Introduction
Ecosystem boundaries are seldom impermeable. For example, extensive transfer of nutrients and energy takes place from terrestrial systems to the ocean, driving high primary and secondary marine productivity (Cloern et al. Reference Cloern, Foster and Kleckner2014). Similarly, terrestrial ecosystems receive marine-derived nutrients from aerosols, shore drift of algae and carrion, marine mammals, and seabirds (Polis et al. Reference Polis, Sànchez-Piñero, Stapp, Anderson and Rose2004). Seabirds, in particular, are important mediators of these interactions. They transfer energy and nutrients from the marine system when they come ashore to breed, moult or rest (Polis et al. Reference Polis, Anderson and Holt1997, Sànchez-Piñero & Polis Reference Sànchez-Piñero and Polis2000), and in doing so substantially influence ecosystem structure and function (Sànchez-Piñero & Polis Reference Sànchez-Piñero and Polis2000).
Seabirds also influence terrestrial systems in other ways, particularly as ecosystem engineers – organisms that modify resources available to other species by either their presence or actions, and thereby create, modify or maintain habitats (Wright & Jones Reference Wright and Jones2006). Seabirds make their ecosystem engineering contribution largely through the excavation of burrows and the construction of other forms of nests. These may result in the displacement of tons of material, creating new habitats for a range of other organisms (Bancroft et al. Reference Bancroft, Garkaklis and Roberts2005).
On sub-Antarctic islands, seabirds are important in transferring nutrients from the sea to terrestrial systems (Smith Reference Smith1978, Joly et al. Reference Joly, Frenot and Vernon1987, Erskine et al. Reference Erskine, Bergstrom, Schmidt, Stewart, Tweedie and Shaw1998). From burrowing petrels to the larger penguins and albatrosses, all surface and sub-surface nesters make significant nutrient transfers (Smith Reference Smith2008), with profound influences on ecosystem structure and function (Crafford & Scholtz Reference Crafford and Scholtz1986, Joly et al. Reference Joly, Frenot and Vernon1987, Gabriel et al. Reference Gabriel, Chown, Barendse, Marshall, Mercer, Pugh and Smith2001, Vincke et al. Reference Vincke, van de Vijver, Ledeganck, Nijs and Beyens2007, Moravcová et al. Reference Moravcová, Beyens and van de Vijver2010).
Sinclair & Chown (Reference Sinclair and Chown2006) proposed that the wandering albatross, Diomedea exulans L., serves as a thermal ecosystem engineer for caterpillars of the detritivorous, tineid moth, Pringleophaga marioni Viette. Caterpillar biomass is, on average, higher in recently abandoned albatross nests, and varies less among nests than it does in other habitats or older albatross nests occupied by the moth caterpillars. Because nutrient availability does not differ appreciably between newer and older nests, it was suggested that the increased temperatures in occupied albatross nests favour caterpillar growth and survival. Sinclair & Chown (Reference Sinclair and Chown2006) indicated that incubating birds raise nest temperatures by c. 5°C compared with the surrounding soils. Limited recordings of nest temperatures suggest that they are close to the optimum temperature for caterpillar growth, and decrease the probability of repeated low temperature stresses which affect growth and survival (Sinclair & Chown Reference Sinclair and Chown2005, Reference Sinclair and Chown2006).
Despite the major empirical requirements of this thermal ecosystem engineering hypothesis being addressed in its initial formulation, two significant pieces of evidence were missing. First, the temperature record on which the assumption of elevated temperatures during incubation was based encompassed only 17 days in the autumn (April 2004). By contrast, wandering albatross nests are occupied for almost a full year (Ryan & Bester Reference Ryan and Bester2008). Therefore, the extent to which a beneficial temperature effect might be realized both across cooler and warmer periods of the year was not determined. Increased temperatures in cool periods could increase the advantage of the nest habitat, but P. marioni growth and survival decreases at and above 15°C, making extensive warming during the summer potentially problematic (Haupt et al. Reference Haupt, Crafford and Chown2014a). If caterpillars are routinely exposed to temperatures which compromise their fitness, wandering albatross nests would clearly be more harmful than beneficial. Second, the original study compared caterpillar biomass in recently abandoned nests (as a proxy for occupied nests which could not be sampled because doing so would disturb the birds) with old nests, making no distinction between nests from which chicks had fledged that season and older nests. This distinction is important because it can provide insight into the duration of the increased abundance of caterpillars in nests. For example, if caterpillar biomass or abundance is high in nests from which chicks have recently fledged (but low in older nests), it can be assumed that the ecosystem engineering effect lasts the entire year of incubation, given that the wandering albatross lays in mid-summer, with eggs hatching in March and birds fledging the next summer (December to February) (Ryan & Bester Reference Ryan and Bester2008). By contrast, if abundance or biomass is low in both the previous season’s and older nests it suggests that the engineering effect is restricted to the period during which incubation is actually occurring, and declines shortly thereafter.
Here we address these gaps by providing temperatures from several nests across a full year of occupation and by comparing caterpillar abundances from old nests (chicks fledged or abandoned two or more seasons previously), those from which chicks have recently fledged (i.e. nests last warmed by birds c. 4 months previously; based on records from the long-term wandering albatross monitoring programme on the island, Ryan & Bester Reference Ryan and Bester2008), and those which have been recently abandoned due to egg loss or young chick mortality (i.e. nests warmed by birds c. 4–6 weeks prior to sampling). Our aim was to assess more comprehensively the extent to which the thermal ecosystem effect is being realized and the duration of carry-over of the effect after chick fledging.
Materials and methods
Study site and species
Marion Island (46°54'S, 37°45'E) is part of the Prince Edward Island group. It is of volcanic origin, dominated by tundra and polar desert biomes, and has a mean annual temperature of c. 6.5°C, 1900 mm total annual precipitation, and a windy climate (Chown & Froneman Reference Chown and Froneman2008). The Prince Edward Islands support c. 7300 adult wandering albatrosses (c. 44% of the global population; Ryan & Bester Reference Ryan and Bester2008). Breeding pairs build nests comprising large mounds of vegetation and peat collected from the surrounding area (Fig. 1). A single egg is laid and incubated over the summer, hatches in March, is incubated until the brood-guard phase is over (c. 4–5 weeks later), and chicks fledge anytime between December and February (Ryan & Bester Reference Ryan and Bester2008). Therefore, nests are occupied for almost a full year. House mice have been introduced to Marion Island (Chown & Froneman Reference Chown and Froneman2008) and are associated with wandering albatross nests, affecting a small proportion of the chicks (Jones & Ryan Reference Jones and Ryan2010).
The flightless moth Pringleophaga marioni is endemic to the Prince Edward Islands, and, apart from their occurrence in wandering albatross nests, all life stages are found in a range of habitats from salt spray plant communities on the coast to microhabitats under stones, and in mosses and cushion plants at elevations up to 800 m a.s.l. Larvae are most abundant in nutrient-enriched areas such as Poa cookii (Hook.f.) tussock grasslands and Cotula plumosa (Hook.f.) herbfield (Burger Reference Burger1978, Crafford & Scholtz Reference Crafford and Scholtz1987). They have a mean critical thermal minimum (CTmin) of -0.6°C, freeze at -5.0°C, and show 100% survival of 12 h exposure to -6.0°C, but have 100% mortality after 18 h at -9.0°C (Klok & Chown Reference Klok and Chown1997). Although they are moderately freeze-tolerant, repeated exposure to low temperatures reduces the growth rate of the caterpillars (Sinclair & Chown Reference Sinclair and Chown2005). Recent evidence has also resolved the life-cycle duration of the species (Haupt et al. Reference Haupt, Crafford and Chown2014a). At 10°C, egg duration is 4–7 weeks, larval duration c. 46 weeks and pupal duration c. 7 weeks, with a total life cycle of c. 1 year. Although larval duration is much reduced (to c. 17 weeks) at 15°C, survival is low.

Fig. 1 Wandering albatross (Diomedea exulans) nests on sub-Antarctic Marion Island illustrating the form and structure of the nests. Note in the occupied nests the presence of Poa cookii, and bryophytes incorporated into the nest shown in the top right image. The image on the bottom left is of a recently abandoned nest (note eggshell fragments in the bottom left corner of the image, and two clear mouse burrows), and the image on the bottom right is of a long-abandoned nest, now overgrown.
Microclimate measurements
During the April–May relief voyage to Marion Island in 2011, ten occupied and ten abandoned wandering albatross nests were identified in the vicinity of the meteorological station and along the route to Macaroni Bay on the east coast (Fig. 2). Two calibrated iButton thermocron dataloggers (Model DS1922L, Maxim Integrated, Fairbridge, South Africa) were attached to plastic cable-ties (to make relocation possible) and were inserted mid-height into the side of each nest (c. 7–10 cm above the surrounding ground level). The loggers recorded temperature (0.5°C resolution) at hourly intervals from May 2011 to March 2012. Care was taken not to damage the nest structure, and albatross researchers assisted with iButton deployment into occupied nests during their routine surveys. Soil temperature was recorded hourly at 2 cm depth by iButtons that were inserted in the side of plastic marker poles and placed into the soil 2 m away from each nest. For occupied nests, chick mortality or fledgling date was obtained from the albatross monitoring programme records (see Crawford & Cooper Reference Crawford and Cooper2003 for programme description).

Fig. 2 Sampling locations of abandoned wandering albatross nests (triangles) along Marion Island’s east coast between the meteorological station and Ship’s Cove (to the north) and East Cape (to the south). The insets show the position of Marion Island in the Southern Ocean (top) and of the location of the study site on Marion Island (bottom).
Sampling of nests and other habitats for caterpillars
During the April–May relief voyage in 2012, 43 abandoned wandering albatross nests were identified between the meteorological station on Marion Island’s east coast to Ship’s Cove in the north and East Cape to the south (Fig. 2), excluding the wandering albatross long-term study colony at Macaroni Bay to which general entry is not permitted. Nests were located mainly in dry or wet mire communities, or along Blechnum penna-marina slopes (Gremmen Reference Gremmen1982). Following Sinclair & Chown (Reference Sinclair and Chown2006), only nests not currently occupied by birds were sampled. These were termed ‘abandoned nests’ and subsequently grouped into three types: i) old, nests that had not been occupied for most of 2011 or the previous seasons, ii) fledged, nests that had been occupied the previous season, i.e. built in November 2010 and from which chicks had fledged between December 2011 and January 2012, and iii) new, nests built for the current season, i.e. in November 2011, but had recently failed due to egg or young chick mortality. Researchers working over the 2011/2012 field season provided this information based on nest monitoring routinely conducted at the island. Fifteen new nests and fourteen of each of the fledged and old nests were selected as we came upon them and sampled across the area, within the constraints of the Prince Edward Islands Management Plan (Chown & Froneman Reference Chown and Froneman2008).
The height and the largest and smallest diameters of each nest were recorded, and the vegetation occurring on and surrounding the nest, the number of mouse burrows visible, and the distance from the coast were noted. The nest material was searched by hand for caterpillars by 2–4 samplers, who completely dismantled the nest and searched all of the material for caterpillars. No time limit was set on the search, which was typically completed in 1–2 hours, after which the material was replaced to form an in situ mound. Caterpillars were placed in groups of c. 20 in 350 ml plastic jars filled with nest material and returned to the laboratory within 6 hours of collection.
Caterpillars were then separated from the nest material, counted and weighed (0.1 mg resolution; Mettler AE163, Mettler-Toledo, Cape Town, South Africa), and returned within 24 h to the nests from which they were collected. Individual caterpillar dry mass was estimated from a linear regression of dry on wet mass by using the mass of 138 individuals dried at 60°C (dry mass (g) =0.113311×wet mass (g); intercept=0) provided by Sinclair & Chown (Reference Sinclair and Chown2005). Because caterpillars are usually concentrated in the surface layers of the nest (T.M. Haupt, S.L. Chown, personal observations), caterpillar biomass and density in nests were expressed as mg m-2 and numbers m-2, respectively, with the surface area of each nest calculated as the area of an ellipse.
Similar to Sinclair and Chown (Reference Sinclair and Chown2006), estimates of caterpillar biomass and density in nests in April and May 2012 were compared to those obtained from invertebrate surveys in April and May 1997 (Hänel Reference Hänel1999). Although a 15 year time difference exists between the habitat and albatross nest sampling events, the recent general decline in invertebrate densities in most habitats on Marion Island, largely owing to predation by introduced mice (Chown et al. Reference Chown, McGeoch and Marshall2002, Chown & Froneman Reference Chown and Froneman2008, also G.T.W. McClelland, A.E. Burger, R.J. van Aarde & S.L. Chown, unpublished data) biases our analysis against finding higher densities of caterpillars in nests. Dry caterpillar biomasses and numbers in nests were compared to those obtained by Hänel (Reference Hänel1999) from hand-sorted 7 cm diameter soil cores on a per-surface-area basis. These previous data were collected in an island-wide stratified approach, covering the mire communities dominated by Sanionia uncinatus (Hedw.) or Blepharidophyllum densifolium (Hook.) Ångström ex C. Massal., and non-mires dominated by P. cookii, C. plumosa or Crassula moschata G.Forst.
Statistical analyses
Microclimate temperature data from occupied and abandoned nests, as well as adjacent soil habitats were divided into months, and for each month the mean, daily minimum and maximum, and absolute minimum and maximum temperatures were obtained. For each month, a generalized linear model (Gaussian distribution of errors, log link in the case of absolute minimum and maximum as data were skewed), implemented in R version 3.0.0 (R Development Core Team 2013), was used to compare temperature variables of occupied nests with those of abandoned nests and the adjacent soil habitats. For each nest and adjacent soil habitat, temperatures at which caterpillars are below their CTmin, i.e. <0.2°C (because of the CTmin range of -1.6°C to 0.1°C), supercooling point, i.e. <-3.3°C (because of the supercooling range of -3.4°C to -7.1°C), and lower lethal temperature of <-8.9°C (based on 100% mortality at -9°C; Klok & Chown Reference Klok and Chown1997), were counted in Microsoft Excel (Microsoft, Seattle, WA, USA).
Caterpillar abundance (i.e. biomass and density) was compared among nest types (old, fledged and new) using a generalized linear model (assuming a quasi-Poisson distribution of errors following Crawley Reference Crawley2012) implemented in R version 3.0.0. Because of relatively high numbers of zero counts in the samples from non-nest vegetation types, caterpillar biomass and density were compared among new nests and the vegetation types using a hurdle model (with negative binomial distribution) (Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009) implemented in R version 3.0.0 using the pscl library (Jackman Reference Jackman2014).
Results
Some iButtons failed to record or were irrecoverable, or the chick died soon after sampling of nest temperatures began (Fig. S1 found at http://dx.doi.org/10.1017/S0954102015000383), thus, temperature data were available for three occupied nests, eight soil habitats adjacent to occupied nests, five abandoned (fledged) nests and five soil habitats adjacent to abandoned nests. Temperatures in occupied nests were higher than in other habitats (Fig. 3) with a broad range of daily minimum and maximum temperatures (Table SI http://dx.doi.org/10.1017/S0954102015000383). Albatross research monitoring records indicate that the chicks began venturing away from the nests at the beginning of December and fledged at the end of the month. A decline in temperature of the now-vacant nests was noticeable at this point (Fig. 3). Whilst nests were occupied (May to December 2011) monthly temperature parameters (i.e. mean, daily minimum and maximum, and absolute minimum and maximum) were mostly significantly higher in occupied nests compared to adjacent soils, and compared to abandoned nests and adjacent soils (Table I). After chicks had fledged (i.e. December 2011 and January 2012), there were mostly no significant differences in the monthly temperature parameters between these habitats (Table I), though where present these were between abandoned nests and soil sites, reflecting spatial variation in microclimate.

Fig. 3 Mean (± standard error) temperature recorded in occupied and abandoned wandering albatross nests and soil habitats adjacent to these nests from May 2011 to March 2012. Note that the period in December 2011 where temperatures begin to converge among nest types is coincident with the period when chicks begin venturing away from nests and fledge.
Table I For each monthly temperature parameter, habitats which had significantly lower temperatures compared to occupied nests are shown. These are: soil adjacent to occupied nests (OS), abandoned nest (AN) and soil adjacent to abandoned nests (AS). Non-significant differences between occupied nests and other habitats are indicated by ‘ns’. The single instance where temperatures were significantly lower in occupied nests compared to other habitats is indicated by * (for full statistical results see Supplemental Table S2 found at http://dx.doi.org/10.1017/S0954102015000383).

In occupied nests, CTmin threshold events (temperatures <0.2°C) occurred on average 50 times over the course of the year compared to 420 in abandoned nests and 842 in soil habitats. The large number of events in the soils and abandoned nests reflect the repeated passing of the threshold given average temperatures close to zero in the winter months and unpredictability of temperature change at the island (Chown & Froneman Reference Chown and Froneman2008). The temperatures in nests or soils never reached the thresholds at which freezing (<-3.3°C) or death (lower lethal temperature <-8.9°C) occur.
Caterpillar biomass and density in new nests was significantly higher than in old and fledged nests (Table II, Fig. 4). Moreover, the coefficient of variation of caterpillar biomass in new nests was lower than that found for fledged and old nests (Fig. 4). Compared to vegetation complexes sampled in April and May 1997, caterpillar biomass in new nests was significantly lower than in S. uncinatus and C. plumosa, no different than in C. moschata, and significantly higher than in B. densifolium and P. cookii (Table III). Caterpillar density in new nests was significantly lower compared to S. uncinatus, C. plumosa and B. densifolium, and no different to that found in C. moschata and P. cookii (Table IV). By contrast, the coefficient of variation in nests, and new nests in particular, was 5–6 fold lower, in the latter case significantly (Fligner-Killeen test: X 2=102.5786, df=16, P<0.0001), than in all other habitats (Fig. 4).

Fig. 4 Mean (± standard error) and coefficient of variation (CV) of biomass (mg m-2) and density (numbers m-2) of Pringleophaga marioni caterpillars in eight habitats on Marion Island. Five vegetation complexes sampled by Hänel (Reference Hänel1999) in April or May 1997 (Poa cookii, Cotula plumosa, Crassula moschata, Blepharidophyllum densifolium and Sanionia uncinatus), and old, fledged and new nests sampled in this study.
Table II Outcomes of the generalized linear models (negative binomial distribution of errors, log link function) comparing caterpillar biomass (mg m-2) or density (numbers m-2) in new, old and fledged nests.
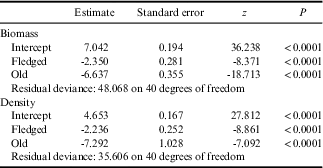
Table III Outcome of the hurdle model comparing caterpillar biomass (mg m-2) in new nests with that found in five vegetation complexes (Blepharidophyllum densifolium, Cotula plumosa, Crassula moschata, Poa cookii and Sanionia uncinatus) in April and May 1997 (Hänel Reference Hänel1999).

Table IV Outcome of the hurdle model comparing caterpillar density (numbers m-2) in new nests with that found in five vegetation complexes (Blepharidophyllum densifolium, Cotula plumosa, Crassula moschata, Poa cookii and Sanionia uncinatus) in April and May 1997 (Hänel Reference Hänel1999).

Discussion
Here we extend previous observations (Sinclair & Chown Reference Sinclair and Chown2006) to show that wandering albatross nests are warmer than surrounding habitats for the duration of the albatross incubation period, and that this increased temperature substantially decreases the incidence of events below the CTmin of caterpillars. Like Sinclair & Chown (Reference Sinclair and Chown2006), we also find higher biomass and lower variance of caterpillars in new versus older nests. However, dividing the ‘old’ nests into those which fledged chicks and those which are more than one season old reveals that the decline in caterpillar abundance once the albatrosses leave is rapid (within several months), suggesting a relatively short cycle during which the nest harbours a high density of caterpillars. These observations are consistent with both the c. 1 year life cycle proposed by Haupt et al. (Reference Haupt, Crafford and Chown2014a), and the thermal ecosystem engineering hypothesis.
Unlike the previous investigation, which proposed the thermal ecosystem engineer hypothesis (Sinclair & Chown Reference Sinclair and Chown2006), we found that caterpillar abundance or biomass is not always higher in newly abandoned nests than in other non-nest habitats. Why is this the case? First, the finding might be a consequence of the difference in sampling methods between those used for nests and those used in the habitat surveys undertaken previously (Hänel Reference Hänel1999). The former included a relatively large area (whole nests, on average 7010 cm2) whereas the latter include quite small areas (7 cm diameter cores, area of 38.5 cm2) which are then scaled up to provide caterpillar abundance per m2. Indeed, larger census areas tend to bias density estimates downwards (Gaston et al. Reference Gaston, Blackburn and Gregory1999), and this may have had an effect here, though the same effect should have been realized for the previous investigation. Second, it is clear that abundances are naturally dynamic and expected to vary from year to year, even though previous studies have suggested lower, short-term interannual abundance variation characterizes many arthropod species on Marion Island compared with those elsewhere (Crafford et al. Reference Crafford, Scholtz and Chown1986, Barendse & Chown Reference Barendse and Chown2001). Alternatively, declining abundances across the island associated with the impact of house mice and their use of different habitats (Chown et al. Reference Chown, McGeoch and Marshall2002, Smith et al. Reference Smith, Avenant and Chown2002) might also be causing variation in abundances among years. Nonetheless, the consistently low coefficient of variation in biomass/density among new nests, compared with other nest types and non-nest habitats, is in keeping with Sinclair & Chown (Reference Sinclair and Chown2006). It shows, together with relatively high abundances, that new nest habitats are consistently good habitats for caterpillars, and more so than might be expected for any other habitat type sampled on the island. In consequence, it appears that wandering albatrosses do serve as ecosystem engineers, although the magnitude of the effect is likely to vary among individual nests.
The thermal ecosystem engineering hypothesis relies on increased temperatures (closer to optima for caterpillar growth and survival) for the duration of incubation (Sinclair & Chown Reference Sinclair and Chown2006). Our year-long temperature dataset confirms that this is the case for the entire period that nests are occupied. Whilst nests were fully occupied (i.e. between May 2011 and November 2012), mean temperatures were anywhere between 8°C and 16°C. By contrast, mean temperatures in abandoned nests and surrounding soils were between 3°C and 10°C during the monitoring period, and when nests were abandoned, or once chicks left the nest, temperatures rapidly converged with those of the surrounding environment. Thus, caterpillars in occupied nests are less likely to encounter critical temperatures that limit activity and are likely to be consistently closer to optima for growth, than caterpillars in other environments. Although temperatures in nests occasionally reach values that are inimical to growth (i.e. ≥15°C), fluctuating temperatures that include these higher values have less impact than constant exposure to these temperatures and seem to result in growth that is close to the optimum rate, with minimal impact on survival (at a fluctuating temperature of 5–15°C the larval stage is 37 weeks) (Haupt et al. Reference Haupt, Crafford and Chown2014a; see also Colinet et al. Reference Colinet, Sinclair, Vernon and Renault2015 for general discussion of fluctuating temperatures).
We expect that these increased nest temperatures will lead to faster development of caterpillars. Based on a sum of effective temperatures of 227 degree days for completion of the larval stage, with a lower development threshold of 0.8°C (Haupt et al. Reference Haupt, Crafford and Chown2014a), the temperature data suggest that caterpillars are likely to complete their life cycle within 30 weeks in occupied nests, but in about a year in unoccupied nest (or non-nest) habitats. This increase in development time is consistent with the thermal ecosystem engineering hypothesis, and also with the relatively low caterpillar abundance in nests from which chicks have fledged. We expect that the majority of caterpillars from those nests have already completed development and left the nest at the time of sampling.
An alternative explanation for the rapid decrease in caterpillar abundance is that albatross adults and later on chicks themselves discourage introduced house mice, which are major predators of P. marioni caterpillars (Smith et al. Reference Smith, Avenant and Chown2002), from occupied nests, reducing caterpillar predation pressure. However, numbers of mouse burrows do not differ among nest categories (mean±standard deviation (n), old: 1.6±1.8 (14); fledged: 2.3±2.7 (14); new: 3.1±3.0 (15); generalized linear model, quasi-poisson distribution, P>0.44), and the impact of mice on albatross chicks (Jones & Ryan Reference Jones and Ryan2010) suggests that albatross presence does not particularly deter the activities of mice at the nest.
The low abundance of caterpillars in nests that have not been recently occupied suggests that there is differential colonization of newly-built albatross nests. Information on caterpillar chemosensory and thermal cue responses show that they are unlikely to seek out nests actively (Haupt et al. Reference Haupt, Sinclair and Chown2014b). It is not known whether adult moths seek out oviposition sites, as is common in other Lepidoptera, but given the short lifespan and low vagility of the adult female moths (Crafford et al. Reference Crafford, Scholtz and Chown1986), coupled with the presence of P. marioni larvae across a range of habitats far from albatross nests (Burger Reference Burger1978, Crafford et al. Reference Crafford, Scholtz and Chown1986), this seems unlikely (Haupt et al. Reference Haupt, Sinclair and Chown2014b). An alternative explanation is that caterpillars or eggs are serendipitously incorporated into the nests as adult albatrosses construct them from the surrounding vegetation (Warham Reference Warham1990, Ryan & Bester Reference Ryan and Bester2008), which often includes P. cookii and bryophytes such as B. densifolium (observation of 60 nests indicated that P. cookii was used for construction of 49 of the nests and mosses in the same number, though not necessarily the same nests; data available on request) which have high densities or biomasses of caterpillars (Burger Reference Burger1978, Crafford & Scholtz Reference Crafford and Scholtz1987, Chown et al. Reference Chown, McGeoch and Marshall2002).
Indeed, this can be estimated based on what is known of albatross nest size and caterpillar biomass per m2. The mean volume of a new nest is 175 235 cm3 (n=15). To construct such a nest, assume an albatross would need to collect 8.7 times the volume of a standardized area used for expressing caterpillar biomass (i.e. 1 m2×2 cm, assuming caterpillars occupy only the top 2 cm of substrate, which=20 000 cm3). Assume that, from Hänel’s (Reference Hänel1999) data, caterpillar density is on average 26 caterpillars per m3 in P. cookii-dominated or 52 caterpillars per m3 in S. uncinata bryophyte-dominated habitats. This suggests that albatrosses might incidentally collect somewhere between 230 and 450 caterpillars during their activity. Making the conservative assumption that somewhere between 10 and 50% of individuals actually end up being incorporated into nests, this suggests that nests should have somewhere between 23 and 200 caterpillars. Our counts from new nests vary between 33 and 148. The estimates are within the same order of magnitude, suggesting that the idea is plausible. Once caterpillars or eggs are added to the nests, favourable nest conditions improve caterpillar growth and survival. Thus, although it is clear that a thermal ecosystem engineering effect on caterpillar performance is realized, the high abundance in nests may be serendipitous.
Acknowledgements
We thank two anonymous reviewers for their helpful comments on the manuscript, Asanda Phiri for her dedicated assistance to the project, and Stephen Kirkman and Fhatani Ramwashe for assistance with Fig. 2. Mashudu Mashau, Kersti Hickley and Charlene Janion-Scheepers assisted with the sampling, and the albatross programme researchers, Maëlle Connan and Mia Cerfonteyn, assisted with logger deployment. This work was supported by a National Research Foundation grant (SNA14071475789) through the South African National Antarctic Programme.
Author contribution
SLC & BJS designed the research. TMH, BJS, JDS & SLC undertook the research. TMH & SLC undertook the statistical analyses. All authors contributed to writing of the manuscript.
Supplementary Material
A supplemental figure and two tables will be found at http://dx.doi.org/10.1017/S0954102015000383.











