Background
Exposure to stress leads to a cascade of physiological events culminating in maladjustment, including alterations in the body's stress response (McEwen, Reference McEwen2007; Lupien et al. Reference Lupien, McEwen, Gunnar and Heim2009; Danese & McEwen, Reference Danese and McEwen2012), lower fittedness to the social ecology (DuBois et al. Reference DuBois, Felner, Brand, Adan and Evans1992), and diminished adaptation to daily life (Delongis et al. Reference Delongis, Coyne, Dakof, Folkman and Lazarus1982; Serido et al. Reference Serido, Almeida and Wethington2004). When stress is chronic and occurs during early sensitive periods, its effects on brain and behavior significantly increase (Hofer & Shair, Reference Hofer and Shair1987; Noonan et al. Reference Noonan, Caldwell, Li, Walker, Pedersen and Mason1994; Coplan et al. Reference Coplan, Andrews, Rosenblum, Owens, Friedman, Gorman and Nemeroff1996; Feldman et al. Reference Feldman, Granat, Pariente, Kanety, Kuint and Gilboa-Schechtman2009; Feldman, Reference Feldman2015b ). Yet, while chronic stress carries lasting effects on the developing brain, there are elements in the rearing environment, particularly the mother's presence and parenting behavior, that provide a regulatory buffer against the effects of stress on offspring adaptation (Hofer, Reference Hofer1994; Sullivan & Holman, Reference Sullivan and Holman2010). These elements can define risk and resilience trajectories in stress-exposed children in ways that require much further research.
Research in psychoneuroimmunology, the study of neural–endocrine–immune system interactions, underscores the endocrine–immune interface as a critical factor in shaping the organism's reactivity to chronic stress (Maier et al. Reference Maier, Watkins and Fleshner1994; Glaser & Kiecolt-Glaser, Reference Glaser and Kiecolt-Glaser2005). While most studies on early-life stress focused on the hypothalamic-pituitary-adrenal (HPA) system and its multi-level manifestations, several studies in animal models addressed the impact of chronic early stress on the developing immune (Coe et al. Reference Coe, Kramer, Kirschbaum, Netter and Fuchs2002; O'Mahony et al. Reference O'Mahony, Marchesi, Scully, Codling, Ceolho, Quigley, Cryan and Dinan2009; O'Connor et al. Reference O'Connor, Winter, Hunn, Carnahan, Pressman, Glover, Robertson-Blackmore, Moynihan, Lee and Caserta2013) and oxytocinergic systems (Liu et al. Reference Liu, Diorio, Tannenbaum, Caldji, Francis, Freedman, Sharma, Pearson, Plotsky and Meaney1997; Francis et al. Reference Francis, Young, Meaney and Insel2002), providing evidence for its lifelong effects on immune system functionality. Studies have repeatedly shown that psychological distress alters immune reactivity (Herbert & Cohen, Reference Herbert and Cohen1993; Engeland et al. Reference Engeland, Hugo, Hilgert, Nascimento, Junges, Lim, Marucha and Bosch2016). Early-life stress enhances inflammatory responsiveness to psychosocial stressors and suppresses inhibitory mechanisms that dampen inflammation primarily by making immune cells insensitive to the anti-inflammatory effects of cortisol (Miller & Cohen, Reference Miller and Cohen2001; Fagundes et al. Reference Fagundes, Glaser and Kiecolt-Glaser2013). This suppression leads to increased inflammation, reduced lymphocytic proliferation rate (Kay et al. Reference Kay, Tarcic, Poltyrev and Weinstock1998), and diminished cytokine response (Coe et al. Reference Coe, Kramer, Kirschbaum, Netter and Fuchs2002). Importantly, the relations between stress and immunity are bi-directional, given that inflammatory processes in general and early-life inflammatory events in particular are involved in inducing various psychopathologies, including schizophrenia, autism, depression, and anxiety disorders (Lucchina et al. Reference Lucchina, Carola, Pitossi and Depino2010; Meyer et al. Reference Meyer, Feldon and Dammann2011; Giovanoli et al. Reference Giovanoli, Engler, Engler, Richetto, Voget, Willi, Winter, Riva, Mortensen, Feldon, Schedlowski and Meyer2013; Musaelyan et al. Reference Musaelyan, Egeland, Fernandes, Pariante, Zunszain and Thuret2014).
Salivary immunoglobulin A (s-IgA) is an important mucosal barrier factor that complexes with luminal antigens, thus disrupting pathogen penetration (Stone et al. Reference Stone, Cox, Valdimarsdottir and Neale1987; Humphrey & Williamson, Reference Humphrey and Williamson2001; De Almeida et al. Reference De Almeida, Grégio, Machado, De Lima and Azevedo2008). Due to its importance and accessibility, human studies on stress and psychopathology have utilized s-IgA as a biomarker of the immune system. Generally, acute psychological stress has been linked with reduced s-IgA levels (Deinzer et al. Reference Deinzer, Kleineidam, Stiller-winkler, Idel and Bachg2000; Ng et al. Reference Ng, Koh, Mok, Lim, Yang and Chia2004), whereas chronic stress with increased s-IgA (Bosch et al. Reference Bosch, Ring, de Geus, Veerman and Amerongen2002). However, results regarding the effects of stress on s-IgA are complex and somewhat inconsistent (Valdimarsdottir & Stone, Reference Valdimarsdottir and Stone1997; Tsujita & Morimoto, Reference Tsujita and Morimoto1999), requiring much further research, particularly in stress-exposed children.
Apart from the immune system, the oxytocinergic system has been extensively studied in relation to social affiliation and stress management in humans and animals (Ring et al. Reference Ring, Malberg, Potestio, Ping, Boikess, Luo, Schechter, Rizzo, Rahman and Rosenzweig-Lipson2006; Ross & Young, Reference Ross and Young2009; Norman et al. Reference Norman, Hawkley, Cole, Berntson and Cacioppo2012; Feldman, Reference Feldman2016; Neumann & Slattery, Reference Neumann and Slattery2016). Rodent studies indicate that hypothalamic oxytocin (OT) mediates the social buffering of the stress response (Smith & Wang, Reference Smith and Wang2014); OT administration to central amygdala of prenatally stressed rats reverses their social deficits (Lee et al. Reference Lee, Brady, Shapiro, Dorsa and Koenig2007); and intranasal OT administration attenuates adrenocorticotropic hormone stress response in squirrel monkeys (Parker et al. Reference Parker, Buckmaster, Schatzberg and Lyons2005). In humans, intranasal OT suppresses cortisol response to stress in emotionally dysregulated (Quirin et al. Reference Quirin, Kuhl and Düsing Rainer2011) and healthy individuals (Heinrichs et al. Reference Heinrichs, Baumgartner, Kirschbaum and Ehlert2003), reduces cortisol levels following parent–infant interaction (Weisman et al. Reference Weisman, Zagoory-Sharon and Feldman2013a ), decreases social stress by increasing activity in the precuneus and cingulate cortex (Eckstein et al. Reference Eckstein, Scheele, Weber, Stoffel-Wagner, Maier and Hurlemann2014), and facilitates autonomic recovery after acute psychosocial stress (Engert et al. Reference Engert, Koester, Riepenhausen and Singer2016).
Maternal physical proximity, stress reactivity, and parenting behavior mediate the effects of stress on the child (Luecken & Lemery, Reference Luecken and Lemery2004; Gunnar & Quevedo, Reference Gunnar and Quevedo2007). Hofer and colleagues discovered a set of ‘hidden regulators’, elements in the mother's physical presence and neuroendocrine signals that function to regulate specific systems in the pup, particularly those associated with the stress response (Hofer, Reference Hofer1994). Importantly, maternal presence exerts not only a general ‘social buffering’ effect but a system-specific regulatory impact on offspring physiology and behavior (Mousseau & Fox, Reference Mousseau and Fox1998; Grindstaff et al. Reference Grindstaff, Brodie and Ketterson2003; Weaver et al. Reference Weaver, Cervoni, Champagne, D'Alessio, Sharma, Seckl, Dymov, Szyf and Meaney2004). Our biobehavioral synchrony model (Feldman, Reference Feldman2012a , Reference Feldman2015a , Reference Feldman2016) extends this system-specific approach and indicates that human mothers and children coordinate their physiological response online during social interactions. We found that the coupling of maternal and child's neurohormonal response, endocrine synchrony, is an important mechanism by which mothers externally regulate children's stress response. Through endocrine synchrony, mothers’ stress-reactive or stress-buffering hormonal systems tune the parallel system in the child, augmenting or attenuating the child's stress reactivity through both hormonal concordance and synchronous behavior (Feldman et al. Reference Feldman, Gordon and Zagoory-Sharon2010, Reference Feldman, Gordon and Zagoory-Sharon2011; Pratt et al. Reference Pratt, Goldstein, Levy and Feldman2017).
In the current study, we utilized a special cohort of children exposed to continuous war-related trauma from birth and followed across the first decade of life. Our cohort affords a unique ‘natural experiment’ in which all children are exposed to the same wartime stressors while biological and contextual factors differentiate those at greater or lesser risk. Extant research has shown that war exposure increases the prevalence of children's psychiatric disorders, particularly anxiety disorders and post traumatic stress disorder (PTSD) (Joshi & O'Donnell, Reference Joshi and O'Donnell2003; Barenbaum et al. Reference Barenbaum, Ruchkin and Schwab-Stone2004), and maternal anxiety augments child anxiety (Kendler et al. Reference Kendler, Myers and Prescott2017), particularly in the context of war (Chemtob et al. Reference Chemtob, Nomura, Rajendran, Yehuda, Schwartz and Abramovitz2010; van Ee et al. Reference van Ee, Kleber and Mooren2012); yet, no study has tested functionality of maternal and child's immune and OT systems as pathways to child psychopathology. Here, we measured s-IgA and OT in mothers and children, observed maternal and child social behavior, and assessed maternal and child's disorders, focusing on anxiety-related symptoms.
Three hypotheses were formulated. As to mean-level differences, we expected war exposure to markedly increase children's psychiatric disorders in general and anxiety-related symptomatology in particular. We also expected chronic war exposure to be associated with higher s-IgA and lower OT in mother and child.
Second, consistent with the biobehavioral synchrony perspective (Feldman, Reference Feldman2015a , Reference Feldman b , Reference Feldman2017), we expected that maternal immune and oxytocinergic biomarkers will correlate with the parallel biomarker in the child and with maternal and child's social behavior so that higher OT and lower s-IgA will be linked with maternal sensitive parenting and greater child social engagement.
Finally, consistent with the ‘hidden regulators’ model (Hofer, Reference Hofer1994), we expected non-redundant, system-specific paths leading from trauma to child anxiety via maternal mediation. Thus, maternal OT and s-IgA would each chart a unique path mediating the effects of war on child anxiety, augmenting risk via stress reactivity or buffering stress via OT functionality.
Methods
Participants
Participants were recruited in early childhood and followed three times across the first decade: in early childhood (M = 2.76 years, s.d. = 0.91), middle childhood (M = 7.68 years, s.d. = 0.7), and late childhood (M = 9.3 years, s.d. = 1.41). The initial cohort included 232 families (47.6% males and 47.1% firstborns) in two groups. The war-exposed group comprised 148 families living in the same frontline neighborhoods in Sderot, Israel, located 10 km from the Gaza border. Individuals in Sderot have been exposed to unpredictable and continuous rocket attacks for more than a decade, leaving only 15 s to enter protected spaces after hearing alert sirens, and exposing citizens to frequent mortar shelling without prior signals. The control group included 84 non-exposed families from comparable towns. Groups were matched on age, gender, birth order, parental age and education, maternal employment, and marital status and controls were screened for other trauma. S-IgA and OT were collected only in late childhood, and thus, the current report utilizes data from this stage. To address consistency over time in available variables, we measured individual stability in maternal and child social behavior and child internalizing symptoms, which were assessed in early childhood (Feldman & Vengrober, Reference Feldman and Vengrober2011; Feldman et al. Reference Feldman, Vengrober, Eidelman-Rothman and Zagoory-Sharon2013c , Reference Feldman, Vengrober and Ebstein2014b ).
Early childhood
Families were visited at home for about 3.5 h during the afternoon. Mothers were asked to play with the child for 10 min using pre-selected toys. Mothers reported on child's internalizing and externalizing symptoms using the Child Behavior Checklist 1.5–5 (Achenbach & Rescorla, Reference Achenbach and Rescorla2000).
Late childhood
Children were 9–11 years old, including 48% males and 45.1% firstborns. Of the initial sample, 177 families participated and dropouts were mainly related to inability to locate families. The war-exposed group included 101 mothers and children, while the control group comprised 76 dyads. No difference was found in any demographic or study variables between those who participated and those who dropped out and their respective groups. While this attrition is sizable, it does not differ from other longitudinal studies following young families over 10 years, particularly in high-risk contexts. The study was approved by the Bar Ilan University's Institutional Review Board and all parents signed informed consent.
Procedure
Families were visited at home for approximately 2.5 h during the afternoon (between 15 : 00 and 19 : 00), to control for diurnal hormonal variability. Following acquaintance, baseline saliva samples were collected from mother and child. Next, mothers were interviewed to determine psychiatric diagnosis of mother and child and reported on child symptoms. Following, a second salivary sample was collected (60 min from baseline) from mother and child. Next, mother and child were videotaped in two well-validated interaction paradigms, each lasting 7 min (Feldman et al. Reference Feldman, Bamberger and Kanat-Maymon2013a , Reference Feldman, Rosenthal and Eidelman2014a ). In the first, dyads were asked to discuss a typical conflict in their relationship (‘conflict interaction’), and in the second, they were asked to plan together their ‘best day ever’ (‘positive interaction’). Ten minutes after the interactions, a third saliva sample was collected.
Psychiatric diagnosis
Child psychiatric diagnosis
Children were tested for current Axis-I disorders using the Developmental and Well-Being Assessment (DAWBA). The DAWBA is a structured interview administered to mothers, generating International Classification of Diseases, 10th Edition (ICD-10) and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) psychiatric diagnoses in 5–17 years old children (Goodman et al. Reference Goodman, Ford, Richards, Gatward and Meltzer2000). It is a well-validated tool, including a large epidemiological study in Israel (Mansbach-Kleinfeld et al. Reference Mansbach-Kleinfeld, Apter, Farbstein, Levine and Poznizovsky2010). DAWBA was administered by clinicians and supervised by child psychiatrist, blind to any other information, with reliability >85% and cases conferred every few weeks (κ = 0.87).
Child anxiety assessment
Mothers completed the Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher et al. Reference Birmaher, Khetarpal, Brent, Cully, Balach, Kaufman and Neer1997), which screens for DSM-IV childhood anxiety disorders in children including Panic Disorder, Generalized Anxiety Disorder, Separation Anxiety and Social Anxiety, in addition to Significant School Avoidance. The SCARED has satisfactory test–retest reliability (Birmaher et al. Reference Birmaher, Khetarpal, Brent, Cully, Balach, Kaufman and Neer1997) and its psychometric properties were reported to be robust in a recent meta-analysis (Hale et al. Reference Hale, Crocetti, Raaijmakers and Meeus2011). A combined total score of these sub-scales goes beyond the dichotomy of Axis-I disorders and enables to compare symptoms severity among participants.
Mother anxiety symptoms
Mothers completed the State-Trait Anxiety Inventory for Adults (STAI: Spielberger et al. Reference Spielberger, Gorsuch and Lushene1970), comprising 20 items of state anxiety (STAI-S), evaluating current symptoms, and 20 items of trait anxiety (STAI-T), referring to feelings in the recent past. As predicted, test–retest reliability is low for the STAI-S and high for the STAI-T, and internal consistency is high (Spielberger et al. Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1983). Mothers’ anxiety was calculated as mean z-scores.
Coding
Mother–child interactions at both early and late childhood were coded using the Coding Interactive Behavior (CIB) Manual (Feldman, Reference Feldman1998), a well-validated rating system for social interactions. The CIB includes multiple codes for mother, child, and dyad with good psychometric properties (Feldman, Reference Feldman2012b ). The CIB has been validated in multiple studies of normative and high-risk populations and across cultures. Coding was conducted by trained coders, blind to any other information, and reliability on 20% of the interactions exceeded 90% on all codes (k = 0.82, range = 0.78–0.96). Two constructs were used at both early and late childhood: Maternal sensitivity comprised the following items: maternal expression of sensitivity to the child's views, emotions, and ideas; mother's maintaining physical proximity and expressing affectionate touch; mother's containment of her own and the child's stress and anxiety; and mother expressing acknowledgement and warmth through positive affect, vocalizations, and supportive presence. Child engagement addresses the child's capacity to express positive emotions, exhibit a range of emotional reactions, child containment of negative affect, child expressing trust toward mother.
Samples collection and measuring
Saliva samples were collected from mother and child by a Salivette (Sarstedt, Rommelsdorft, Germany) in mother/child's mouth for 1 min. Salivates were kept cooled and then stored at −20 °C until centrifuged twice at 4 °C at 1500 g for 20 min.
OT
The liquid samples were stored at −80 °C. To concentrate the samples by three or four times, the liquid samples were lyophilized for 3–4 days and kept in −20 °C until assayed. Determination of OT levels was performed using a commercial OT Enzyme-Linked Immunosorbent Assay (ELISA) kit (Assay Design-ENZO, New York, USA). The kit provides quantitative in vitro assay for free OT in human saliva. The dry samples were reconstructed in the assay buffer immediately before and further measured according to the kit's instructions. Measurements were performed in duplicate and the concentrations of samples were calculated by using MatLab-7 according to relevant standard curves. The intra-assay coefficient of samples and controls is <12.4% and 14.5%, respectively.
s-IgA
Determination of s-IgA was performed using a commercial s-IgA ELISA kit (EUROIMMUN AG: 23560 Luebeck, Germany). The kit provides quantitative in vitro assay for s-IgA in human saliva. On day of assay, samples were thawed completely and diluted 1 : 201 in sample buffer and further measured according to the kit's instructions. Measurements were performed in duplicate and the concentrations of samples were calculated by using MatLab-7 according to relevant standard curves. The intra-assay coefficient of samples and controls is 8.1%, and inter-assay coefficient for samples and controls are 11.1% and 15.5%, respectively.
Statistical analysis
OT and s-IgA were measured, consistent with prior research, by computing area under the curve with respect to the ground (Pruessner et al. Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003), to more accurately assess total overall hormonal production. The χ2 and t tests compared study variables between exposed and control groups. Pearson correlations tested relationships among variables. Finally, for a comprehensive model of the direct and mediated paths from war exposure to children's anxiety via maternal and child hormones and behavior, we conducted a path analysis using lavaan 0.5-23.1097 package (Rosseel, Reference Rosseel2012) in R 3.3.2 (R Core Team, 2014; RStudio, 2015). Path analysis was based on maximum likelihood estimations and the following indicators were used to evaluate the model fit: χ2 values, and their degrees of freedom and p values, with good fit indexed by non-significant values; root mean square error of approximation (RMSEA), values that are <0.06 are considered to indicate a good fit; comparative fit index (CFI), and Tucker-Lewis index (TLI), values >0.95 are considered to indicate a good fit (Hu & Bentler, Reference Hu and Bentler1999). To assess significance of the mediation effects, we used Hayes's (Reference Hayes2013) procedure and calculated the 95% confidence intervals (CIs) of 5000 bias-corrected and accelerated bootstrapping analyses (MacKinnon et al. Reference MacKinnon, Lockwood and Williams2004; Hayes, Reference Hayes2013). In cases where the value zero is not included in the CI, this indicates significant effect at α < 0.05.
Results
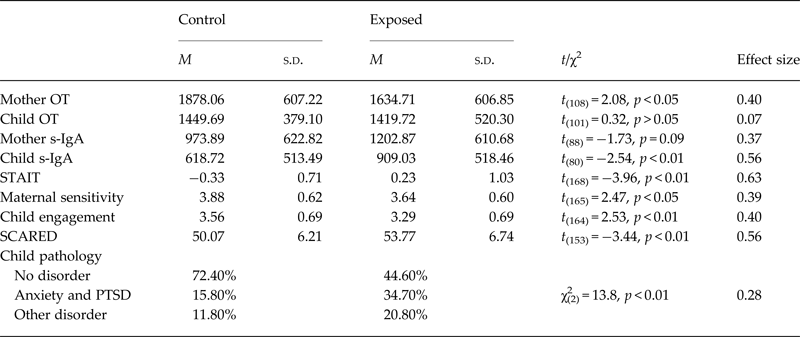
Differences between war-exposed and controls on study variables appear in Table 1 and Fig. 1. Using t tests, it was found that exposed mothers reported higher anxiety, showed lower sensitivity during interactions, and had lower OT. War-exposed children had higher s-IgA, displayed lower social engagement, and had higher SCARED scores. Using χ2 tests, we found that prevalence of anxiety-related disorders and PTSD according to psychiatric diagnosis (DAWBA) was higher in the exposed compared with the control group (34.7% v. 15.8%). Prevalence of other disorders was also higher in the exposed group (20.8% v. 11.8%), mainly conduct disorders, oppositional defiant disorder, and attention deficit hyperactivity disorder.
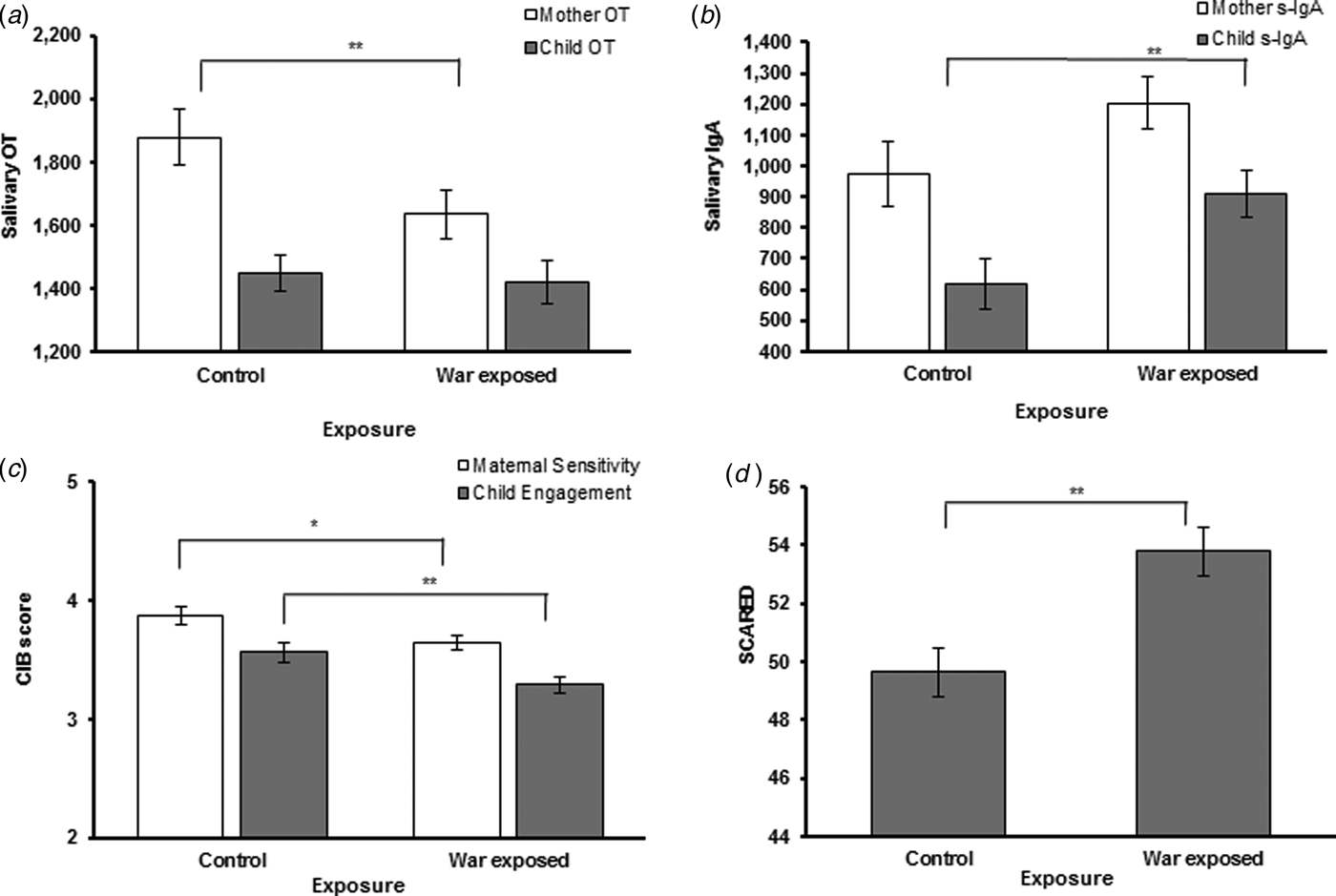
Fig. 1. Differences between exposure groups in hormones, behavior, and child SCARED levels. The t tests for hormones, behaviors, and children SCARED levels by exposure groups. (a) Mothers in the control group had significantly more salivary OT. (b) Children in the war-expososed group had significantly more s-IgA. (c) Mothers and children in the control group showed significantly more senetivity and engagemnt, respectivly. (d) Children in the exposed group had significaly higher SCARED levels. *p < 0.05, **p < 0.01.
Table 1. Differences between exposure groups in hormones, behaviors, and pathologies

Hormonal, behavioral, and SCARED variables were examined using t tests with Cohen's d as effect size. Child pathology was examined using χ2 and Cramer's V as effect size. *p < 0.05, **p < 0.01.
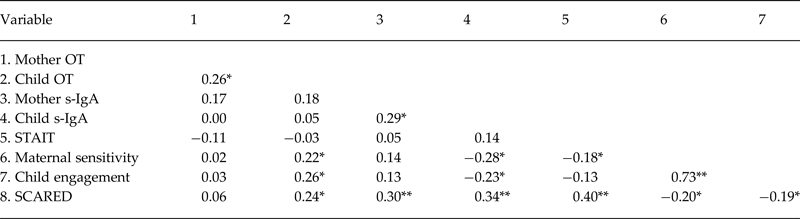
Correlational analysis among study variables across the entire sample appears in Table 2 and shows endocrine synchrony between maternal and child OT and s-IgA. Higher child OT correlated with maternal sensitivity and child engagement and with SCARED scores. Higher s-IgA was associated with lower maternal sensitivity, decreased child engagement, and higher SCARED scores. In addition, maternal anxiety correlated with low maternal sensitivity and higher child SCARED scores. SCARED scores correlated with mother s-IgA levels, lower maternal sensitivity, and reduced child engagement. We also examined the correlations for each group separately and these are presented in online Supplementary Tables S1a and S1b and computed Fisher's Z tests to assess differences in the magnitudes of the correlations. While some correlations were significant in one group or another, none of the correlations showed significant differences in magnitude among groups as indicated by Fisher's Z tests (all p > 0.05).
Table 2. Pearson correlations between hormones, behaviors, and SCARED
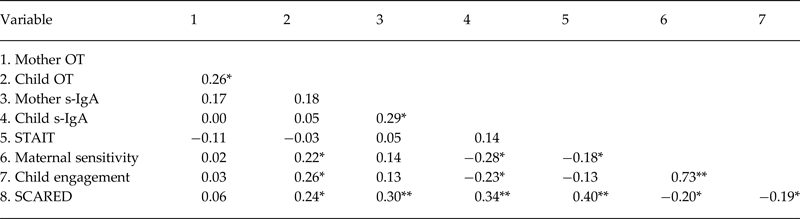
*p < 0.05, **p < 0.01.
We next examined longitudinal associations in mother and child's social behavior and mother and child's symptoms from early to late childhood. Maternal sensitivity (r = 0.23, p < 0.01), child social engagement (r = 0.24, p < 0.01), and mother anxiety (r = 0.53, p < 0.01) were individually stable from early to late childhood. In addition, we found correlations between child internalizing symptoms in early childhood and SCARED scores in late childhood (r = 0.18, p < 0.05), highlighting the stability of the variables measured here across the first decade of life.
Finally, we used path analysis to test our model on the role of maternal hormones, behavior, and anxiety in mediating the link between war exposure and child symptoms (Fig. 2). The overall model provided good fit to the data: χ2 (31) = 41.38, p = 0.10, RMSEA = 0.04 with lower 90% CI 0.00 and higher 90% CI 0.08 PCLOSE = 0.59, CFI = 0.95, TLI = 0.91. We used gender to control the model, although no gender differences were found among groups.
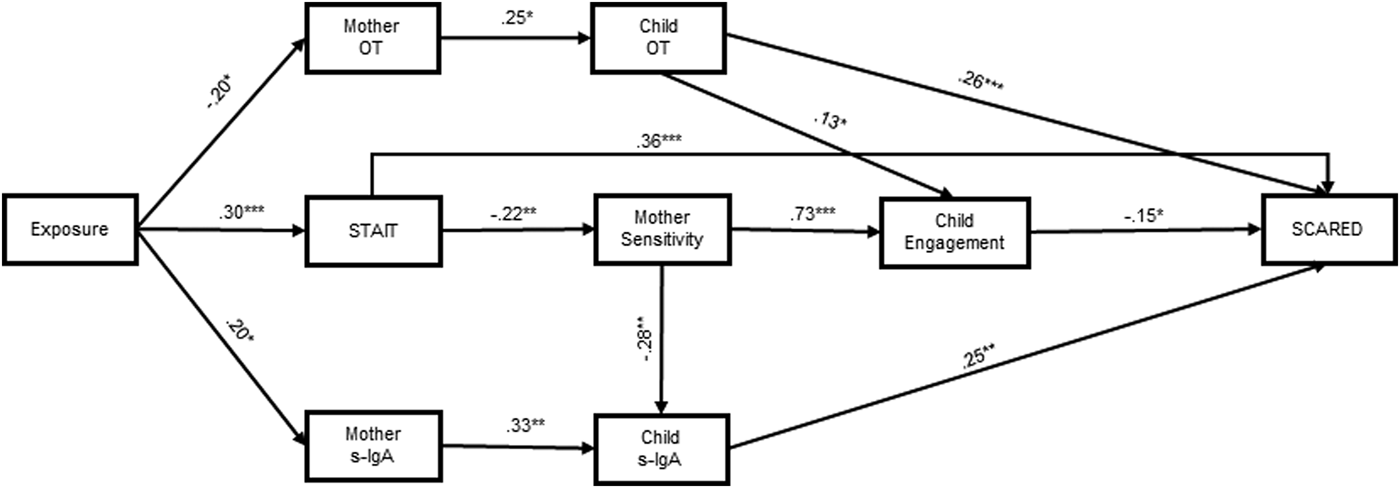
Fig. 2. Path analysis with exposure, hormones, behavior, and mother anxiety to predict children's SCARED levels. Coefficients represent standardized regression weights. We used gender to control the model, even though no differences were found between exposure groups for gender. We used the total score for SCARED. The overall model provided an adequate fit to the data: χ2 (31) = 41.38, p = 0.10, RMSEA = 0.04 with lower 90% CI 0.00 and higher 90% CI 0.08 PCLOSE = 0.59, CFI = 0.95, TLI = 0.91. *p < 0.05, **p < 0.01, ***p < 0.001.
Three parallel paths were identified leading from war exposure to child anxiety, and two additional paths converged with these paths. In the first, war exposure linked with higher maternal s-IgA, leading to higher child s-IgA, which was associated with higher SCARED scores. Test of mediation indicated that this indirect path was significant (95% CI 0.002–1.156). The second path linked exposure with decrease in maternal OT, which correlated with lower child OT, culminating in higher SCARED scores, and test of mediation showed significant indirect path (95% CI −0.709 to −0.013). The third path, via maternal anxiety, included two trajectories; the first directly linked maternal anxiety with children's SCARED scores (95% CI 0.024–1.147); the second initiated a behavioral trajectory leading to lower maternal sensitivity, lower child engagement, leading to higher SCARED scores.
Two pathways converged on these paths. First, increase in mother sensitivity led to decreased child s-IgA, which then combined with the first path, and test of mediation confirmed significant mediation (95% CI 0.010–0.280). Second, children's OT predicted increased child social engagement, which converged with the third path via its biobehavioral trajectory, resulting in reduced SCARED symptoms, and test of mediation confirmed significant mediation (95% CI 0.001–0.118).
Finally, to support our model, we explored an alternative model suggesting direct impact of war on child hormones and behavior without maternal mediation. In this model, exposure did not predict children's OT but predicted children's s-IgA. Test of mediation showed that the OT indirect path was non-significant (95% CI −0.365 to 0.039), and the s-IgA path was significant (95% CI 0.067–1.559). The path from exposure to mother STAI was significant (95% CI 0.098–1.506) and so was the convergent path with s-IgA (95% CI −0.240 to −0.003). This model had lower model fit measurements [χ2 (31) = 50.26, p = 0.02, RMSEA = 0.06 with lower 90% CI 0.03 and higher 90% CI 0.09 PCLOSE = 0.28, CFI = 0.91, TLI = 0.84] supporting the current model.
Discussion
Although millions of children across the globe are exposed to chronic war, ethnic strife, and political violence, no study to our knowledge examined the development of children's anxiety symptoms in the context of war integrating endocrine and behavioral measures from mother and child. Our study – the first to assess biomarkers of the immune and affiliation systems in mothers and children – highlights the pervasive impact of continuous trauma on functionality of the immune and OT systems, social behavior, and, ultimately, on child anxiety, and charts pathways leading from trauma to child symptoms via maternal and child's hormones and behavior. Guided by the ‘hidden regulators’ (Hofer, Reference Hofer1994) and ‘biobehavioral synchrony’ (Feldman, Reference Feldman2015a , Reference Feldman2016, Reference Feldman2017) frames, we explored system-specific mechanisms by which maternal stress- and affiliation-related systems exacerbate or attenuate the child's stress reactivity, defining trajectories of risk and resilience.
War-exposed children had significantly more anxiety disorders and PTSD, higher anxiety symptoms, and higher immune system activation. Furthermore, immune system activation, indexed by higher s-IgA levels, was directly linked with increased anxiety-related symptoms. These findings highlight the specific associations among chronic stress, anxiety disorders, and immune system functionality and define the utility of s-IgA as a biomarker of anxiety in stress-exposed children.
The findings on the increase in s-IgA in children with anxiety disorders are consistent with prior research in chronically stressed populations. Following lengthy military deployment in Afghanistan, soldiers exhibited higher s-IgA (Kvietkauskaite et al. Reference Kvietkauskaite, Vaicaitiene, Girkontaite and Labeikyte2014); individuals exposed to occupational stress had elevated s-IgA (Henningsen et al. Reference Henningsen, Hurrell, Baker, Douglas, MacKenzie, Robertson and Phipps1992; Kugler et al. Reference Kugler, Reintjes, Tewes and Schedlowski1996; Zeier et al. Reference Zeier, Brauchli and Joller-Jemelka1996); and elevated s-IgA levels were linked with increased daily hassles (Bosch et al. Reference Bosch, Brand, Ligtenberg, Bermond, Hoogstraten and Nieuw Amerongen1998). Other studies found that work-related stress is inversely correlated with s-IgA (Fujimaru et al. Reference Fujimaru, Okamura, Kawasaki, Kakuma, Yoshii and Matsuishi2012) and immediate stress, such as exams (Deinzer & Schüller, Reference Deinzer and Schüller1998), or chronic stressors, such as caring for sick/disabled relatives (Phillips et al. Reference Phillips, Carroll, Evans, Bosch, Clow, Hucklebridge and Der2006; Gallagher et al. Reference Gallagher, Phillips, Evans, Der, Hunt and Carroll2008) correlated with decreased s-IgA. Such inconsistencies may relate to methodological issues (Segerstrom & Miller, Reference Segerstrom and Miller2004) or to differential modulation of the immunoglobulin transport kinetics (Engeland et al. Reference Engeland, Hugo, Hilgert, Nascimento, Junges, Lim, Marucha and Bosch2016). Another factor may be the time from the last stressful/traumatic event, as s-IgA levels increase shortly after stress and decrease thereafter (Tsujita & Morimoto, Reference Tsujita and Morimoto1999), and type of stressor (acute v. mild) (Viena et al. Reference Viena, Banks, Barbu, Schulman and Tartar2012). Our findings show that when stress is chronic and characterized by unpredictable eruptions, it activates children's immune system. This accords with studies on the presence of immune activation/low-grade inflammation, evidenced by increased C reactive protein, interleukin-6, and inflammatory cytokines, in patients with anxiety disorders, such as general anxiety disorder, panic disorder, or social anxiety disorder (Hoge et al. Reference Hoge, Brandstetter, Moshier, Pollack, Wong and Simon2009; Copeland et al. Reference Copeland, Shanahan, Worthman, Angold and Costello2012), as well as in anxious individuals (O'Donovan et al. Reference O'Donovan, Hughes, Slavich, Lynch, Cronin, O'Farrelly and Malone2010; Liukkonen et al. Reference Liukkonen, Räsänen, Jokelainen, Leinonen, Järvelin, Meyer-Rochow and Timonen2011; Vogelzangs et al. Reference Vogelzangs, Beekman, de Jonge and Penninx2013). Notably, other studies demonstrated opposite results (Rider et al. Reference Rider, Achterberg, Lawlis, Goven, Toledo and Butler1990; Rohrmann et al. Reference Rohrmann, Hennig and Netter2000), suggesting the need for further research. Together, these findings suggest a complex pattern of relationship between anxiety and immune functioning, whereby different forms of anxiety under a variety of contexts can either augment or suppress immunity.
The direction of the relationship between anxiety and immunity is still inconclusive (Jensen, Reference Jensen2016), although research in both humans and animal models suggests that immune activation may play a causal role in anxiety disorders. Using a prospective experimental design, it was found that activation of the immune system can induce elevated anxiety (Reichenberg et al. Reference Reichenberg, Yirmiya, Schuld, Kraus, Haack, Morag and Pollmächer2001; Lasselin et al. Reference Lasselin, Elsenbruch, Lekander, Axelsson, Karshikoff, Grigoleit, Engler, Schedlowski and Benson2016). In animals, immune activation was associated with anxiety-like symptoms (Gibney et al. Reference Gibney, McGuinness, Prendergast, Harkin and Connor2013; Yang et al. Reference Yang, Wang, Guo, Sun, Li, Yang, Zhang, Liu, Zhao and Wu2016), and direct causal links from chronic stress to immune activation to subsequent anxiety was reported in a model of repeated social defeat stress (Wohleb et al. Reference Wohleb, Powell, Godbout and Sheridan2013). Importantly, exposure to inflammatory challenge in early life was found to induce an anxious phenotype in adulthood (Walker et al. Reference Walker, March and Hodgson2004), particularly following a ‘second hit’ of stress exposure later in life (Walker et al. Reference Walker, Nakamura, Byrne, Naicker, Tynan, Hunter and Hodgson2009). These findings are particularly relevant to our cohort and other conditions of chronic and repeated early stress.
Our study is the first to measure s-IgA in chronically stressed mothers and children and to show that when individuals are exposed to chronic stress, there are stable alterations in immune system functionality. While endocrine fit emerged between mother and child's s-IgA levels, there were also important immune-behavior links and children who experienced more sensitive parenting had lower s-IgA levels. This suggests that when mothers are less physiologically reactive to the chronic condition, it carries a buffering effect on their children's stress response both directly and via the expression of more optimal parenting that functions to attenuate stress. Furthermore, the finding that maternal and child social behaviors were individually stable from early to late childhood suggests that children's immune response matures in the context of the parent's ongoing sensitive style across the entire first decade of life.
Findings for OT showed both similarities and differences from those of the immune system. First, we found endocrine coupling between mother and child for both biomarkers. Such endocrine synchrony may relate not only to contextual and dyadic factors, but may also express shared genetic dispositions that shape social behavior and OT functionality (Knafo & Plomin, Reference Knafo and Plomin2006). Notably, while war-exposed mothers had lower OT than controls, no difference emerged in children's OT suggesting that the development of the child's oxytocinergic system in the context of war is fully mediated by its effects on the mother. Interestingly, OT's links with child anxiety symptoms were described by two paths. First, children's OT linked to higher social engagement, a social competence showing individual stability from infancy to adolescence and across attachment relationships with mother, father, and close friends (Feldman, Reference Feldman2010; Feldman et al. Reference Feldman, Bamberger and Kanat-Maymon2013a , Reference Feldman, Gordon, Influs, Gutbir and Ebstein b ). Social engagement, in turn, predicted lower anxiety symptoms and was reduced in children diagnosed with anxiety disorders and PTSD. It appears that at this age, the child's socially focused and engaged style that supports the capacity to form and maintain social relationships and express a range of emotions provides a resilience buffer in stressful contexts (Halevi et al. Reference Halevi, Djalovski, Vengrober and Feldman2016). Such engaged style was predicted by greater maternal sensitivity and maternal OT, highlighting the maternal contribution to children's social skills. Associations between maternal OT and sensitive parenting has been repeatedly shown in animal and human studies (Bale et al. Reference Bales, Boone, Epperson, Hoffman and Carter2011; Feldman et al. Reference Feldman, Gordon, Influs, Gutbir and Ebstein2013b ) and the current findings detail how maternal and child's OT is integrated with child social behavior to buffer anxiety in the context of wartime trauma. Interestingly, while war exposure directly impacted children's s-IgA, its impact on OT was mediated by maternal OT, consistent with the cross-generational effects of OT described in humans and animal research (Feldman et al. Reference Feldman, Gordon and Zagoory-Sharon2010) and with Hofer & Shair (Reference Hofer and Shair1987) initial description on the role of OT as the first feeding-based ‘hidden regulator’ of the offspring's stress response.
In addition to mediated effects on reducing anxiety by increasing children's social repertoire, OT had a direct impact on increasing child anxiety symptoms. These findings illustrate the two-pronged role of OT in the context of stress and affiliation; while OT is associated with attachment and its stress-reducing attributes, it is also linked with anxiety, particularly social anxiety (Kirsch et al. Reference Kirsch, Esslinger, Chen, Mier, Lis, Siddhanti, Gruppe, Mattay, Gallhofer and Meyer-Lindenberg2005; Hoge et al. Reference Hoge, Pollack, Kaufman, Zak and Simon2008; Labuschagne et al. Reference Labuschagne, Phan, Wood, Angstadt, Chua, Heinrichs, Stout and Nathan2010; Weisman & Feldman, Reference Weisman and Feldman2013). The literature on OT and anxiety are mixed; while some found correlations between OT and anxiety (Tops et al. Reference Tops, Van Peer, Korf, Wijers and Tucker2007; Holt-Lunstad et al. Reference Holt-Lunstad, Birmingham and Light2011), others showed that OT is low in anxious children (Carson et al. Reference Carson, Berquist, Trujillo, Garner, Hannah, Hyde, Sumiyoshi, Jackson, Moss, Strehlow, Cheshier, Partap, Hardan and Parker2014), particularly those with separation anxiety (Lebowitz et al. Reference Lebowitz, Leckman, Feldman, Zagoory-Sharon, McDonald and Silverman2016), while still others found sex-related differences, such that women's OT was linked with higher attachment anxiety while in men OT correlated with lower trait anxiety (Weisman et al. Reference Weisman, Zagoory-Sharon, Schneiderman, Gordon and Feldman2013 b). One explanation for our findings may draw on the ‘social saliance’ model of OT (Shamay-Tsoory & Abu-Akel, Reference Shamay-Tsoory and Abu-Akel2016), which suggests that OT increases the saliance of social events. When children experience sensitive parenting and are socially engaged, the OT system directs attention to positive social exchanges and this may function to attenuate child anxiety. When children grow in the context of chronic stress that is not buffered by positive parenting, OT may enhance the child's social vigilance, fear, and stress leading to greater anxiety symptoms.
Several study limitations should be mentioned. First, we did not measure saliva flow. We found that measuring flow rate in our sample would decrease children's willingness to participate, divert their attention, and increase their resistance. Under such conditions our ability to collect saliva would result in the loss of many participants. Second, we focused on the mother–child relationship and did not include other attachments that may serve as protective buffers, including fathers, siblings, grandparents, teachers, or close friends. We acknowledge that paternal care could have critical influence on the child's behavior (Ramchandani et al. Reference Ramchandani, Domoney, Sethna, Psychogiou, Vlachos and Murray2013) and our choice to focus on mother–child dyad was due to recruitment and technical issues. The third limitation considers the inability to control for the family's choice of place of living. While our study is ecologically valid, we accept the possibility that there could be other personal factors predisposing people to live in safe or unsafe areas, although we tried to control for the city size and demographic conditions by recruiting controls from towns of similar sociodemographic background to Sderot. Moreover, attrition rate from the exposed group, despite our efforts, is sizeable, and while it is not different from other longitudinal follow-up of high-risk family, it could have impacted the findings. Finally, salivary OT measurements are relatively new and we did not collect measures of OT and s-IGA in early childhood, precluding our ability to chart longitudinal links of these hormones.
Child exposure to continuous war, chronic stress, and domestic and political violence is a global epidemic and understanding how such conditions impact children's immune and affiliative functioning, ultimately shaping their physical health and psychological well-being, requires much further research in age-specific, culture-specific, and context-specific studies. Our findings lend support to models that underscore the critical importance of the mother on the system-specific development of stress-reactive and stress-buffering systems in children as mediated by attuned and sensitive parenting. Much further research is required to test other biomarkers of the immune system, their cross-talk with other components of the stress response, and their interplay with the oxytocinergic system to further define the less researched, albeit critically important construct of resilience in order to develop system-specific interventions for children growing up amidst adversity.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717002550.
Acknowledgements
This work was supported by NARSAD Independent Investigator Award to Ruth Feldman and by the Simms-Mann Foundation.
Declaration of Interest
None.