Due to advances in cancer therapy, the 5-year relative survival rate following childhood cancer has improved to 83%.1 Of the >300,000 childhood cancer survivors in the United States, one-fourth are now living >30 years after diagnosis.Reference Mariotto, Rowland and Yabroff2 Anthracyclines are among the most effective chemotherapeutic agents and are widely used to treat childhood malignancies, including leukemia and osteosarcoma.Reference Weiss, Sarosy and Clagett-Carr3 However, the use of anthracyclines is limited by short- and long-term cardiotoxicities,Reference Lipshultz, Lipsitz and Sallan4 ranging from subtle left ventricular dysfunction to overt heart failure needing long-term medical management and in some cases heart transplantation.Reference Lipshultz, Adams and Colan5 Even though higher doses of anthracyclines are a major risk factor for the development of left ventricular dysfunction, there is no safe dose of anthracyclines free from long-term cardiotoxicity.Reference Aggarwal, Pettersen and Bhambhani6–Reference Nysom, Holm and Lipsitz8 Therefore, childhood cancer survivors who received anthracyclines need lifelong monitoring of left ventricular function with serial echocardiograms. The Children’s Oncology Group recommends the use of left ventricular fractional shortening or left ventricular ejection fraction as long-term echocardiographic screening tools in this population.9 It is important to understand if childhood cancer survivors have abnormalities involving certain left ventricular segments, as there can be cardiomyocyte ischemic damage and necrosis due to various mechanisms. Changes in left ventricular ejection fraction and left ventricular fractional shortening can reflect functional changes in isolated segments of the left ventricular, which are important since anthracyclines can reduce both regional and global myocardial function.Reference Yu, Yu and Cheuk10
Speckle-tracking echocardiography is a newer echocardiographic technique to assess the left ventricular systolic function. The longitudinal, circumferential, and radial strain can be measured to assess regional myocardial deformation and global left ventricular function.Reference Geyer, Caracciolo and Abe11 In adults with heart failure and a reduced left ventricular ejection fraction, left ventricular global longitudinal strain independently predicts all-cause and cardiovascular mortality and is superior to other conventional echocardiographic measures.Reference Sengelov, Jorgensen and Jensen12 Global and regional longitudinal strain, as assessed by two-dimensional Speckle-tracking echocardiography, was reduced in adult women with breast cancer after exposure to anthracyclines, even when left ventricular ejection fraction was normal.Reference Stoodley, Richards and Hui13 In 19 children, global longitudinal strain and mid- and apical-segmental strain were significantly lower than in age- and sex-matched controls as early as 4 months after beginning chemotherapy. The acute changes in strain measurements during anthracycline therapy predicted the likelihood of a later decrease in left ventricular ejection fraction.Reference Poterucha, Kutty and Lindquist14 Yu et al evaluated, using three-dimensional echocardiography, 53 anthracycline-treated childhood cancer survivors aged 18.6 ± 5.1 years. They reported abnormal regional strain in all the left ventricular segments except the basal anteroseptal segment (p < 0.05) compared to matched controls.Reference Yu, Yu and Cheuk10 Data on the effect of anthracyclines at the segmental level in children who have completed chemotherapy are limited. Therefore, we sought to evaluate the left ventricular segmental changes using two-dimensional speckle echocardiography in asymptomatic childhood cancer survivors with normal left ventricular fractional shortening and who had completed anthracycline chemotherapy ≥1 year prior to enrollment.
Methods
This is a study involving anthracycline-treated childhood cancer survivors for various paediatric cancers at the Children’s Hospital of Michigan. The patients were identified from echocardiography and oncology databases in our centre. Echocardiographic studies performed between January 2014 and December 2015 were analysed offline. Children were enrolled if they had completed anthracycline chemotherapy >1 year before enrollment, had no symptoms of heart failure, and had a normal left ventricular fractional shortening (>28%) as assessed by M-mode echocardiography. Children still receiving chemotherapy, who had undergone bone marrow transplantation, who had congenital cardiovascular malformations (except a patent foramen ovale), who had a decreased left ventricular function (a left ventricular fractional shortening ≤28%), or who had incomplete echocardiographic data (more than two segments are missing in the Speckle-tracking echocardiography evaluation), were excluded. For the control group, we enroled children who presented to our cardiology clinic with a chief complaint of musculoskeletal chest pain, an innocent murmur, or vasovagal syncope with normal cardiac anatomy and left ventricular systolic function (left ventricular fractional shortening ≥28%) by echocardiography. Demographic and clinical data, such as cancer diagnosis, date of cancer diagnosis, date of completion of cancer therapy, and the cumulative anthracycline dose, were also collected. The study, as well as the waiver of parental consent and patient assent, were approved by the Wayne State University Institutional Review Board and authorised by the Detroit Medical Center.
Speckle-tracking echocardiography
All echocardiograms were obtained using a Philips iE33 Ultrasound System (Philips Medical, Andover, Massachusetts, United States of America) and stored in the Digital Imaging and Communications in Medicine format. The studies were downloaded and analysed offline using a vendor-independent, two-dimensional Cardiac Performance Analysis software program (TOMTEC Imaging Software, Unterschleissheim, Germany). Peak systolic longitudinal strain from the apical two-, three-, and four-chamber views and peak systolic radial and circumferential strain from the parasternal short-axis views at the level of the papillary muscles were traced semi-automatically. The best single cardiac cycle was selected for analysis as validated by several studies.Reference Amzulescu, De Craene and Langet15, Reference Johnson, Kuyt and Oxborough16 After the tracing was complete, the images were played frame by frame, and the tracing was manually adjusted if necessary. This software measures the different segmental speckle strains and strain rates. It depicts the various segments according to the American Heart Association’s segmental criteria (Fig 1).Reference Cerqueira, Weissman and Dilsizian17 The average measurement of all 16 segments is the global longitudinal strain. The parasternal short-axis view at the level of the papillary muscle covers six segments and assesses radial and circumferential strain. A single reader (GK), who was blinded to the patient’s clinical data, performed all echocardiographic measurements. Twenty random study echocardiograms were selected and read by a second reviewer (JA-V) who was blinded to the data of the first reviewer to assess reproducibility of the data.

Figure 1. The 16 segment left ventricular “bulls eye” model.
Conventional echocardiographic assessment of left ventricular function
Each cancer survivor and control subject underwent a complete echocardiographic exam (including M-mode, spectral Doppler, and tissue Doppler imaging) for assessment of left ventricular systolic, diastolic, and global function. All measurements were analysed offline with the Xcelera cardiovascular ultrasound imaging software program (R4.1; Philips Healthcare, Eindhoven, The Netherlands). Left ventricular fractional shortening was calculated from M-mode measurements taken from the parasternal short-axis view at the level of the papillary muscle, according to the standard American Society of Echocardiography guidelines.Reference Lang, Badano and Mor-Avi18 Mitral inflow was imaged with spectral and tissue Doppler echocardiography to assess early left ventricular diastolic filling velocity (E), late diastolic left ventricular filling caused by atrial contraction (A), and mitral valve septal and lateral annulus tissue Doppler velocities of early and late diastolic left ventricular filling and left ventricular systole (E′, A′, and S′, respectively). These measurements were used to calculate the E/A ratio and the E/E′ ratio. The myocardial performance index was measured as the ratio of the time spent in diastole (the isovolumetric contraction time plus the isovolumetric relaxation time) to the systolic ejection time by standard methods.Reference Tsutsumi, Ishii and Eto19
Statistical methods
Continuous variables are reported as means and standard deviations, and categorical variables as numbers and percentages. Various conventional and speckle echocardiographic measurements from childhood cancer survivors and controls were compared with students t-tests, Mann–Whitney tests, and chi-squared tests, as appropriate. The relationships between cumulative anthracycline dose and various echocardiographic measurements were assessed with Pearson’s correlation coefficient. Statistical significance was set at a p-value <0.05. Intra-class correlation coefficients were calculated to assess the inter-observer variability in a random sample of 20 study patients. We also ran the post hoc power analysis using our global longitudinal strain in these two groups. We used an alpha error of 0.05 and also 0.01, in both the situation the power was 100% with our sample size to detect the mean difference of 3.7. All data were analysed with the SPSS statistical analysis program, version 20 (IBM SPSS Inc., Chicago, Illinois, United States of America).
Results
Of the 113 children enroled, 41 (36.2%) were childhood cancer survivors and 72 were controls. The mean (standard deviation) age of the childhood cancer survivors at echocardiography was 12.7 (3.8) years. The median (interquartile range, 25th–75th percentile) age at cancer diagnosis was 4.53 (2.17–9.26) years, and the median (interquartile range, 25th–75th percentile) duration of follow-up after anthracycline chemotherapy was 4.73 (2.15–8) years. The mean (standard deviation) cumulative anthracycline dose was 197.9 (94.3) mg/m2, with the median (range) being 160.2 (60 to 396.9) mg/m2. Baseline clinical characteristics were similar in both groups (Table 1). The most common cancers were neuroblastoma and B-type acute lymphoblastic leukaemia. The various other malignancies with their frequencies are listed in Table 2.
Table 1. Clinical characteristics of childhood cancer survivors after anthracycline chemotherapy and controls.

SD = standard deviation.
Table 2. Cancer diagnosis in 41 childhood cancer survivors treated with anthracycline chemotherapy.

Left ventricular strain analysis
In childhood cancer survivors, the peak systolic longitudinal strain was significantly lower in the two- (−18.6 [3.2] versus −21.3 [2.5], p < 0.001), three- (−16.3 [6.0] versus −21.7 [3.0], p < 0.001), and four- (−17.6 [2.7] versus −20.8 [2.0], p < 0.001) chamber views as well as for the global longitudinal strain (−17.6 [2.7] versus −21.3 [2.0], p < 0.001) when compared to controls with normal echocardiograms (Table 3). The circumferential strain was also significantly lower in childhood cancer survivors (−20.8 [4.3] versus −23.5 [2.6], p < 0.001) compared to controls with normal echocardiograms (Table 3). Left ventricular radial strain was not different between the groups (40.1[14.6] versus 40.8 [14.1], p = 0.79). The intra-class correlation coefficients for global longitudinal strain was 0.94 (95% confidence interval 0.86–0.98, f-value 17.16, p < 0.001) indicating minimal inter-observer variability.
Table 3. Left ventricular strain characteristics measured by 2D speckle-tracking echocardiography among childhood cancer survivors and controls.
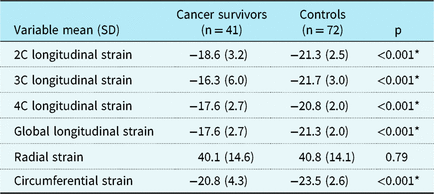
C = chamber; SD = standard deviation.
* p < 0.05
Left ventricular segmental analysis
Survivors had lower systolic longitudinal strain measurements in all but four left ventricular segments (basal inferior, basal anterior, mid-inferior, and mid-inferolateral) (Table 4 and Fig 2). The circumferential strain measured at the level of the mid-papillary muscle in the anterior, septal, anterior-septal, and the inferior segments were significantly lower in childhood cancer survivors than in controls (Table 5). Radial strain in the posterior segment was lower in childhood cancer survivors, with all the other segments showing similar radial strain (Table 6).
Table 4. Longitudinal strain measurements in childhood cancer survivors and controls obtained with 2D speckle-tracking echocardiography, by heart segment.
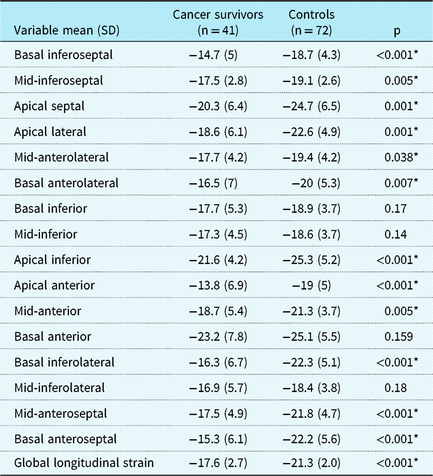
SD = standard deviation.
* p < 0.05

Figure 2. Comparison of the longitudinal strain in the 16 left ventricular segments between cancer survivors and controls.
Table 5. Circumferential strain measurements in childhood cancer survivors and controls obtained with 2D speckle-tracking echocardiography, by heart segment.

SD = standard deviation.
* p < 0.05
Table 6. Radial strain measurements in childhood cancer survivors and controls obtained with 2D speckle-tracking echocardiography, by heart segments.

SD = standard deviation.
* p < 0.05
Conventional assessment of left ventricular function
Diastolic functional measurements (E/E′ ratio at the septal and lateral mitral annulus) did not differ between groups (Table 7). The E/A ratio was higher in the normal controls compared to the childhood cancer survivors; however, this was within the normal range (Table 7). Systolic functions, as assessed by fractional shortening, and the global myocardial function, as assessed by myocardial performance index, were normal and similar between groups (Table 7).
Table 7. Doppler velocities measured in childhood cancer survivors and controls with conventional echocardiography.

A = late diastolic left ventricular filling caused by atrial contraction; E = early left ventricular diastolic filling velocity; E′ = tissue Doppler velocity of the mitral valve annulus due to early diastolic left ventricular filling; MPI = myocardial performance index; SD = standard deviation.
* p < 0.05.
Effect of anthracycline dose on speckle measurements
Longitudinal global strain had a weak correlation with cumulative anthracycline dose (r = 0.284, p = 0.098). There was no correlation of doses of anthracycline with circumferential and radial strain. Also, there was no correlation of global longitudinal strain with age at cancer diagnosis (r = −0.15, p = 0.13) or with the duration of follow-up (r = 0.098, p = 0.27). Interestingly, both global circumferential strain (r = 0.27, p = 0.02) and global radial strain (r = −0.31; p = 0.008) had significant but weak correlations with the duration of follow-up.
Discussion
With two-dimensional Speckle-tracking echocardiography, we found subtle myocardial deformational abnormalities in asymptomatic childhood cancer survivors with a median follow-up duration of 4.73 (2.15–8) years after anthracycline chemotherapy, while simultaneous conventional echocardiographic measures of systolic left ventricular function were normal. Global systolic longitudinal strain was significantly lower in childhood cancer survivors than in controls. Also, the longitudinal and circumferential strains were significantly lower in the majority of left ventricular segments in the childhood cancer survivors group when compared to controls with normal echocardiograms. Similarly, global circumferential strain was significantly lower in childhood cancer survivors than in controls, whereas radial strain was unaffected. A previous study of 53 childhood cancer survivors aged 18.6 [5.1] years (mean [standard deviation]) showed abnormalities in the left ventricular global longitudinal strain at a median of 7.2 years (range from 2.4 to 16.4 years) after exposure to a median cumulative anthracycline dose of 229 mg/m2 (range from 40 to 644 mg/m2).Reference Yu, Yu and Cheuk10 Mean (standard deviation) left ventricular global three-dimensional strain in the childhood cancer survivors was lower than that of age-matched controls (35.4% [7.5%] versus 44.6% [7.8%], p < 0.001), even though they had no symptoms of heart failure and normal left ventricular ejection fraction.Reference Yu, Yu and Cheuk10. In childhood cancer survivors, three-dimensional segmental strain measurements in all of the segments of the left ventricular, except the basal anteroseptal segments, were significantly lower than those in controls.Reference Yu, Yu and Cheuk10 In another study, among 19 childhood cancer survivors exposed to anthracyclines (mean [standard deviation] cumulative dose of 296 [103] mg/m2), left ventricular peak global longitudinal strain was decreased from baseline as early as 4 months after the start of anthracycline chemotherapy and preceded the decrease in the left ventricular ejection fraction at 8 months after the start of chemotherapy.Reference Poterucha, Kutty and Lindquist14 Peak longitudinal systolic strain was decreased in the apical, apical septal, apical anterior, and apical lateral segments and in the mid-inferior, mid-inferior septal, and mid-anterior segments of the left ventricular 4 months after anthracycline chemotherapy was completed.Reference Poterucha, Kutty and Lindquist14 Among 70 breast cancer survivors exposed to anthracyclines 6 years before echocardiographic assessment, global longitudinal strain and longitudinal strain in the anterior, lateral, and septal segments were lower than in 50 healthy control women, whereas radial strain did not differ, despite both groups having normal left ventricular systolic function.Reference Ho, Brown and Barrett20 Pignatelli et al evaluated 25 children (aged 9.8 ± 5.8 years) with various cancers, of whom 15 (60%) showed abnormal peak systolic global longitudinal strain and 19 (76%) had abnormal peak circumferential strain compared to their age-matched controls (p = 0.005).Reference Pignatelli, Ghazi and Reddy21
There are only a few paediatric studies evaluating the effect of anthracycline chemotherapy on the function of different left ventricular segments in the long-term follow-up of childhood cancer survivors.Reference Yu, Yu and Cheuk10, Reference Toro-Salazar, Gillan and O′Loughlin22
Given the unique architecture of the myofibres, contraction of the left ventricular is a complex, three-dimensional movement that involves left ventricular longitudinal shortening and thickening in the circumferential and radial directions. In left ventricular systole, the shortening and thickening of the muscle fibres can be measured as left ventricular systolic strain and can indicate early changes in cardiac dysfunction.Reference Sengupta, Korinek and Belohlavek23 The contraction of the left ventricle along the long axis is primarily regulated by the sub-endocardial myofibres, and the contraction of the left ventricle along the short axis is regulated by the mid- and the sub-epicardial myofibres. The sub-endocardial fibres are thought to be the most vulnerable to damage due to ischemia or toxicity and are the first to be affected in left ventricular systolic dysfunction. The mid- and sub-epicardial myofibres compensate for the left ventricular dysfunction in the earlier stages, and their contractility may decline only after substantial myocardial damage has occurred.Reference Bansal and Sengupta24 This process may explain the abnormalities we found in left ventricular longitudinal strain in majority of the segments, and the more limited changes in circumferential and radial strain were found only in certain segments.
One meta-analysis (16 studies; 5721 patients with acute coronary syndrome, heart failure, valvular heart disease, etc.) found that a decline in global longitudinal strain was independently associated with death and that global longitudinal strain was impaired, even in childhood cancer survivors with normal left ventricular ejection fraction.Reference Kalam, Otahal and Marwick25 Furthermore, strain measurement is independent of preload, heart rate, and angle.Reference Amundsen, Helle-Valle and Edvardsen26 Strain is also easy to measure and fairly reproducible, with measures of longitudinal strain having the best reproducibility.Reference Becker, Bilke and Kuhl27 Additionally, global longitudinal strain is independent of maturational changes and thus can be used to study myocardial function in different age groups.Reference Lorch, Ludomirsky and Singh28 With all of these advantages, Speckle-tracking echocardiography could be a readily available bedside technology for assessing regional and global left ventricular systolic function in childhood cancer survivors. The expert consensus report from the American Society of Echocardiography and the European Society of Cardiovascular Imaging recommends using two-dimensional Speckle-tracking echocardiography to evaluate childhood cancer survivors and recommends global longitudinal strain as the optimal deformation measure for detecting early subclinical left ventricular dysfunction.Reference Plana, Galderisi and Barac29 A reduction in global longitudinal strain greater than 15% from baseline is likely to be abnormal.Reference Plana, Galderisi and Barac29 Our study adds to the limited body of literature by verifying the feasibility of using two-dimensional Speckle-tracking echocardiography in children with cancer and that the speckle changes happen even when the fractional shortening is normal. However, further long-term studies are needed to assess whether these early changes in speckle-tracking echocardiography in childhood cancer survivors correlate with worsening of left ventricular function.
Very limited data are found in the literature that has evaluated the segmental strain during the long-term follow-up of childhood cancer survivors.Reference Yu, Yu and Cheuk10 In our childhood cancer survivors, longitudinal strain was lower in 12 of the 16 segments of the left ventricular, circumferential strain was lower in four of the six segments, and radial strain was lower in the posterior segment. We speculate that only the septal portion of the longitudinal strain measured by two-dimensional speckle imaging is affected in the early stages during the chemotherapy,Reference Poterucha, Kutty and Lindquist14 but this eventually progresses to all segments, as found in our study.
Although changes in left ventricular ejection fraction and left ventricular fractional shortening remain the conventional focus of monitoring left ventricular function in various populations, the improper shortening and thickening of the myofibres are the preliminary changes in compensated cardiac dysfunction. This may be indicated by changes in strain and strain rate, which can be detected by speckle-tracking echocardiography.Reference Longobardo, Suma and Jain30 However, at present in children, there are no studies utilising the speckle strain imaging to predict the outcomes in childhood cancer survivors. Therefore, a longitudinal study to predict the role of early longitudinal strain changes on the short or long-term outcome is warranted.
There is no evidence of left ventricular diastolic dysfunction in the childhood cancer survivors in our cohort. The left ventricular fractional shortening (a conventional measure of systolic function) and myocardial performance index (a marker of global left ventricular function) were normal in childhood cancer survivors at a mean of 5.6 (4) years after chemotherapy. Our findings are similar to those of our previous study of the diastolic function in 63 childhood cancer survivors who completed anthracycline chemotherapy (median cumulative dose 165 mg/m2) at a median duration of 5.2 years.Reference Aggarwal, Pettersen and Bhambhani6 In these childhood cancer survivors, all measures of diastolic dysfunction (velocity of E, A, E/A ratio, tissue Doppler velocities E′, A′, E/E′ ratio, and E′/A′ ratio at the septal and lateral annulus of the mitral valve) were normal 5 years after treatment. These measures were also similar in the subgroups exposed to median doses of <150 mg/m2, between 150 and 300 mg/m2, and >350 mg/m2.Reference Harahsheh, Aggarwal and Pettersen31 Our results are contrary to those of Ganame et al, who found left ventricular diastolic dysfunction and an abnormal myocardial performance index, along with abnormalities in left ventricular strain, after about 5 years in asymptomatic childhood cancer survivors exposed to anthracyclines (median cumulative dose 240 mg/m2).Reference Ganame, Claus and Uyttebroeck32 These differences may be explained by the lower median cumulative anthracycline dose (160.2 mg/m2) in our childhood cancer survivors compared to the patients in the above study. The myocardial performance index was abnormal and higher (0.51 versus 0.46) in childhood cancer survivors 12 years after a cumulative anthracycline dose >300 mg/m2 than it was in those 13 years after a cumulative anthracycline dose <300 mg/m2.Reference Armenian, Gelehrter and Vase33 Our study differs from the above studies in that the median cumulative anthracycline dose in our childhood cancer survivors was lower, at 160.2 mg/m2. This difference could explain the findings of normal left ventricular fractional shortening, diastolic function, and myocardial performance index in our childhood cancer survivors cohort.
Limitations
Our study is limited by its retrospective nature and a moderate sample size. Moreover, the study was not designed to look at the implication of longitudinal strain changes on the long-term outcomes in childhood cancer survivors. However, there is very limited data on the long-term changes in the strain at the various segments of the left ventricle in children exposed to anthracycline. Due to our small sample size, the effects of different types of cancer on changes in left ventricular strain were not analysed; correlations with age at cancer diagnosis and duration of follow-up were similarly limited.
Conclusions
Even within a year after completing anthracycline chemotherapy, asymptomatic childhood cancer survivors with a normal left ventricular fractional shortening nevertheless had abnormal global longitudinal strain measurements. Changes in left ventricular peak systolic longitudinal strain were global and affected the majority of the left ventricular segments. Changes in global circumferential strain were apparent as well. A longitudinal follow-up study is needed to assess the long-term clinical implications of abnormalities in segmental and global systolic strain on the left ventricular function and progression to symptoms to determine if these strain abnormalities have increased predictive value for the subsequent development of cardiovascular morbidity and mortality.
Acknowledgment
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of Interest
None.
Ethical Standards
This study does not involve human and/or animal experimentation.











