Staphylococcus aureus is recognized as the major contagious mastitis pathogen worldwide (Zecconi et al. Reference Zecconi, Calvinho and Fox2006a). Moreover, Staph. aureus is associated with both human and foodborne diseases (Balaban & Rasooly, Reference Balaban and Rasooly2000; Honeyman et al. Reference Honeyman, Friedman and Bendinelli2001). The large number of diseases caused in different species can be related to the vast array of virulence factors expressed by Staph. aureus and by the ability to colonize different sites both on the body (skin, nares, udder) and can be found also in devices such as prosthesis and milking machines (Roberson et al. Reference Roberson, Fox, Hancock, Gay and Besser1994; Montanaro et al. Reference Montanaro, Arciola, Baldassarri and Borsetti1999).
To control the disease at herd level and to reduce the risk of milk contamination with Staph. aureus enterotoxin it is important to identify the epidemiological pattern and hence the sources of infection and contamination (Zecconi et al. Reference Zecconi, Calvinho and Fox2006a; Andre et al. Reference Andre, Campos, Borges, Kipnis, Pimenta and Serafini2008). These kinds of epidemiological studies currently rely on molecular characterization of isolates. Several molecular methods have been proposed to characterize Staph. aureus, or to identify virulence patterns (Matthews et al. Reference Matthews, Kumar, O'Conner, Harmon, Pankey, Fox and Oliver1994; Aarestrup et al. Reference Aarestrup, Dangler and Sordillo1995; Fitzgerald et al. Reference Fitzgerald, Meaney, Hartigan, Smyth and Kapur1997; Rivas et al. Reference Rivas, Gonzalez, Wiedmann, Bruce, Cole, Bennett, Schulte, Wilson, Mohammed and Batt1997; Zecconi et al. Reference Zecconi, Binda, Borromeo and Piccinini2005; Zecconi et al. Reference Zecconi, Cesaris, Liandris, Daprà and Piccinini2006b) in isolates from dairy cows. Among all these methods pulsed field gel electrophoresis (PFGE) is recommended for Staph. aureus typing (Tenover et al. Reference Tenover, Arbeit, Archer, Biddle, Byrne, Goering, Hancock, Hebert, Hill and Hollis1994; McDougal et al. Reference McDougal, Steward, Killgore, Chaitram, McAllister and Tenover2003) but it discriminates the different isolates without giving information on the presence of the different virulence factors. Such problems could be overcome by combining PFGE with a validated method to identify virulence gene pattern (Zecconi et al. Reference Zecconi, Binda, Borromeo and Piccinini2005; Zecconi et al. Reference Zecconi, Cesaris, Liandris, Daprà and Piccinini2006b).
This paper reports the results of a study applying both PFGE and the assessment of the presence of a selected number of virulence genes to investigate the potential role of teat skin on Staph. aureus transmission among cows and on the source of contamination of milk and milk products.
Material and Methods
Herds and sampling
Nine herds following a mastitis control programme based on segregation of infected cows as described in previous papers (Zecconi et al. Reference Zecconi, Piccinini and Fox2003, Reference Zecconi, Piccinini and Fox2004, Reference Zecconi, Calvinho and Fox2006a) were included in the study. Quarter milk samples were taken from all the infected cows, following the procedures described by National Mastitis Council (NMC, 1999).
Based on the owners' permission and on herd size (range 50–100 lactating cows), teat swabs were also taken from cows confirmed free from Staph. aureus intramammary infection (IMI) in five of the herds considered. Teat skin swabs were taken from all the four quarters of ten cows housed in the Staph. aureus-free pen. These cows had at least two consecutive negative quarter-milk samples. Teat swabs were taken by a cotton-tipped stick, rotating the cotton-tip on the teat barrel covering an area of about 2 cm2.
All the herds sold raw milk direct to the consumers, and three of them were characterized by on-site production of raw-milk cheese. In these herds, raw milk collected during evening milking is mingled with the milk of the morning milking and directly processed in an on-site small scale cheese plant. In these herds curd samples were also taken and cultured. Practically, about 100 g of curd samples were taken after ripening, when the temperature was below 40°C, using a sterile plastic bottle.
Isolates
Curd, milk samples and teat swabs were cultured on blood agar plate with 5% (v/v) bovine blood. Isolates were presumptively identified as Staph. aureus according to the following scheme: catalase-positive, Gram-positive cocci, haemolytic on blood agar, and coagulase-positive in 4–18 h. The presumptive identification was confirmed by API ID32 Staph (BioMerieux, Marcy L'Etoile, France). A minimum of two isolates was selected from each herd, based on phenotype characteristics. Practically, when the isolates were phenotipically identical only two isolates were considered, while in all the other cases, an isolate for each phenotype was selected. Isolates were stored at −20°C in BHI broth with 15% (v/v) glycerol.
PFGE and virulence gene fingerprint
PFGE procedure followed the method described by McDougal et al. (Reference McDougal, Steward, Killgore, Chaitram, McAllister and Tenover2003). Briefly, a single colony of each isolate was inoculated in 5 ml of BHI broth at 37°C for 24 h. The concentration of cells was adjusted spectrophotometrically and plugs were prepared after lysostaphin treatment; DNA was cut with SmaI restriction enzyme (Fermentas, Vilnius, Italy). PFGE was performed on CHEF II Instrument (Biorad, Hercules CA, USA) with running parameters: 6 V/cm, temperature 14°C and run time 21 h.
Virulence genes fingerprint was performed following the procedure described elsewhere (Zecconi et al. Reference Zecconi, Binda, Borromeo and Piccinini2005; Zecconi et al. Reference Zecconi, Cesaris, Liandris, Daprà and Piccinini2006b). For further analysis we considered the number of repetitions of spa gene X region (1–12), the presence (2) absence (1) of cna gene, and the efb enzyme restriction pattern (3 classes).
Data analysis
The electrophoretic patterns of both PFGE and virulence genes were analysed using BioNumerics software package (version 5.0; Applied-Maths, Sint-Martens-Latem, Belgium). The basis of the software is a relational database consisting of entries corresponding to individual organisms. Each organism was initially characterized by PFGE fingerprint. PFGE bands were assigned by manual editing of the software-identified bands, and the position tolerance and optimization were set to 2%. Similarity coefficients of PFGE fingerprints were calculated based on band position. PFGE patterns were clustered by the unweighted pair group method (UPGAMA) applying Dice coefficient.
Isolates from the three herds where both skin and milk samples were available (1, 4, 5), were also analysed for virulence genes pattern (efb, spa, cna). The multistate categorical coefficient with a tolerance level of zero was used to calculate the similarity matrix (Ramisse et al. Reference Ramisse, Houssu, Hernandez, Denoeud, Hilaire, Lisanti, Ramisse, Cavallo and Vergnaud2004). This categorical parameter treats each different value as a different state, implying that character sets are unordered: the same weight is therefore given to large or small numbers of differences.
Similarity matrices obtained from PFGE fingerprint and virulence genes analysis were then combined in a single matrix (composite data set). The ‘take from experiments’ and ‘correct for internal weights’ methods were used to calculate similarity coefficients. With this method the matrices from the individual experiments are averaged according to weights automatically assigned by the software.
The patterns were then clustered by the UPGAMA method. Cluster were defined by the presence of a relatedness ⩽80% between isolates. In other words, if the cluster analysis gave a relatedness >80% the isolates were genetically considered to be from the same clone. Within a cluster, subtypes were defined by the presence of difference in PFGE bands between 4 and 6 (Tenover et al. Reference Tenover, Arbeit, Goering, Mickelsen, Murray, Persing and Swaminathan1995).
Data were also analysed by SAS 9.1 (SAS Institute, Cary NC, USA) procedure FREQ to compare cluster distribution frequency and by procedure GLM to assess the relationship between clusters and spa gene pattern. The model included herd, spa gene pattern and random error, and an α=0·05 was considered for statistical analysis.
Results
Sixty-one isolates were considered, 24 from teat skin, 32 from milk samples and 5 from curd samples. Teat swabs were taken in five herds, but in only three of them could Staph. aureus be isolated from them. Curd was sampled in three herds, but Staph. aureus could be isolated in only 2 herds.
PFGE fingerprint
The analysis of PFGE fingerprints (Fig. 1 and Table 1) showed the presence of 5 different clusters (C, D, F, G, H) characterized by a relatedness ⩽80%. Most of the isolates (82%) were included in two clusters, C and G, with respectively 30 and 23 isolates. Moreover, when the number of fragment differences was considered, isolates in cluster C can be classified in two subgroups, C1 and C2, including respectively 19 and 11 isolates. Application of the same approach to cluster G allowed 3 subgroups (G1, G2 and G3) to be identified with respectively 2, 1 and 20 isolates.

Fig. 1. Dendrogram of 61 Staphylococcus aureus isolates as determined by PFGE clustering (A–H) based on a threshold ⩽80%. Subclustering (C1, C2; G1–3) was based on the presence of a 4–6 PFGE bands difference.
Table 1. Distribution of 61 Staphylococcus aureus isolates by source and PFGE fingerprint results. PFGE clustering (A–H) based on a threshold ⩽80%. Subclustering (C1, C2; G1–3) was based on the presence of a 4–6 PFGE bands difference

Independently of the source of the isolates, in six herds at least two different strains could be identified; in one herd (3) the isolates were associated with both C subgroups, while a unique strain was isolated from two herds (2, 9). Moreover, a unique skin isolate was classified in cluster D, while all the other isolates form the same herd were classified in a different cluster (G3).
When the source of isolates was considered, milk isolates were classified in any of the five clusters, teat skin in three clusters (C, D, G) and curd isolates were found only in two clusters, respectively C and G.
Teat skin and milk isolates characteristics
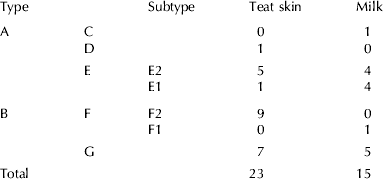
To assess the role of teat skin as a reservoir of Staph. aureus IMI, we considered 38 isolates from three herds where both skin and milk samples were available. Both PFGE and PFGE plus virulence genes pattern analysis were performed. However, patterns observed using only PFGE results and PFGE plus virulence gene did not statistically differ (data not shown); therefore we considered the latter for further investigations (Fig. 2). This analysis identified five clusters, and two of them (E and F) included two subtypes each. Two clusters (C and D) included a single strain, respectively from milk and teat skin sample.

Fig. 2. Dendrogram of 38 Staphylococcus aureus isolates as determined by PFGE clustering and virulence genes pattern (A–G) based on a threshold ⩽80%. Subclustering (E1, E2; F1, F2) was based on the presence of a 4–6 PFGE bands difference.
Table 2 reports the distribution of isolates by cluster and source of isolates. The analysis of this distribution by Fisher's exact test did not show any significant difference either when clusters or sources were compared. However, it should be noted that teat skin isolates are more frequently clustered in a subtype (F1 and E1) and in cluster G.
Table 2. Distribution of 38 Staphylococcus aureus isolates where both milk and teat skin samples were taken, by source and PFGE fingerprint results. PFGE clustering (A–F) based on a threshold ⩽80%. Subclustering (E1, E2; F1, F2) was based on the presence of a 4–6 PFGE bands difference
Role of spa gene
To assess the role of spa gene in isolates distribution, we compared the number of repetitions observed in isolates classified by cluster and by source. Analysis by source showed skin isolates with a higher mean number of repetitions of spa gene X region (8·22±3·01) when compared with milk isolates (6·07±3·53); the t test for this difference has a P value of 0·05.
Analysis of the three clusters with a sufficient number of isolates showed that cluster G had a significantly lower mean number of repetitions (5·08±0·82), when compared with the other two clusters E (7·30±0·76) and F (9·60±0·89). Statistical analysis by general linear model showed that this difference was statistically significant, while the difference between cluster E and F was not significant.
Discussion
The importance of epidemiological investigations based on PFGE fingerprint of Staph. aureus isolates has been demonstrated by several studies both in human and veterinary medicine (Middleton et al. Reference Middleton, Fox, Gay, Tyler and Besser2002; Montesinos et al. Reference Montesinos, Salido, Delgado, Cuervo and Sierra2002; Zadoks et al. Reference Zadoks, van Leeuwen, Kreft, Fox, Barkema, Schukken and van Belkum2002). Indeed, PFGE has been shown to be the most suitable method for these kinds of studies and standardized protocols are available (McDougal et al. Reference McDougal, Steward, Killgore, Chaitram, McAllister and Tenover2003).
However, when PFGE analysis is performed, the information on the presence of virulence genes is not available. In our study we combined PFGE analysis with virulence genes fingerprint pattern, selecting three genes previously shown to be of importance in Staph. aureus virulence (Zecconi et al. Reference Zecconi, Binda, Borromeo and Piccinini2005; Zecconi et al. Reference Zecconi, Cesaris, Liandris, Daprà and Piccinini2006b).
Distribution of isolates among herds confirmed the presence of herd-specific Staph. aureus strains for most of the herds (Larsen et al. Reference Larsen, Sloth, Elsberg, Enevoldsen, Pedersen, Eriksen, Aarestrup and Jensen2000; Joo et al. Reference Joo, Fox, Davis, Bohach and Park2001; Middleton et al. Reference Middleton, Fox, Gay, Tyler and Besser2002; Tenhagen et al. Reference Tenhagen, Scheibe, Zucker, Koster and Heuwieser2007). The same pattern was observed both in teat skin and in milk samples, and in the few curd samples.
The presence of isolates with genetic characteristics unique for each herd suggests that reservoirs within the herd could help in maintaining the infection among cows. Therefore, the control of Staph. aureus IMI depends on identifying these reservoirs. Many potential sources have been identified: from air to bedding, from equipment to teat skin (Roberson et al. Reference Roberson, Fox, Hancock, Gay and Besser1994; Zadoks et al. Reference Zadoks, van Leeuwen, Kreft, Fox, Barkema, Schukken and van Belkum2002). Our study focused on the role of teat skin as a potential reservoir and the genetic analysis of the isolates confirmed that teat skin could be a potential source for IMI development (Fox & Norell, Reference Fox and Norell1994; Roberson et al. Reference Roberson, Fox, Hancock, Gay and Besser1994). It should be noted that in our study Staph. aureus was isolated from teat skin of confirmed Staph. aureus-negative cows that were segregated from infected ones.
However, isolates form teat skin showed some specific genetic characteristics, being mainly classified in the same subtype. Moreover, these strains are characterized by a number of spa gene X region repetitions significantly higher than the one of milk isolates. These data suggest that some virulence factors could be involved in the persistence of Staph. aureus on teat skin and potentially on its diffusion among cows, as has been shown for several diseases (Frenay et al. Reference Frenay, Theelen, Schouls, Vandenbroucke-Grauls, Verohef, VanLeeuwen and Mooi1994; Montesinos et al. Reference Montesinos, Salido, Delgado, Cuervo and Sierra2002; El-Sayed et al. Reference El-Sayed, Alber, Lämmler, Bonner, Huhn, Kaleta and Zschock2006; Zecconi et al. Reference Zecconi, Cesaris, Liandris, Daprà and Piccinini2006b).
The present study suggests that once Staph. aureus IMI have been established in a herd, the bacteria can be isolated also from teat skin of negative cows. Therefore, Staph. aureus infecting the udder or colonizing teat skin could be easily transferred to raw milk, mainly through milking. In the presence of high levels of contamination, enterotoxin could be produced and this could increase the risk for foodborne diseases (Zecconi & Hahn, Reference Zecconi and Hahn1999).
Conclusions
The role of teat skin contamination in Staph. aureus IMI epidemiology is still a matter of discussion. This study supports the role of teat skin contamination in maintaining the infection among cows. It also suggests that some strains have higher chances to survive on teat skin and to increase the risk for contamination of milk and milk products.






