pashley et al., Reference Pashley, Ardí and Hammond1995; Bentancourt et al., Reference Bentancourt, Scatoni, Gonzalez and Franco2003; Bong et al., Reference Bong, Er, Yiu and Rajan2008). Variations in the duration of immature stages of S. cerealella might be attributed to the differences in the macronutrients (especially starch), inhibitors and weight of tested wheat cultivars.
The results regarding the incubation period of S. cerealella agreed with those achieved by Saikia et al. (Reference Saikia, Goswami and Bhattacharyya2014), who reported incubation periods of 2.8 ± 0.8 and 3.3 ± 0.5 days for S. cerealella reared on maize and rice, respectively. The developmental time of whole larval and pupal stages of S. cerealella, in this study, is not in agreement with the findings of Shukle & Wu (Reference Shukle and Wu2003), who noted that the developmental time of the whole larval and pupal stages of S. cerealella was 46.6 ± 5.6 days on wheat seeds mixed with Brewer's yeast. Such discrepancy might be attributed to either genetic differences in populations or variations in experimental conditions and cultivars used for feeding of this pest.
In agreement with previous research (Consoli & Filho, Reference Consoli and Filho1995; Ashamo, Reference Ashamo2010), the results of this study showed that tested wheat cultivars had significant effects on the longevity of S. cerealella adults. The shortest adult longevity of S. cerealella on cultivar Sepahan suggested that this cultivar did not contain all of the ingredients necessary for the optimal development of S. cerealella larvae, which led to the emergence of adults with shorter longevity.
The reduced survival rates of this pest found on cultivars Nai 60 and Sepahan might be attributed to their negative correlation (r = −0.900) with inhibition of α-amylase (table 3). The range of survival rates of S. cerealella, in the current study, is in agreement with those reported for S. cerealella reared on paddy varieties (Ashamo, Reference Ashamo2010) and corn genotypes (Consoli & Filho, Reference Consoli and Filho1995). Gatehouse & Boulter (Reference Gatehouse and Boulter1983) suggested that the level of inhibitors in legumes is related to the observed resistance to pests. In this study, the digestive α-amylase activities of S. cerealella larvae reared on different wheat cultivars were affected by the proteinaceous extract of these cultivars (fig. 6) that led to the reduced growth and increased mortality of this insect on some tested cultivars. Studies on plant inhibitors and insect digestive enzymes indicated that insect enzymes responded to exposed inhibitors by changing the complement of midgut proteinases (Dunse et al., Reference Dunse, Kaas, Guarino, Barton, Craik and Anderson2010; Lomate & Hivrale, Reference Lomate and Hivrale2011). In some insects, the produced proteinases are able to degrade inhibitors, and reduce the adverse effect on pest growth and development (Bown et al., Reference Bown, Wilkinson and Gatehouse1997; Giri et al., Reference Giri, Harsulkar, Deshpande, Sainani, Gupta and Ranjekar1998).There was also a positive correlation between food availability (weight of each cultivar) and survival of the pest (table 3). An increased food availability implies a change in the activity of digestive enzymes to ensure proper nutritional requirements (Kotkar et al., Reference Kotkar, Sarate, Tamhane, Gupta and Giri2009; Borzoui et al., Reference Borzoui, Naseri and Namin2015).
The performance of lepidopteran adults can directly be influenced by larval food (Nay & Perring, Reference Nay and Perring2008; Sarate et al., Reference Sarate, Tamhane, Kotkar, Ratnakaran, Susan, Gupta and Giri2012). The results of this study showed a small body size in adults reared on cultivar Sepahan (an unsuitable host); however, no significant correlation was found between the adult weight of S. cerealella and the dietary factors of tested cultivars (table 3). This result is consistent with the data obtained by Ahmed & Raza (Reference Ahmed and Raza2010), who reported a significant difference in the adult weight of S. cerealella fed on various maize varieties. Furthermore, the realized fecundity and egg fertility of S. cerealella was higher in females fed on cultivar Bam because of the higher nutritional value of this cultivar, particularly in relation to its weight, starch content and level of inhibitors. Similar results were observed by Shobana et al. (Reference Shobana, Murugan and Kumar2010), with Papilio polytes Linnaeus (Lepidoptera: Papilionidae) reared on a nutrient-rich diet.
The results of the mean hundred-wheat weight of each tested cultivar showed that the physical characteristics of wheat cultivars had an apparent effect on the biological and physiological aspects of S. cerealella. Shazali (Reference Shazali1987) showed that S. cerealella consumed significantly more food in the larger grain type than in the smaller one. In the present study, the direct correlations (table 3) were clear, even though there were differences in life history, realized fecundity, egg fertility and α-amylase activity of S. cerealella among the large and small size of the wheat cultivars (of viewpoint the mean hundred-wheat weight). The insects reared on cultivar Bam (with the highest weight) completed their immature stages faster than those reared on the other cultivars (table 1, fig. 8). Moreover, realized fecundity and egg fertility were elevated with increase in the weight of wheat cultivars (tables 2 and 3). Similarly, an increase in the level of α-amylase activity was obtained with increase in the weight of wheat cultivars (table 3). It seems that when seed weight is increased, more food is available for the feeding of S. cerealella larvae; therefore, the larvae elevated α-amylase levels to get more nutrients. Furthermore, the larvae could have been used these optimal food conditions for optimal fitness. This finding is also supported by previous studies (Behmer, Reference Behmer2009; Kotkar et al., Reference Kotkar, Sarate, Tamhane, Gupta and Giri2009; Karasov et al., Reference Karasov, Martinez del Rio and Caviedes-Vidal2011; Borzoui et al., Reference Borzoui, Naseri and Namin2015) that have stated that the kind and level of digestive enzymes in many insects can be largely explained by the interaction between diet macronutrients and insect food needs, which ultimately affect the life cycle of these insects.
This study is the first attempt to characterize amylolytic activity in larvae of S. cerealella. High amylolytic activity was found in the midgut of larvae of S. cerealella as reported for other lepidopteran grain pests (Bouayad et al., Reference Bouayad, Rharrabe, Ghailani and Sayah2008; Pytelkova et al., Reference Pytelkova, Hubert, Lepsik, Sobotnik, Sindelka, Krizkova, Horn and Mares2009). The pH is a key factor affecting biochemical reactions of digestive enzymes. The results of this study showed that the α-amylase enzyme was active over an alkaline pH range (pH 9–12). According to the results of Tabatabaei et al. (Reference Tabatabaei, Hosseininaveh, Goldansaz and Talebi2011), the α-amylase enzyme from Ectomyelois ceratoniae (Zeller) (Lepidoptera: Pyralidae) was active in the pH range of 7–11.
Results from the current study on the digestive α-amylase of S. cerealella showed that this lepidopteran pest replies to different wheat cultivars by changing digestive enzymes. Correlation analyses and comparisons of α-amylase activities of the fourth instar larvae fed on tested cultivars suggested that factors found in the cultivars (weight and α-amylase inhibitors) influenced the α-amylase activity. This result coincides with the outcomes reported by Harsulkar et al. (Reference Harsulkar, Giri, Patankar, Gupta, Sainani, Ranjekar and Deshpande1999) and Kotkar et al. (Reference Kotkar, Sarate, Tamhane, Gupta and Giri2009), for H. armigera. However, Bouayad et al. (Reference Bouayad, Rharrabe, Ghailani and Sayah2008) reported that the level of α-amylase activity in larvae of P. interpunctella was dependent upon the dietary carbohydrate source.
In this study, a difference in the expression level of α-amylase isozyme was detected in the PAGE. Considering the relationship between the intensity of α-amylase band and the hundred-wheat weight of each cultivar, it seems that there is a mechanism to precisely discover the diet content and adjust the level of this major digestive enzyme (Behmer, Reference Behmer2009; Kotkar et al., Reference Kotkar, Sarate, Tamhane, Gupta and Giri2009; Sarate et al., Reference Sarate, Tamhane, Kotkar, Ratnakaran, Susan, Gupta and Giri2012). Silva et al. (Reference Silva, Terra, Xavier-Filho, Grossi de Sa, Isejima, DaMatta, Miguens and Bifano2001) showed that the α-amylase gene was regulated according to the amount of starch in the diet. Interestingly, although starch contents were not significantly different between cultivars Arg and Bam (fig. 7), the midgut α-amylase from larvae fed on cultivar Bam was higher than those fed on cultivar Arg (fig. 4), suggesting the hyperproduction of digestive amylases from midgut cells of S. cerealella. Correlation coefficient given in table 3 between amylolytic activity of larvae and inhibition of α-amylase supported this finding.
According to the correlation analysis, there is a negative correlation between the duration of immature stages of S. cerealella and the starch content of tested cultivars (table 3). Behmer (Reference Behmer2009) reported that herbivorous insects regulate their nutrient intake using pre- and post-ingestive mechanisms. Since the significant correlation was not established between the amylolytic activity of S. cerealella larvae and the starch content of the tested cultivars, it seems that the larvae consumed more food until the requirement for carbohydrate was reached. More feeding by S. cerealella larvae may lead to the intake of a high level of enzyme inhibitors into the digestive system that could affect the life history of this pest. As seen in table 3, a significant positive correlation was detected between the duration of immature stages and inhibition of α-amylase.
Concluding remarks
The results of this study showed that S. cerealella not only tried to complete its life cycle on nutritionally unsuitable wheat cultivars but also developed its immature stages to achieve necessary nutrition for further survival. Moreover, wheat proteinaceous extract, acting as an inhibitor, affected nutrient regulation by S. cerealella. The larvae of S. cerealella fed on heavier wheat (cultivar Bam) completed their immature stages faster than those reared on lighter cultivars. The results of this study showed that the insects fed on cultivar Sepahan (with the lightest weight) had the longest immature stages, the highest mortality and lowest weights of adult males and females, fecundity and fertility along with a lower amylolytic activity. Therefore, it can be suggested that cultivar Sepahan is an unsuitable host for S. cerealella, which could be recommended for cultivation as the most resistant for this pest. An explained biochemical and molecular analysis of midgut α-amylase enzyme upon disposal of S. cerealella to a specific diet will highlight their particular roles. It would be interesting to focus on the digestive proteases of S. cerealella and their interactions with the protein content of different wheat cultivars. Also, the insect's response to the α-amylase inhibitors should be essentially reviewed for the selection of appropriate α-amylase inhibitors and their transgenic expression for resistance to this pest.
Acknowledgement
We thank the University of Mohaghegh Ardabili (Ardabil, Iran), for cooperation by support for the experiment.














