INTRODUCTION
Sex ratio theory, a productive focus in evolutionary biology, seeks to understand how natural selection leads to an optimal allocation of reproductive effort to male and female offspring among individuals of a dioecious species and what ecological and evolutionary factors lead to variation in the population sex ratio (Charnov, Reference Charnov1982). Although the theory was originally developed for large multicellular organisms such as plants and animals, one influential model, Local Mate Competition (LMC) (Hamilton, Reference Hamilton1967), applies well to the reproductive system of malaria parasites (Plasmodium and related genera). This model posits that any local population with limited genetic variation and high levels of inbreeding should produce a female-biased sex ratio that will reduce competition among brothers for matings with sisters. For a strictly clonal population in which all individuals are genetically identical, the optimal sex ratio would be just enough males to mate with all females, and the sex ratio would therefore be related to the number of mates possible for a single male. In contrast, genetically diverse populations, which experience low levels of inbreeding, should produce more equal proportions of males and females (Read et al. Reference Read, Narara, Nee, Keymer and Day1992; West et al. Reference West, Reece and Read2001).
Malaria parasites undergo multiple cycles of asexual replication within the vertebrate host, eventually producing male or female sex cells, the microgametocytes and macrogametocytes. Within the vector's midgut, each female gametocyte develops into a single female gamete and each male gametocyte produces one to several flagellated male gametes. Male fecundity is therefore the number of successful male gametes produced by a single microgametocyte. The male and female gametes quickly combine within the midgut of the vector, completing the sexual stage of malaria's life cycle (Garnham, Reference Garnham1966). The gametes competing for fertilization are limited to those taken up by a single vector, and therefore the breeding population of a malaria parasite is partitioned among individual insect vectors where LMC will occur (West et al. Reference West, Reece and Read2001). The algebra of sex ratio theory predicts the expected gametocyte sex ratio in the vertebrate hosts based on the genetic (clonal) diversity present and likely degree of inbreeding. For example, when a single clone of parasites is present, just enough male gametocytes should be produced to mate with all the female gametocytes within the vector, and this value would depend on the fecundity of male gametocytes (Read et al. Reference Read, Narara, Nee, Keymer and Day1992; Reece et al. Reference Reece, Drew and Gardner2008; Schall, Reference Schall2009).
West et al. (Reference West, Reece and Read2001) and Gardner et al. (Reference Gardner, Reece and West2003) noted that environmental conditions that reduce the probability of male gametes finding and combining with female gametes would favour ‘fertility insurance’ with an increase in investment in male gametocytes. For example, as the vertebrate host mounts an immune response to an infection, immune factors from the vertebrate carry over into the blood meal and attack the parasite's male gametes (the ‘transmission blocking immunity’ of Carter et al. Reference Carter, Gwadz and McAuliffe1979). This effect should be more pronounced late in the course of an infection when the host immune system detects the presence of the parasite. Low density of gametocytes in the vertebrate blood, a common situation for many malaria infections (Taylor and Read, Reference Taylor and Read1997), would also lead to an apparent ‘surplus’ of male gametocytes relative to that expected by the simple LMC model because of the difficulty faced by the very few male gametes in finding a female gamete during the few minutes available for mating in the vector.
In summary, the LMC model with fertility insurance predicts that single-clone infections of a malaria parasite will produce a female-biased sex ratio (with the precise bias depending on the fecundity of the males). This bias should be reduced or absent when gametocyte density is low, and will fade over the course of an infection due to immune system attack and resulting transmission blocking immunity. Elegant experimental studies of rodent (Reece et al. Reference Reece, Drew and Gardner2008) and avian (Paul et al. Reference Paul, Raibaud and Brey1999, Reference Paul, Coulson, Raibaud and Brey2000) malaria parasites support LMC with fertility insurance. Infections of P. chabaudi established in laboratory mice produce a sex ratio early in an infection that matches the algebraic predictions of the theory depending on the number of clones and their relative proportions in the mouse blood. For both P. chabaudi and P. gallinaceum in domestic chickens, single-clone infections are strongly female-biased early in the infection, but switch to greater production of male gametocytes over time, perhaps because the host mounts an immune attack that could reduce the fecundity of male gametocytes. The rodent malaria experiments, however, present another intriguing result. The well-characterized laboratory clones of P. chabaudi tend to produce female-biased sex ratios early in an infection, as predicted by the theory, but there is consistent variation among clones in precise sex ratio. The only other suggestion of variation in gametocyte sex ratio among clones is from Burkot (Reference Burkot, Williams and Schneider1984) who found variation in a few laboratory clones of P. falciparum, although these were clones maintained in vitro. Variation among clones for gametocyte sex ratio could be a result of genetic variation for some factor that drives sex ratio among clones, perhaps male gametocyte fecundity, gametocyte density, or effect on erythrocyte density (Reece et al. 2008).
We investigated the gametocyte sex ratio of a natural parasite-host association, P. mexicanum in its reptile host, the western fence lizard, Sceloporus occidentalis. We studied replicate experimental single-clone infections of 10 genetic clones to test if (a) infections with only a single clone produce female-biased gametocyte sex ratios; (b) the gametocyte sex ratios of infections change over time to produce more males; (c) gametocyte density is correlated with sex ratio; (d) there is clonal (genetic) variation for sex ratio. This is the first such study of single-clone infections of a natural parasite-host system.
MATERIALS AND METHODS
Experimental infections
Blood dots and smears for this study were obtained from 2 experiments, both conducted at the University of California Hopland Research and Extension Center near Hopland California in southern Mendocino County, California, USA. Detailed descriptions of the experimental design of these experiments can be found in Osgood and Schall (Reference Osgood and Schall2004) (henceforth referred to as Study I) and Vardo-Zalik and Schall (Reference Vardo-Zalik and Schall2008, Reference Vardo-Zalik and Schall2009) (henceforth referred to as Study II). Briefly, blood from western fence lizards (S. occidentalis) naturally infected with P. mexicanum was used to initiate replicate infections in previously non-infected recipient lizards. Blood from a donor lizard with a standard number of asexual-stage parasites mixed with saline was injected into the peritoneal cavity of recipient lizards. This method of initiating infections has been described in detail by Eisen and Schall (Reference Eisen and Schall2000). Experimental lizards were kept outdoors in vector-proof cages and blood was taken from a toe clip every 7 (Study I) or 10 (Study II) days for 4 (Study I) or 3 (Study II) months during the summer. Blood dots were dried and frozen and fresh blood was used to prepare a thin smear, which was treated with Giemsa stain (Schall, Reference Schall1989).
For our study we used only the infections initiated from donors that were judged to contain a single parasite genotype. These single-clone infections were identified by analysis of 5 (Study I) or 3 (Study II) microsatellite markers using PCR primers and conditions presented by Schall and Vardo (Reference Schall and Vardo2007). Study I had 7 single-clone donors, that were each used to initiate 3, 4 or 5 recipient infections, for a total of 31 induced infections. Study II had 3 single-clone donors, which were used to initiate 3 or 7 recipient infections each, for a total of 17 induced infections. Examination of the microsatellite allele data showed that all clones were distinct for the two studies. Large and equal sample sizes were not possible due to the small size of donor lizards and the relative scarcity of single-clone infections at the study site (Vardo-Zalik and Schall, Reference Vardo-Zalik and Schall2009).
Gametocyte sex ratio
We determined gametocyte sex ratio by scanning blood smears at 1000x and scoring each gametocyte as male or female, taking care to sample gametocytes from all areas of the smear. Each slide was examined until 100 mature gametocytes were scored or a maximum of 1 hour. Male and female gametocytes can be readily differentiated based on their appearance after staining, as noted by Schall (Reference Schall1989). To assure accurate scoring of gametocytes, all slides were counted by 1 investigator (A.T.N.), and 2 other investigators scored samples of the smears; all 3 investigators produced very similar results.
Time and gametocytaemia effect on sex ratio
Gametocyte sex ratio and gametocytaemia (gametocytes per 1000 erythrocytes examined under 1000x) were determined for every recipient infection in Study I 5, 6, 10 and 11 weeks after each lizard was inoculated with infected blood to investigate possible time and gametocytaemia effects on sex ratio. In addition, 1 recipient infection per donor from Study I was selected and the sex ratio was determined for every available slide to determine if sex ratio varied over the course of an infection.
Genetic variation for sex ratio
We used gametocyte sex ratio counts from Study I 5, 6, 10 and 11 weeks post-inoculation (above) as well as 3 and 9 weeks after gametocytes became patent in the blood to determine if there was genetic variation for sex ratio. Gametocyte patency was based on a 1 min scan at 1000x. Gametocytes first became patent in the blood 1 to 7 weeks post-inoculation, with the majority of infections (18 out of 31) becoming patent 3 to 4 weeks post-inoculation. We used sex ratio counts from a series of weeks after gametocyte patency in addition to a series of weeks post-inoculation because we noted that infections differed in the time it took for gametocytes to become patent in the blood of the host and concluded that this difference in establishment time might affect the sex ratio of the infection.
Multiple dates were sampled in case confounding factors early in an infection (such as a long establishment time) or late in an infection (such as interactions with the lizard immune system) might mask a clone effect. We also used sex ratio counts from Study II 11 weeks post-inoculation and 3 weeks post-gametocyte patency (fewer blood smears were made in Study II, so fewer dates were possible) to determine if the presence or absence of a genetic effect on sex ratio was also observed in a different set of clones.
Statistical analysis
Statistical analysis was performed using JMP 8 or SAS 9.2 (SAS Institute, Cary, NC, USA). To test for any role of age of the infection, gametocytaemia, and clone on gametocyte sex ratio, data for Study I (for which a longer series of sample dates was available) 5, 6, 10 and 11 weeks post-inoculation were analysed using a Generalized Estimating Equation (GEE) fitted to a binomial error and logit-link function for gametocyte sex ratio (as the dependent variable) (SAS). GEE take into account repeated measurements of the same infection over a series of weeks. Entered into the analysis for each slide examined were the number of male and total number of gametocytes counted, sample time, gametocytaemia (gametocytes per 1000 erythrocytes), and clone. To further examine a possible genetic influence on gametocyte sex ratio, all clones (Studies I and II separately) were analysed using a Generalized Linear Model (GLM) at each date, again with sex ratio modelled with binomial errors and logit-link function (JMP statistical package, SAS Institute). For 7 infections with gametocyte sex ratio for each sample period, we plotted 90% confidence intervals for proportion data for each sample.
RESULTS
Overall gametocyte sex ratio
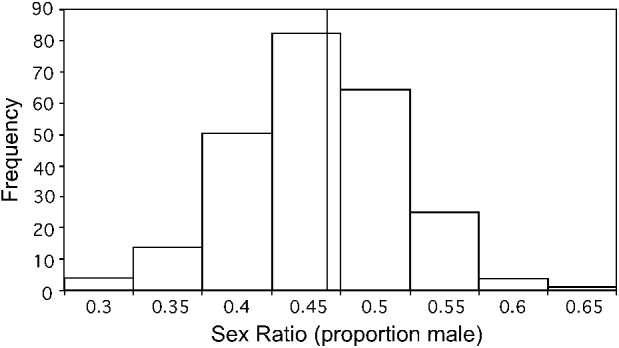
A total of 48 experimentally induced infections were followed from Study I and Study II, with 297 blood samples examined. Some lizards died before the entire sequence of smears was taken over time, and some smears did not contain sufficient gametocytes to calculate sex ratio. Of the 297 smears examined, 287 allowed sex ratio counts, with a sample size of at least 100 for 245 smears, 75–99 for 4, 50–74 for 20, and <50 for 18 (these had low parasite densities). Gametocyte sex ratios had a mean/median/mode proportion male of 0·44/0·44/0·42 and infections in which at least 100 mature gametocytes were counted ranged from the proportion male gametocytes of 0·28 to 0·63 (Fig. 1).
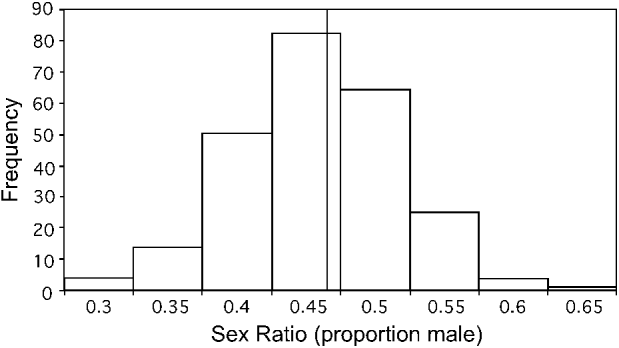
Fig. 1. Sex ratios of single-clone replicate infections of the malaria parasite Plasmodium mexicanum in its natural host, the western fence lizard Sceloporus occidentalis. The graph includes sex ratios of all experimentally induced single-clone infections from Studies I and II in which at least 100 mature gametocytes were scored as male or female. Data are from 48 infections over time for a total of 245 gametocyte sex ratios scored. A reference line is included at the mean/median value (0·44).
Gametocyte sex ratio over time and effect of gametocytaemia
Overall, gametocyte sex ratio was not related to sample time (GEE, P=0·144). Because of low gametocyte numbers early in some infections and the death of a few lizards, the number of samples available for counting gametocytes was reduced at different time periods (post-inoculation and post-patency).
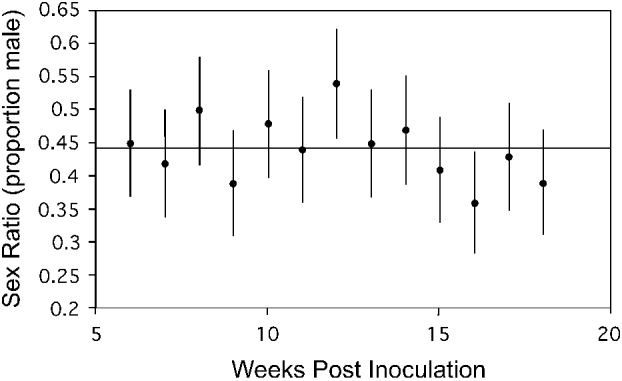
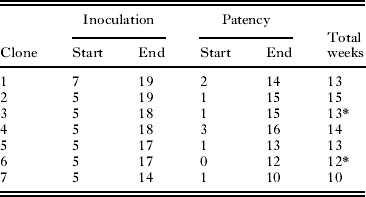
Gametocyte sex ratio of 7 infections was followed for ∼13 weeks (∼5–18 weeks post-inoculation, 1–14 weeks post-gametocyte patency). Table 1 shows the start and end times for each infection. Each of these 7 infections was initiated with blood taken from different donor lizards (and thus different parasite genotypes) from Study I, and thus represented different genetic clones in each infection. The start and end dates of our counts are not constant because the time of first patency differed somewhat among infections and infection initiation dates varied (altering the end dates possible). Fig. 2 shows a sample infection of the 7 with 90% confidence intervals for the calculated proportion of male gametocytes. The result drawn from this figure is consistent across all 7 infections: confidence intervals overlapped broadly. Note that if there were errors in counting immature gametocytes (that appear more female in morphology (Schall, Reference Schall1989)), then sex ratio should increase in proportion of males over time; this was not observed.
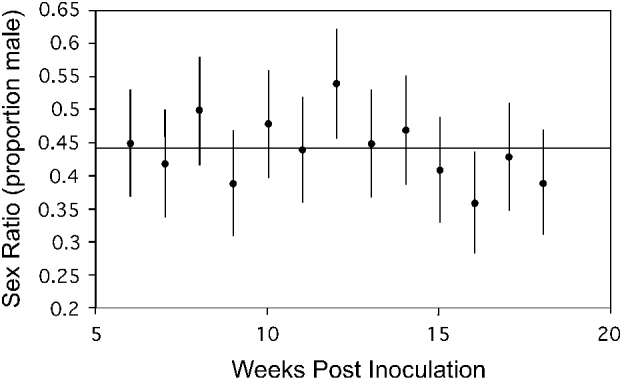
Fig. 2. Gametocyte sex ratio of the malaria parasite Plasmodium mexicanum in an experimentally infected lizard (Sceloporus occidentalis) 6–18 weeks after initiation of infection via injection of infected blood from a donor lizard. Sex ratio counts are from an individual lizard from Study I infected with a single clone of a parasite. Six additional infections of 6 different clones from Study I were also sampled and showed a similar lack of trend in sex ratio over time. Each point represents a single count and error bars represent a 90% confidence interval (calculated for proportion data) and a reference line showing the grand mean for all samples is shown.
Table 1 Summary of seven infections of different clones of the malaria parasite Plasmodium mexicanum in its natural lizard host followed over time

Median gametocytaemia was 4·5 gametocytes per 1000 erythrocytes and ranged from 0 to 117 gametocytes per 1000 erythrocytes. Sex ratio was not related to gametocytaemia (114 samples for the 31 experimental infections of Study I; GEE P =0·402). Sample sizes for each date are given in Table 2.
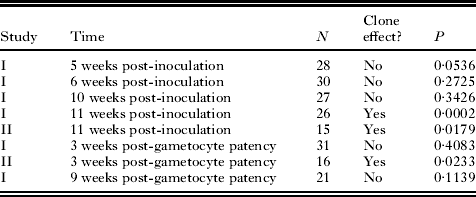
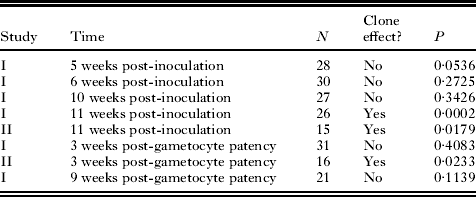
Table 2 Summary of the presence or absence of a clone effect on the sex ratio of single-clone infections of the malaria parasite Plasmodium mexicanum in its natural host, the western fence lizard Sceloporus occidentalis
Genetic variation in gametocyte sex ratio
When clone was entered into the GEE analysis with time and gametocytaemia for Study I, there was no significant effect (P=0·058). However, the P value suggested that a Type II error was possible by combining all time periods. Separating time periods for both studies, a GLM showed a significant clone effect in Study II for gametocyte sex ratio at both times at which counts were performed. Study I showed a significant clone effect on gametocyte sex ratio at the latest date sampled (11 weeks post-inoculation), but not at any of the other dates (5, 6, and 10 weeks post-inoculation, 3 and 9 weeks post-patency). These findings are shown in Table 2 and Fig. 3.

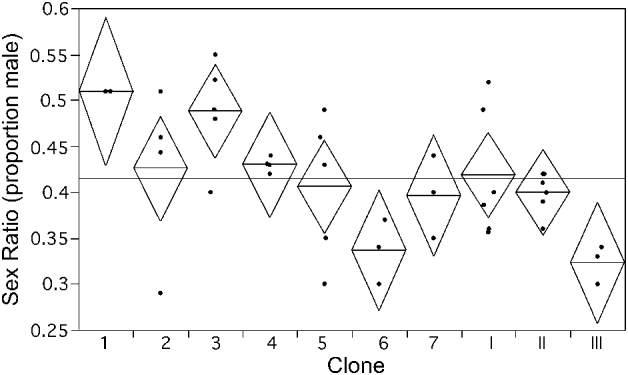
Fig. 3. Clone effect on gametocyte sex ratio of the malaria parasite Plasmodium mexicanum in experimental single clone infections 11 weeks after initiation of the infection. Clones 1–7 are from Study I. Clones I–III are from Study II. Points represent individual infections and are slightly shifted horizontally to make all points visible. Diamonds show mean line at the centre and 95% confidence interval vertically between tips. The grand mean is displayed as a reference line.
DISCUSSION
Predictions of sex ratio theory
Within sex ratio theory, the model of LMC predicts that a single clone of malaria parasites replicating within a vertebrate host will produce a female-biased gametocyte sex ratio. However, several factors would drive gametocyte sex ratio towards a greater production of male cells. First, male fecundity, or the number of gametes produced by a male gametocyte, could be low, and thus even the process of LMC would result in no female bias. Second, if the mobility or viability of male gametes is reduced by any environmental factor in the blood meal taken by the vector, an apparent ‘surplus’ of males will be produced, even in single-clone infections. For example, immune system carry-over in the vector could become more severe over time, thus favouring production of more male gametocytes. Last, low density of gametocytes in the vertebrate host's blood would also result in fertility insurance and shifting of the sex ratio away from a female bias.
These predictions assume that malaria parasites use a phenotypically plastic strategy such that the parasite can monitor and respond to changing environmental conditions within the vertebrate host, including clonal diversity. This appears to be the case for P. chabaudi in experimental infections in laboratory mice (Reece et al. Reference Reece, Drew and Gardner2008). The rodent malaria parasite appears to detect both the number of coexisting clones and their relative abundance in an infection, and shifts gametocyte sex ratio to match very closely the algebraic predictions of LMC (Schall, Reference Schall2009). The P. chabaudi infections also shift gametocyte sex ratio over time towards more male gametocytes (consistent with an induced immune attack, although still not conclusively demonstrated), as well as differences in gametocyte density in the blood. The avian malaria parasite, P. gallinaceum, appears to monitor indirectly the activity of the bird host's immune system and shifts to a less female-biased gametocyte sex ratio when erythrocytes are being rapidly cleared and replaced (Paul et al. Reference Paul, Raibaud and Brey1999, Reference Paul, Coulson, Raibaud and Brey2000). In contrast, malaria parasites could also evolve a fixed strategy of an optimal gametocyte sex ratio based on prevailing clonal diversity and predictable changes in environmental conditions over the course of the infection. In this case, some infections may reveal what appears to be a ‘surplus’ of male gametocytes. Selection may favour a balanced genetic polymorphism for gametocyte sex ratio if conditions within infections vary substantially and only a fixed strategy is possible. We tested these predictions by producing replicate single-clone infections of P. mexicanum in its natural lizard host, the western fence lizard. This study has the advantage of being a natural parasite-host system, but also suffers additional complexity compared to the rodent or bird malaria laboratory model systems (below).
Overall sex ratio
The gametocyte sex ratios observed in this study averaged only marginally female biased.
The distribution of sex ratios determined for the experimental infections with a single clone of parasite is similar to that seen for natural infections of P. mexicanum previously sampled (1 sample for each infected lizard) at the field site (mean 0·46 males, range 0·24–0·72; Schall (Reference Schall1996)). These results predict an effective male fecundity ranging from 0·61 to 2·6 gametes, with the most common male fecundity of 1·3 gametes based on the LMC model (Schall, Reference Schall2009). The only estimate of male fecundity for P. mexicanum is presented by Osgood and Schall (Reference Osgood and Schall2004), who reported the number of gametes produced by a small sample (only 19) of male gametocytes from 5 natural infections, with a mode of 5 and range of 1–6 gametes. This would predict a gametocyte sex ratio of 0·17 (1 male: 5 females); none of the experimental infections produced a gametocyte sex ratio this strongly female biased. This result contrasts with the generally significantly female bias in gametocyte sex ratios for malaria parasites of humans (Read et al. Reference Read, Narara, Nee, Keymer and Day1992; West et al. Reference West, Reece and Read2001), birds (Paul et al. Reference Paul, Coulson, Raibaud and Brey2000), and rodents (Reece et al. Reference Reece, Duncan, West and Read2003), and suggests that male fecundity of P. mexicanum is low, fertility insurance is strongly active, or the parasite has evolved a fixed strategy that will not produce a female-biased sex ratio even when only a single clone of parasites is present.
Fertility insurance: infection over time
We noted no increase in the proportion of male gametocytes over the course of the P. mexicanum infections. This contrasts with the pattern seen in bird or rodent malaria infections in which gametocyte sex ratio shifts from a strong female bias to a more equal proportion of male and female cells over time (Paul et al. Reference Paul, Raibaud and Brey1999, Reference Paul, Coulson, Raibaud and Brey2000; Reece et al. Reference Reece, Drew and Gardner2008), and is particularly apparent for infections that are self curing (Paul et al. Reference Paul, Raibaud and Brey1999, Reference Paul, Coulson, Raibaud and Brey2000). Nothing is known concerning the response of the reptilian immune system to infection with malaria parasites (or for that matter, very little is known concerning the lizard immune response to any parasite infection). Plasmodium mexicanum occurs in chronic infections in fence lizards under natural conditions, with the course of infection resulting in a stable parasitaemia lasting at least several months (Bromwich and Schall, Reference Bromwich and Schall1986). The parasitaemia in these stable infections ranges over 3 orders of magnitude. Likewise, gametocyte sex ratio also varies among infections and remains constant for most infected lizards (Schall, Reference Schall1989). These findings suggest that lizards do not mount a strong immune response to the parasite, at least not one that interferes with the male gametes in the vector's blood meal. Osgood et al. (Reference Osgood, Eisen, Wargo and Schall2003) found that experimental manipulation of testosterone levels in the lizards, which should have an effect on the lizard's immune system, had no effect on the sex ratio, growth rate, or peak parasitaemia of P. mexicanum, further suggesting that the lizard's immune system does not mount a strong attack on the parasite. A weak or absent immune response would explain why sex ratio did not increase over the course of an infection.
An alternative explanation for why sex ratio did not increase over the course of an infection for P. mexicanum is that this parasite's gametocytes are long-lived such that most gametocytes, even those produced very early in an infection, may eventually experience attack of immune products in the vector. Reece et al. (Reference Reece, Duncan, West and Read2003) found that the half-life of gametocytes of Plasmodium chabaudi is only 14 h, but Schall (Reference Schall1989) observed that P. mexicanum gametocytes increase in size over the course of 3 months in individual infections, which could indicate that gametocytes of P. mexicanum are significantly longer lived. More research into the longevity of gametocytes in this species needs to be conducted before we can fully evaluate this hypothesis.
Fertility insurance: gametocyte density
We found no relationship between gametocytaemia and sex ratio for the experimental P. mexicanum infections. Previous studies have found a correlation between sex ratio and gametocytaemia (Pickering et al. Reference Pickering, Read, Guerrero and West2000; Robert et al. Reference Robert, Sokhana, Rogier, Ariey and Trape2003; Reece et al. Reference Reece, Drew and Gardner2008), including for P. mexicanum (Schall, Reference Schall2000). The correlation between gametocytaemia and sex ratio noted in previous studies with P. mexicanum (Schall, Reference Schall2000) could have been a result of differences in genetic diversity of the infections (Schall, Reference Schall, Lewis, Campbell, Sukhdeo and 2002) rather than of fertility insurance. In general, lizard malaria parasites tend to produce far more gametocytes in the host's blood than do those of bird or mammal malaria parasites (Schall, Reference Schall1996; Taylor and Read, Reference Taylor and Read1997). If this aspect of fertility insurance only becomes important below some lower threshold of absolute gametocyte number in the blood meal, P. mexicanum infections may seldom have gametocyte densities below this level. That is, gametocytes will simply be common in most infected lizards, with large numbers taken up by the vector.
Experimental studies of transmission success of the parasite to the sandfly vectors (2 species of Lutzomyia) reveal that almost all sandflies become infected after feeding on an infected lizard's blood, and the midgut is often covered with oocysts (Fialho and Schall, Reference Fialho and Schall1995; Schall, Reference Schall2000). Another possible confounding factor is that we measured the relative numbers of gametocytes per number of erythrocytes (gametocytaemia) rather than actual density of the gametocytes per volume of blood taken by a vector. Previous studies have found rather little variation in erythrocyte numbers per volume of blood in both infected and non-infected fence lizards (Schall, Reference Schall1990), so we suspect that the gametocytaemia measured here is relevant for fertility insurance.
Fertility insurance: blood clotting
Another unexplored factor that could favour fertility insurance is the clotting of blood within the vector. For male gametes to locate female gametes, they must travel through the vector's blood meal to a female gamete within a few minutes. If lizard blood taken up by a vector rapidly clots, this would reduce the mobility of male gametes and could explain the apparent surplus of male gametocytes in single-clone infections (Alano and Billker, Reference Alano, Billker and Sherman2005). We know of no comparative studies of blood clotting for reptiles vs mammals and birds. Anecdotally we have noted that fence lizard blood clots more rapidly than bird or mammal blood, and Vardo-Zalik and Schall (Reference Vardo-Zalik and Schall2008) reported that blood from infected fence lizards clots more quickly than blood from lizards not infected with P. mexicanum. Even in heparin-treated capillary tubes, the blood often formed complete clots within seconds, making measurement of haemoglobin difficult. This would be a result strictly of the density of the clotting blood, rather than the known killing of malaria parasite cells by platelets (Pleass, 2009) because reptiles do not produce platelets in their blood. Clearly, measurement of gamete mobility within vector blood meals to compare different species of malaria parasites would be a technical challenge.
Genetic variation in sex ratio
The experimental P. mexicanum infections provide evidence of genetic variation in sex ratio for a malaria parasite in its natural host from 2 independent studies. The clones selected for Study II appear to be more differentiated with respect to sex ratio than those in Study I since a clone effect was detectable at both dates examined in Study II. Study I showed no genetic variation for sex ratio when the 4 dates were analysed together (possibly a type II error, see Results section), but when each date was analysed separately, a significant clone effect on the latest date (11 weeks post-inoculation) emerged. Reece et al. (Reference Reece, Drew and Gardner2008) found evidence that rodent malaria parasites are able to recognize related from non-related conspecifics and adjust their sex allocation strategies accordingly. If this is also the case for P. mexicanum and if recognition cues are density dependent, it is possible that these cues are weak early in the infection, making it difficult for the parasite to detect the clonal diversity of the infection and respond accordingly. Such a situation could lead to a clone effect being hidden early in an infection and emerging only when the parasite is able to detect that it is alone.
Previously, such variation was found for P. chabaudi infections in laboratory mice (Reece et al. Reference Reece, Drew and Gardner2008) and for a few cloned lines of P. falciparum (Burkot, Reference Burkot, Williams and Schneider1984). This variation could be due to variation in male gametocyte fecundity among clones. If so, why should there be a genetic polymorphism for such an important life-history trait? What would be the selective advantage for any cell to produce fewer gametes? Gametocytes that produce more gametes are able to fertilize more females, but male gametocytes that produce fewer might produce gametes more quickly or of higher quality, making them more successful in finding female gametes (Alano and Billker, Reference Alano, Billker and Sherman2005). Additional research into the fecundity of different genotypes is necessary to determine whether variable fecundity is a probable source of variation in sex ratio between genotypes.
Phenotypic plasticity or fixed strategy?
Malaria parasites could achieve an adaptive sex ratio either by having the ability to alter gametocyte sex ratio based on conditions within each infection (such as clonal diversity or host immune attack) or by having a fixed sex ratio that is adaptive for the most common conditions within infections. There could also be a polymorphism for a range of fixed strategies. The rodent malaria parasite studies argue for a plastic response by P. chabaudi, with a remarkable ability to monitor and respond to clonal diversity within infections (Reece et al. Reference Reece, Drew and Gardner2008). The P. gallinaceum studies show that the parasite monitors changes in hormonal levels of its bird hosts which allows sex ratio adjustment based on the level of immune attack (Paul et al. Reference Paul, Coulson, Raibaud and Brey2000). The P. mexicanum experiments have the advantage of using a natural parasite-host system, but the disadvantage of lacking well-characterized clones of parasite to induce infections in hosts with minimal genetic variation. Our results are therefore equivocal. Plasmodium mexicanum may have phenotypic plasticity for sex ratio but produce so few gametes per male gametocyte that even single-clone infections produce close to 50:50 sex ratios, or the parasite may follow a fixed strategy of fertility insurance because of factors that consistently severely limit male gamete survival or mobility, such as quickly clotting blood. A fixed sex ratio near 50% male may also be favoured because multi-clone infections are common (Vardo and Schall, Reference Schall and Vardo2007).
Sex ratio theory is among the most productive and successful programs in evolutionary biology, and reveals how selection acting on individual organisms leads to the variation in sex ratios seen in populations (Charnov, Reference Charnov1982). This theory has now been usefully applied to malaria parasites, suggesting that a central feature of the parasites’ life history can now be understood (Read et al. Reference Read, Narara, Nee, Keymer and Day1992; Reece et al. Reference Reece, Duncan, West and Read2003, Reference Reece, Drew and Gardner2008; West et al. Reference West, Reece and Read2001, Reference West, Smith, Nee and Read2002). Studies across species with different hosts and evolutionary histories, such as P. mexicanum, are necessary before making any general conclusions on allocation of gametocytes to male and female cells.
ACKNOWLEDGEMENTS
We thank A. Vardo-Zalik, S. Osgood and all of their assistants for their hard work at collecting and preserving the blood dots and smears used in this study as well as the staff of the Hopland Research and Extension Center for their continued friendly support of the ongoing study of Plasmodium mexicanum and its hosts. A. Howard provided much appreciated help with the statistical analysis in SAS.
FINANCIAL SUPPORT
The research was supported by fund from the US NSF (J.J.S., grant number DEB-0813832) and the University of Vermont APLE program (A.T.N.).